New Concept to Non-Invasively Screen Iron Deficiency in Patients
2020-04-16AliDabiriErikSamwelandGhassanKassab
Ali E.Dabiri,Erik Samwel and Ghassan S.Kassab
1California Medical Innovation Institute, San Diego,CA, 92121, USA
23DTholdings, 11107 Roselle St., San Diego, CA, 92121,USA
3MicroSense, Lowell, MA, 01852,USA
Abstract:Nearly two billion people are afflicted with iron deficiency and approximately 300 million children globally have anemia.Most of those affected are unaware of their lack of iron, in part because detection of iron deficiency requires a blood test.It is becoming increasingly important to screen these individuals to reduce medical cost and avoid chronic disease conditions.There are limited settings of laboratory infrastructure for standard blood-based tests around the world to routinely accomplish this important screening test.We propose a new concept to use either human hair or nail as a screening method to detect steady state iron content in patients utilizing a sensitive portable magnetometer.The optimum hair/nail sample weight should be determined for acceptable detection resolution(i.e.,acceptable correlation with the results of blood test).The advantages of a portable device are as follows:non-invasive approach,point of care(on the spot)response with patient hair/nail sample to make screening much faster, and make screening for iron deficiency more available and affordable for patients around the world by eliminating current shortcomings of blood-based iron screening.The potential outcome of this concept is of great value to iron deficient patients.An easy,and cost-effective screening system will reduce morbidity, chronic diseases and medical cost substantially throughout the world.
Keywords: Iron deficient patient;screening;hair;nail;magnetometer
1 Introduction
Globally, anemia affects 1.6B people which corresponds to 25% of the population.The highest prevalence is in preschool-age children and the lowest prevalence is in men.The population group with the greatest number of individuals affected is non-pregnant women (https://apps.who.int/iris/bitstream/handle/10665/43894/9789241596657_eng.pdf?sequence=1).The data suggests that the median value of productivity losses due to iron deficiency in world is about $4 per capita or 0.9% of gross domestic product (https://idl-bnc-idrc.dspacedirect.org/bitstream/handle/10625/25059/109343.pdf?sequence=1).Deficiency in iron, a mineral necessary to carry oxygen in hemoglobin, is thought to be the most common cause of anemia.Iron deficiency can result from inadequate intake or absorption of dietary iron,increased need in periods of growth, increased losses from menstruation in adolescent girls, or infection by intestinal helminths, such as schistosomiasis or hookworm infestation, in areas endemic to these parasites.Dietary iron insufficiency is the most common cause of iron deficiency in infants and young children, while blood loss is responsible for iron deficiency in older children and adults.In premenopausal women, menstrual bleeding is the most common cause of iron deficiency.Since iron is required for the survival and virulence of many pathogens, concerns have been expressed on possible increased risk of malaria, particularly among iron-replete children.Screening to identify iron deficiency in children prior to iron supplementation, however, is not feasible in many malaria-endemic settings.Given the importance and magnitude of anemia globally, particularly in areas where malaria transmission is intense, an assessment of available evidence has been carried out, to examine the safety and effectiveness of iron supplementation in children, including in malaria endemic areas (file:///C:/Users/Calmi2/OneDrive%20-%203DT%20Holdings%20Inc/ali/IRON/2017 WHO.pdf).According to NHLBI report, an estimated 20% women of childbearing age have iron-deficiency anemia (https://www.transparencymarketresearch.com/iron-deficiency-anemia-market.html).
2 Diagnostic Methods
2.1 Current Practice
Human serum is recognized as the“gold standard”to determine iron and other mineral levels.Various invasive laboratory tests are currently used to test the body iron level.The conventional tests are total blood count,serum iron concentration,serum ferritin level,and total ironbinding capacity(TIBC).It is important to note that in the most widely used test of serum ferritin level, the body iron status may not be accurately reflected due to various conditions, including pregnancy, acute or chronic inflammatory disease,malignancy, infection, renal failure or malabsorption syndrome [1].Although conventional tests are normally available in developed countries, it is rather difficult to provide a network of diagnostic laboratories in underdeveloped nations due to the high cost of capital, supplies, and lack of trained medical technicians to screen iron deficient patients.
