miR-449对骨髓间充质干细胞成骨分化调控的机制研究
2020-04-09苏华斌胡波涌叶俊杰
苏华斌 胡波涌 叶俊杰

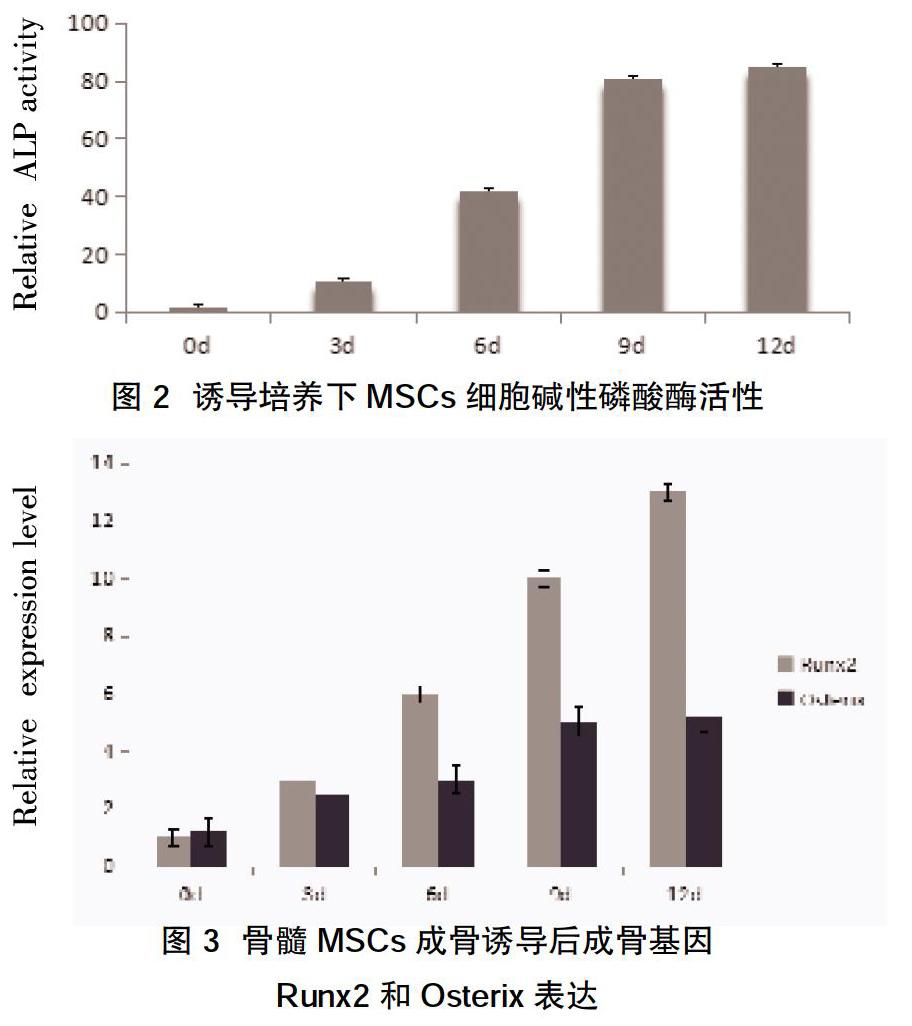
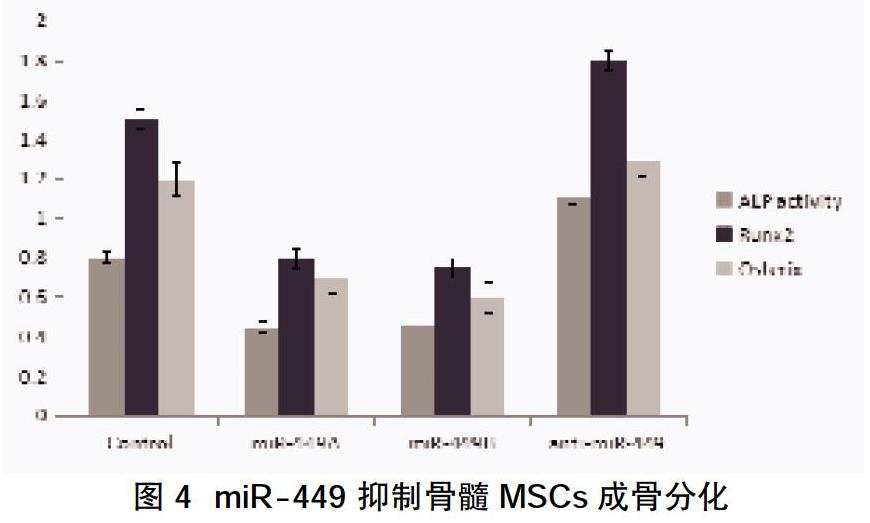
摘要:目的 探究miR-449是否具有調控骨髓间充质干细胞(MSCs)成骨分化的作用。方法 分离、培养骨髓MSCs,采用流式细胞仪检测CD44、CD29、CD34、CD45等表面相关标志抗原的表达鉴定骨髓MSCs;将MSCs分为对照组、miR-449a组、miR-449b组及anti-miR-449组,对照组用成骨诱导培养基培养,其余各组分别经miR-449a/b或inhibitors转染后在成骨诱导培养基培养,分别于培养第3、6、9、12天检测各组碱性磷酸酶活性,qRT-PCR检测miR-449a/b对成骨特异性基因Runx2、Osterix表达的影响。结果 ①第4代间充质干细胞CD44、CD29表达阳性,而CD34 和CD45表达阴性,符合骨髓间充质干细胞的特征。②成骨诱导培养3、6、9、12 d后,MSCs细胞碱性磷酸酶活性较上一时间点升高,且呈时间依赖性,差异有统计学意义(P<0.05);MSCs成骨诱导培养3、6、9、12 d后,Runx2、Osterix表达水平逐渐升高。③miR-449a组和miR-449b组AKP活性低于对照组,而anti-miR-449组AKP活性升高,差异有统计学意义(P<0.05);miR-449a组和miR-449b组 Runx2、Osterix表达较对照组下调,差异有统计学意义(P<0.05)。结论 本次实验成功分离、培养了骨髓MSCs,其具有体外成骨分化能力,而miR-449能抑制骨髓MSCs向成骨分化。
关键词:miR-449 a/b;间充质干细胞;成骨分化
中图分类号:R329 文献标识码:A DOI:10.3969/j.issn.1006-1959.2020.04.021
文章编号:1006-1959(2020)04-0069-04
Abstract:Objective To investigate whether miR-449 can regulate the osteogenic differentiation of bone marrow mesenchymal stem cells (MSCs). Methods Bone marrow MSCs were isolated and cultured. Flow cytometry was used to detect the expression of surface-associated marker antigens such as CD44, CD29, CD34, and CD45. Bone marrow MSCs were identified. The MSCs were divided into control group, miR-449a group, miR-449b group,and anti-miR-449 group, the control group was cultured with osteogenic induction medium, and the remaining groups were cultured in