2.2 New Concept
2.2.1 Background
Recently,it has been reported that human hair may be an alternative approach to detect the human body iron level, replacing the use of a blood sample.In 1956, Duffield et al.[2] concluded that hair iron concentration may not provide adequate information regarding total body iron.In 1971, Lovric et al.[3]measured the iron content of various hair segments in children with iron deficiency and iron overload and concluded that there was no significant association between the groups with respect to hair iron concentration.In subsequent years, however, Bisse et al.[4] concluded that hair iron concentration is useful in the evaluation of body iron status.Sahin et al.[1] studied the possible association between blood parameters and hair iron concentration in patient groups with different body iron contents through chemical analysis.The study was comprised of patients with iron deficiency anemia, as well as patients with transfusionrelated anemia.A control group was selected with no history of underlying disease.They studied 25 patients (mean age 33 years) with iron deficiency anemia, 20 patients (mean age 22 years)with transfusionrelated anemia that showed a difference in body iron content, and 21 healthy controls(gender-matched, mean age 28 years) with no history of underlying disease.The results showed measured mean hair iron56Fe and57Fe concentrations of the iron deficiency group were 5.08 and 6.03 μg/g,respectively, and in the transfusionrelated anemia group these values were 28.9 and 29.4 μg/g,respectively.In the control group, the mean hair iron56Fe and57Fe concentrations were measured as 12.0 and 17.6 μg/g, respectively.The highest hair iron concentration (89.4 μg/g) was observed in transfusionrelated anemia patients, whereas the lowest hair iron concentration (0.77 μg/g) was determined in the iron deficiency anemia group.The differences between the three groups with respect to hair iron56Fe and57Fe concentrations were found to be statistically significant.In addition, a positive correlation was found between hair iron56Fe and57Fe concentrations and serum iron, ferritin level, transferrin saturation,and MCVand MCH values,which are the most important parameters showing body iron content.This study concluded that patient groups with different body iron content had a significant difference in hair iron concentration and these values were correlated with laboratory markers of body iron content.These results support the view that hair sampling can be used as a marker of body iron content.In another study, Donnici et al.[5] developed a method to determine iron in human hair samples by graphite furnace atomic absorption spectrometry (GF AAS).They measured iron levels in hair samples from 20 preadolescent, menstruating girls in schools in Brazil.The concentration range was 14-26 µg/g.Baranowska et al.[6] analyzed hair samples collected from the inhabitants of Poland by x-ray fluorescence spectrometry and obtained an average concentration of 36.3µg/g for Fe in hair samples.
Human nails have also been proposed as a less expensive, non-invasive method of assessing the iron status of individuals [7].Sobolewski et al.[7] measured the iron content of healthy and iron deficient individual nails by atomic absorption spectrophotometry and compared those results with other measurements of iron status including the bone marrow.The iron content of the nails ranged from 6 to 26 μg/g of nail for women and 6 to 23 μg/g of nail for men in healthy individual group.This value dropped to 1-2 μg/g for the women iron deficient group.In iron-depleted and iron-sufficient subjects there was a correspondence between iron content of the nails and bone marrow iron,serum iron,and TIBC.
The major disadvantage of this method is the need to transport the hair/nail samples to an analytical laboratory for testing which is time consuming, expensive, and such a facility may not be accessible in developing countries.Nail is more desirable for the proposed concept as opposed to hair to reduce number of variables like hair thickness, color, curvature or how close the hair samples are from the scalp and sampling difficulties.
2.2.2 Forms of Iron Content
As mentioned above, the iron in nail could be either in the form of pure iron, iron oxides, or a combination of pure iron, and its associated oxides.Aside from hematite (α-Fe2O3), the range of specific magnetization is from 74 to 210 emu/g, a factor of about 3.We will use the lower number for the feasibility analysis.Given that pure iron very easily oxidizes, it is likely that the iron will be primarily in the form of one or more oxide(s), although Sahin et al.[1] reported that the iron is in the form of56Fe and57Fe as discussed above.It is desirable to perform Mossbauer spectroscopy (https://en.wikipedia.org/wiki/M%C3%B6ssbauer_spectroscopy) of the nail to determine the oxidation state of iron: a) What form iron and iron oxide(s) are in? and b) Is there a large variation in the form of the iron and iron oxide(s)and the proportions of the different oxides from one sample (person) to another.This analysis is only needed to develop an accurate conversion formula to calculate the iron density from the measured magnetic moment to create a protocol that is based on the specific forms of iron that we find in the nail.
2.2.3 Feasibility Calculation
The density of human nail is about 0.65 g/cc(measured in our lab.).The average nail growth is about 0.8 mm/ week [8] which translates into 5 mg/week for average size of a finger or 50 mg for 10 fingers.The average iron concentration in human nail for the iron deficiency anemia patient is about 1 µg/g according to previous research [7].One gram of nail should have a magnetic moment of 1 µg × 210 emu/g =0.0002 emu or ~200 µemu [9].Although Sahin et al.[1] reported that the iron is stored in the hair as pure iron, this may not be exactly the case.If iron is not embedded in nail, iron particles could quickly oxidize.Some iron oxides are magnetic but not all.For example, hematite (α-Fe2O3) is only weakly ferromagnetic (0.4 emu/gvs.210 emu/g), maghemite (γ-Fe2O3) and magnetite (Fe3O4) have lower magnetization than pure iron, 74 and 84 emu/gvs.210 emu/g respectively.The magnetic moment for a week of nail growth is about 10 µemu assuming the iron is in the form of pure iron.For our type of device, as described below, this is at least one order of magnitude higher than what we can measure assuming the iron is in the form of pure iron.Therefore,there should be adequate sensitivity if some of the iron is in the form of oxides.
2.2.4 Sample Preparation
The nail sample should be prepared in such a manner to eliminate all possible environmental contaminations.Any coatings may interact with the surface atoms of the magnetic core and form a magnetically disordered layer, reducing the total amount of the magnetic phase.Prior to the nail sampling process, there should not be any cosmetic nail applications, such as nail polish.We need to prevent additional contamination during the process used to cut nail into small pieces to place them inside sample holder.An example could be stainless scissors (or similar instruments) which could potentially have minimum particle loss while cutting the nail.While stainless steel has much weaker magnetic properties than iron, any steel contamination may be problematic.Custom cutters may need to be used if this process ends up not to be desirable.Samples collected should be stored in an auto-sealable polythene bag and should be acid washed(nitric and hydrochloric) to remove surface iron contamination.
2.2.5 Proposed Device
Hair/nail can be an attractive alternative due to its simplicity as a sample(easy to obtain,without trauma and/or discomfort),storage,transport and handling[10].The determination of hair/nail iron concentration necessitates a strict sampling regime.Although there are more investigations on the existence of iron in hair than nail,there is evidence to show the existence of iron in the nail.It is less complicated to use nail rather than hair due to less potential of contaminations and fewer sampling issues such as handling and securing the nail in the sample holder for the magnetic measurement.
We propose a new concept to use human nail as a screening method to detect deficient iron content in patients using a novel cost-effective magnetic approach.The proposed concept is novel in the following aspects: 1) Non-invasive, 2) It is carried out magnetically , rather than chemically for the first time, 3)Portable ultra-sensitive magnetometer with detection limit of about 0.1 µg for pure iron corresponding to signal level as low as 1 µemu and noise level around 20 nemu which is fundamentally a new technological innovation, 4) On the spot screening, 5) Addresses present shortcomings of screening iron deficiency anemia patients around the world,and 6) Can be operated by a technician.