osteogenic induction medium after transfection with miR-449a / b or inhibitors, respectively in culture 3, 6, 9, and12 d, the alkaline phosphatase activity was detected in each group, and the effect of miR-449a / b on the expression of osteogenic specific genes Runx2 and Osterix was detected by qRT-PCR. Results ①The fourth generation of mesenchymal stem cells was positive for CD44 and CD29, while the expression of CD34 and CD45 was negative, which is in line with the characteristics of bone marrow mesenchymal stem cells. ②After 3, 6, 9, 12 d of osteogenic induction culture, the alkaline phosphatase activity of MSCs cells increased compared with the previous time point, and it was time-dependent,the difference was statistically significant (P<0.05). After 3,6,9,12 d of osteogenic induction of MSCs, the expression levels of Runx2 and Osterix gradually increased. ③The AKP activity of miR-449a group and miR-449b group was lower than the control group, while the anti-miR-449 group increased AKP activity, the difference was statistically significant (P<0.05); miR-449a group and miR-449b group Runx2, Osterix expression was down-regulated compared with the control group,the difference was statistically significant (P<0.05). Conclusion This experiment successfully isolated and cultured bone marrow MSCs, which has the ability to differentiate into bone in vitro, and miR-449 can inhibit the differentiation of bone marrow MSCs into osteogenesis.