Sensitive magnetometer is based on sample vibration.It is called Vibrating sample magnetometer(VSM).A VSM is a scientific instrument that measures magnetic properties and is shown schematically in Fig.1.Simon Foner at MIT Lincoln Laboratory invented VSM in 1955 and reported it in 1959(https://en.wikipedia.org/wiki/Vibrating-sample_magnetometer).A sample is first magnetized in a uniform magnetic field.It is then sinusoidally vibrated, typically through the use of a voice coil actuator.The induced voltage in the pickup coil is proportional to the sample's magnetic moment, but does not depend on the strength of the applied magnetic field.In a typical setup, the induced voltage is measured with a lock-in amplifier using the vibration frequency as the reference.
The VSM is commercially available through MicroSense (http://www.microsense.net/products-vsm.htm) which is shown in Fig.2.Some of the relevant features of the unit are as follows: 1) Highest field electromagnet based VSM, up to 3.7 Tesla, 2) Easy to use, 3) Fast, with sweep rates up to 1 T/s and 1000 points/second, and 4) VSM has a noise level around 20 nemu (~20 × 10-9emu) under certain conditions.This VSM uses an air-cooled magnet power supply and has a space saving, flexible design with all components mounted on wheels for easy lab reconfiguration.The VSM is one of the most versatile types of systems to measure magnetic properties of any type of material and very suitable to determine nail iron stored in a magnetic form.The disadvantage of this system is that it is expensive($100K),heavy and has many options that are unnecessary for this application.
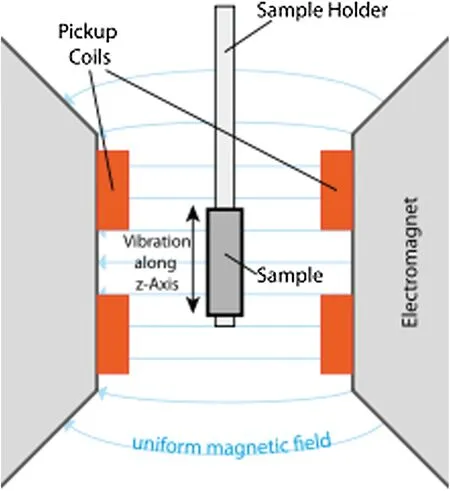
Figure 1: Vibrating sample magnetometer schematic

Figure 2: Vibrating sample magnetometer
To do an iron content measurement, we only need a very small fraction of the VSM capabilities.We require a relatively simple measurement at a field that is much lower than the maximum field offered by a VSM and this field needs to be applied only for a very small period of time.Such a field can be created using a pulsed current through a simple coil.To measure the magnetic moment only at a single field point at room temperature, most of the VSM features are not needed and much more simple inductive measurement may be used.This method is similar to the method employed in Hysteresis graph system,sometimes also called a BH Looper.In such a system the sample is inserted inside a detection coil which itself is placed inside an electro-magnet or magnet coil.The electric signal of the detection coil with the sample is compared with the signal from a reference coil without the sample and from this the sample magnetization is determined.No vibrator (+ vibrator controller + lock-in amplifier) is needed because the pulsed field produces a very quick flux change, which generates the measured voltage.To generate the pulsed current, a capacitor is first charged relatively slowly through a very cheap power supply and this capacitor is then quickly discharged through the magnetizing coil.Because the current is applied for only a short period of time, no expensive water-cooling system is required (unlike in a VSM).By comparing the measured voltage of a reference sample and the sample under test we can determine the iron content.Therefore, a miniaturized system would consist of a solenoidal coil with a detection coil and sample insert + a capacitor + charging circuit + some data acquisition and control electronics.A simplified schematic is shown in Fig.3.Measurement of the whole hysteresis loop requires powering the magnet coil with an AC current.If a single point measurement of the saturation induction of the sample is sufficient, this could potentially be measured by applying a single high-current pulse to the magnet coil.This would significantly reduce the size and cost of such a device.If it is determined that such a pulsed measurement is sufficient, the design target would be to fit a final product into a rolling case and strive to make it possible to power it from a solar panel charged battery pack.This would present a technological innovation of a portable ultra-sensitive magnetometer with detection limit of about 0.1 µg for pure iron corresponding to signal level as low as 1 µemu and noise level around 20 nemu.The nail iron content varies from about 1-2 μg/g for iron deficiency group to ~26 μg/g for the control group[7].Therefore,the detection unit should have a dynamic range of ~30.