3讨论
BMP/ Smad信号通路调控成骨化和成软骨化,是骨形成中最重要的一条信号通路。其中Smad4(mothers against decapentaplegic homolog 4)是BMP/Smad骨形成信号通路的中心介导者。Smadl/5/8 磷酸化后与Smad4形成复合物后进入到细胞核内调控成骨分化关键基因Runx2、Osterix等的转录,从而实现对成骨分化和骨成熟的调控[4,5]。本研究通过Targetscan等在线软件预测miR-449靶基因,发现Smad4是miR-449a/b潜在的靶基因。因此推测miR-449可能通过靶向调节Smad4表达来调控成骨分化关键基因Runx2、Osterix等的转录,从而影响MSCs成骨分化。
miRNAs作为一种新型的基因转录后调控方式,与组织器官发育、细胞生长、分化、凋亡、细胞运动、新陈代谢和疾病发生等生命活动密切相关[6]。越来越多的研究表明miRNA 在干细胞的自我更新和多向分化过程中发挥重要的调控作用[7]。已有研究[9]发现miRNAs在调控MSCs或前体细胞成骨、成软骨及成脂分化过程中也发挥重要生物学功能。miR-449能抑制MSCs向成骨分化,但具体的分子机制或靶基因调控作用尚不明确。
本研究通过分离、培养骨髓MSCs,鉴定其成骨分化能力,在骨髓MSCs转染经特殊化学修饰的miR-449 a/bmimics或inhibitors,通过qRT-PCR检测过表达miR-449a/b对成骨特异性基因Runx2、Osterix表达的影响。结果显示过表达miR-449a/b后Runx2及Osterix表达较对照细胞下调,表明miR-449能通过下调Runx2及Osterix表达抑制骨髓MSCs细胞向成骨分化。从而可能在骨质疏松、骨折愈合与骨不连的发生发展中发挥重要作用,因此,以miR-449为靶点开发的药物有望用于骨质疏松等多种骨代谢疾病的治疗或者逆转骨质疏松患者自体分离的间充质干细胞的成骨能力表现出明显的缺陷,从而将自体干细胞治疗应用于骨质疏松条件下的骨修复成为现实。
综上所述,miR-449能抑制骨髓间充质干细胞向成骨分化,未来可以miR-449为靶点开发相关药物治疗多种骨代谢疾病。
参考文献:
[1]Jie W,Dandan Y,Xuhong H,et al.Association of bone turnover markers with glucose metabolism in Chinese population[J].Acta Pharmacologica Sinica,2017,38(12):1611-1617.
[2]Fan J,An X,Yang Y,et al.MiR-1292 Targets FZD4 to Regulate Senescence and Osteogenic Differentiation of Stem Cells in TE/SJ/Mesenchymal Tissue System via the Wnt/β-catenin Pathway[J].Aging and Disease,2018,9(6):1103-1121.
[3]Kalladka D,Muir KW.Brain repair:cell therapy in stroke[J].Stem Cells Cloning,2014(7):31-44.
[4]Chew E,Prakash R,Khan W,et al.Mesenchymal stem cells in human meniscal regeneration:A systematic review[J].Annals of Medicine&Surgery,2017,24(C):3-7.
[5]Higashi K,Matsuzaki E,Hashimoto Y,et al.Sphingosine-1-phosphate/S1PR2-mediated signaling triggers Smad1/5/8 phosphorylation and thereby induces Runx2 expression in osteoblasts[J].Bone,2016(93):1-11.
[6]Tan J,Xu X,Tong Z,et al.Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis[J].Bone Research,2015(3):15003.
[7]Chen J,Deng S,Zhang S,et al.The Role of miRNAs in theDifferentiation of Adipose-Derived Stem Cells[J].Curr Stem Cell Res Ther,2014,9(3):268-279.
[8]Jihong Yan,Duo Guo,Shu Yang,et al.Inhibition of miR-222-3p activity promoted osteogenic differentiation of hBMSCs by regulating Smad5-RUNX2 signal axis[J].Biochem Biophys Res Commun,2016,470(3):498-503.
[9]彭俊,劉英杰,宗阳,等.miR-125b调控Runx2/Osx表达对骨髓间充质干细胞成骨机制的影响[J].东南国防医药,2019,21(2):124-129.
收稿日期:2019-12-04;修回日期:2019-12-23
编辑/成森