3 Discussion
The rationale for this concept is that the non-invasive detection of iron level in human hair or nail can be made much more readily and inexpensively than the current methods where the blood sample needs to be submitted to a diagnostic laboratory for detection/screening.There are other methods in addition to the magnetic approach that may have the potential to either non-invasively or mini-invasively detect iron level of anemic patients.These methods are discussed in this section.
3.1 Non-invasive Methods
Donnici et al.[5] developed a method to determine iron in human hair samples by graphite furnace atomic absorption spectrometry (GF AAS).The drawbacks are the need for dissolution of hair samples in order to determine iron level which are normally associated with cost of reagents and time-consuming process in sample preparation and the need to transport the hair samples to an analytical laboratory for testing which is time consuming and the facility may not be accessible in developing countries.The system is expensive and cannot be afforded for each individual clinic.
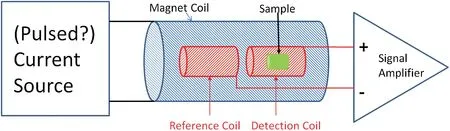
Figure 3: Schematic of the portable device to measure the iron in human hair
Hennig et al.[11]measured an established indicator of iron status,red blood cell zinc protoporphyrin,in the microcirculation of the lower lip.An optical fiber probe was used to illuminate the lip and acquire fluorescence emission spectra.With blue light excitation,zinc protoporphyrin fluoresces,while haem does not.To detect fluorescence, they used an optical fiber probe to illuminate and acquire the fluorescence emission spectra from the lower lip, where only a thin, nonpigmented epithelial layer covers the bloodfilled capillaries perfusing the underlying tissue.Although the method is simple, real time and the device can be portable, the iron is not measured directly and also the different melanin pigmentations of the patient's lips makes the process more complex.It is also important to recognize that iron deficiency can be present without low hemoglobin levels since about 70% of the body's iron is found in the red blood cells of your blood called hemoglobin.However, progressive depletion of iron stores without replacement will eventually result in anemia.
Terahertz (THz) spectroscopy (https://en.wikipedia.org/wiki/Terahertz_spectroscopy_and_technology)also has a potential to detect iron level patients.Whole blood and its components were studied by the THztime domain spectroscopy method [12].They found that change of composition caused by pathological processes in the organism can considerably affect the optical properties of blood components in the THz frequency range to create new rapid diagnostic method.An optical fiber probe could be used to illuminate and acquire the terahertz emission spectra from the lower lip like fluorescence spectroscopy method discussed above with the difference that this directly measures RBC concentration.There is also a potential to use THz directly to measure iron level by measuring THz emission spectra from the THz incident beam on the nail.It remains to be seen if this method is feasible.Portable THz spectroscopy systems are becoming commercialized (https://www.terahertzstore.com/store/products/terahertz-spectrometers/terahertzfiber-coupled-spectrometer-at-1550-nm-rigel-1550.htm).
3.2 Minimally- Invasive Methods
Small blood samples are necessary for in vitro analysis in these methods.In the methods noted below,blood samples can be used directly rather than serum.
Inductively Coupled Plasma Atomic Emission(or Optical)Spectroscopy(ICP-AES,or ICP-AOS),are emission spectrophotometric techniques for chemical analysis of elements by the fact that excited electrons emit energy at a given wavelength as they return to a ground state after excitation by high temperature argon plasma (https://en.wikipedia.org/wiki/).The rationale of this process is that each element emits energy at specific wavelengths peculiar to its atomic character.The energy transfer for electrons when they fall back to the ground state is unique to each element as it depends upon the electronic configuration of the orbital.This technique has been used to analyze biological samples.The analysis can be made in real time with high detection sensitivity but has too many spectra peaks for iron which makes the analysis complicated.The unit size is tabletop and this technique can be utilized to detect iron and its sensitivity with different blood samples.The drawbacks are the cost of sample preparation and the cost of the unit.This method could be used for either blood or solid sample like nail or hair.Another shortcoming is the need for dissolution of solid samples in order to determine iron level which are normally associated with cost of reagents and time-consuming process in sample preparation.In these methods, solid samples must be digested before analysis, generally requiring the use of acids, microwave ovens and high temperatures.Solid sample preparation is laborious,creates contaminations and is costly.
Tao and Huang[13]reported that blood viscosity can be reduced with magnetic fields of 1 T or above in the blood flow direction.One magnetic field pulse of 1.3 T lasting ∼1 min can reduce the blood viscosity by 20-30%.After the exposure,in the absence of magnetic field,the blood viscosity slowly moves up,but takes a couple of hours to return to the original value.The process is repeatable and reapplying the magnetic field reduces the blood viscosity again.In addition,such viscosity reduction does not affect the red blood cells'normal function and the donated blood sample is used to measure the viscosity in a small tube.The rationale comes from the blood cells clumping together, mostly in a line, like box cars on a train.The cells moving together as a train produces less resistance than if they were all bouncing around separately.Further, they tend to flow more down the middle of the tube, reducing friction with the tube wall.It can be postulated that the viscosity is directly proportional to the iron content of the RBC in the blood.This method can be used to determine the iron deficiency of the blood.The change of viscosity can be measured by a viscometer.There is more research to be performed to confirm the hypothesis.
A bio-impedance method can also be used to detect iron levels in real time.Iron is electrically conductive, and the concentration of iron is proportional to electrical conductance (inverse of impedance);i.e., less iron implies lower electrical conductance.As such, operating a conductance device on a blood sample can result in obtaining conductance data, and relatively low conductance data is indicative of low iron concentration.This also needs to be proven feasible.
There are presently three hemoglobin sensors[14]that is usually used for blood donor screening.Two of the sensors (Portable Hemoglobinometer HemoCue and Automated Hematology Analyzer, LH500) use finger prick and third sensor is non-invasive (Hemoglobin Sensor (NBM-200) that is not as accurate as the two sensors.As noted above, it is important to recognize that iron deficiency can be present without low hemoglobin levels since about 70% of the body's iron is found in the red blood cells of your blood called hemoglobin.However, progressive depletion of iron stores without replacement will eventually result in anemia.
The application of the portable device will be to measure the steady state body iron level averaged over about two months.This feature of the device is not a shortcoming since the iron level of people with iron deficiency or anemia will not materially change over two months and the condition tends to be chronic rather than transient.There are situations where this device may not diagnose the patients properly.For example, the patients with thalassemia (it is an inherited blood disorder where the red blood cell is destroyed) are anemic but have normal or high body iron level.The total annual incidence of symptomatic individuals is estimated at 1 in 100,000 throughout the world and 1 in 10,000 people in the European Union and they have many features that can be easily diagnosed[15].
4 Conclusion
Iron is an essential nutrient for development and cell growth in the immune and neural systems,as well as in regulation of energy metabolism and exercise.Anemia affects roughly one third of the World's population.In response to the need for better technology to screen iron deficiency anemia patients, we have conceptualized a novel simple portable medical device to non-invasively detect body iron level using human nail.We can in principle measure a signal as low as 1 µemu.When configured for capsules containing pieces of nail, the detection limit for pure iron is below 0.1 µg, corresponding to 5 mg of nail of an anemic patient which is about the weight of one fingernail growth in a week (if nail iron is pure iron).This technology has the potential to make the screening tests more readily available and inexpensive than the current blood tests or other analytical methods which necessitate diagnostic laboratories.An easy, cost-effective and on the spot screening system will reduce morbidity, chronic diseases and reduce medical cost substantially throughout the world.
Acknowledgement:This work is funded by 3DT Holdings.
Conflicts of Interest:The authors declare that they have no financial conflicts of interest to report regarding the present study.Mr.Erik Samvel is the general manager of MicroSense LLC where vibrating sample magnetometer is manufactured for various applications.
