Amiodarone-induced hepatotoxicity — quantitative measurement of iodine density in the liver using dual-energy computed tomography:Three case reports
2020-04-08HaiJuanLvHongWeiZhao
Hai-Juan Lv, Hong-Wei Zhao
Hai-Juan Lv, Hong-Wei Zhao, Department of Radiology, The Second Affiliated Hospital of Jiaxing University, Jiaxing 314000, Zhejiang Province, China
Abstract BACKGROUND Amiodarone is the drug most commonly used to manage arrhythmias. Long-term amiodarone administration causes hepatotoxicity due to iodine accumulation in the liver. Here, we present three cases of amiodarone-induced hepatotoxicity in patients on long-term oral amiodarone therapy who underwent dual-energy computed tomography (DECT).CASE SUMMARY We report the clinical and iodine density in the liver using DECT in three patients with amiodarone-induced hepatotoxicity. Liver enzymes were increased in these three patients, and abdominal DECT without contrast medium showed highly increased attenuation in the liver. Furthermore, the iodine concentration in the liver was increased. The first patient with amiodarone-induced reversible hepatotoxicity, showed a reversible course of liver function and a decrease in CT values after discontinuation of amiodarone. The second patient on long-term oral amiodarone had increased iodine concentration in the liver and liver damage, the patient eventually developed rapidly progressive pneumonia and died of multiple organ failure. The third patient, showed an increased iodine concentration in the liver and elevated liver enzymes. However, the patient refused radiofrequency ablation for atrial fibrillation and continued oral amiodarone to control atrial fibrillation, and routine liver function tests were required every 3-6 mo in this patient.CONCLUSION DECT is a potentially noninvasive diagnostic tool for quantifying iodine concentration in the liver and monitoring adverse reactions due to amiodarone.
Key Words: Case report; Amiodarone; Hepatotoxicity; Iodine density; Computed tomography; Dual energy; Arrhythmias
INTRODUCTION
Amiodarone is the drug most commonly used to manage arrhythmias[1]. Amiodarone has a long list of potential adverse effects. Hepatotoxicity and elevated transaminase levels are commonly observed with long-term amiodarone use and occur in 15% to 50% of patients[2]. Increases in liver density on computed tomography (CT) are also seen with long-term usage of amiodarone[3]. To the best of our knowledge, the measurement of iodine concentrations in patients with amiodarone-induced liver damage has not previously been reported. Here, we present three cases in which the quantitative measurement of iodine concentration in the liver was performed using dual-energy computed tomography (DECT) in patients receiving low-dose oral amiodarone therapy for the long-term prevention of paroxysmal atrial fibrillation.
CASE PRESENTATION
Chief complaints
Case 1:A 66-year-old male was admitted to the hospital with a 2-wk history of episodic palpitations.
Case 2:An 85-year-old male presented with a 4-wk history of progressively worsening dyspnea on exertion and fatigue.
Case 3:A 71-year-old female was admitted to our hospital due to worsening palpitations.
History of present illness
Case 1:The patient was admitted to hospital with a 2-wk history of episodic palpitations. He noted that each episode was abrupt in onset and lasted approximately 2 h to 1 d before gradually abating.
Case 2:The patient presented with a 4-wk history of progressively worsening dyspnea on exertion and fatigue. He also suffered from a dry cough at night. He denied fevers,chills, night sweats, and chest pain.
Case 3:The patient had episodic palpitations for years, which worsened in the previous 3 d. She denied dyspnea and chest pain.
History of past illness
Case 1:The patient had been diagnosed with paroxysmal atrial fibrillation when he was 56 years old. Since then, he had been treated with amiodarone (200 mg/d) for 10 years. He had no apparent history of liver disease. He reported no nausea, vomiting,or diarrhea. He had no history of smoking-related chronic obstructive pulmonary disease.
Case 2:The patient’s medical history included hypertension and atrial fibrillation, for which he took a thiazide diuretic and amiodarone (200 mg/d). The antiarrhythmic drug had been started 6 years earlier for rhythm control.
Case 3:The patient’s past medical history revealed atrial fibrillation for approximately 6 years that was managed with amiodarone. She used 200 mg of amiodarone in tablet form per day.
Physical examination
Case 1:He had no abnormal findings on abdominal examination.
Case 2:His pulmonary exam revealed significant bibasilar inspiratory crackles. His abdominal examination was unremarkable.
Case 3:She presented with a blood pressure of 145/88 mmHg and a regular pulse of 62 bpm. Holter monitoring confirmed the presence of paroxysmal atrial fibrillation but no signs of ischemic injury. During her examination, she reported decreased appetite without significant weight loss. There were no abnormal findings on abdominal examination.
Laboratory examinations
Case 1:Direct bilirubin, 11.2 μmol/L (reference range, 0-4 μmol/L); γ-glutamyl transpeptidase, 85 U/L (reference range, 10-60 U/L); alanine aminotransferase, 127 IU/L (reference range, 9-50 IU/L); aspartate aminotransferase, 101 IU/L (reference range, 15-40 IU/L); and γ-glutamyl transferase, 1080 IU/L (reference range, 120-250 IU/L). Elevations of these enzymes indicated liver damage and hepatotoxicity.
Case 2:Direct bilirubin, 9.3 μmol/L (reference range, 0-4 μmol/L); γ-glutamyl transpeptidase, 112 U/L (reference range, 10-60 U/L); alanine aminotransferase, 144 IU/L (reference range, 9-50 IU/L); and aspartate aminotransferase, 240 IU/L(reference range, 15-40 IU/L). Elevations of these enzymes indicated drug-induced liver damage.
Case 3:Direct bilirubin, 3.3 μmol/L (reference range, 0-4 μmol/L); γ-glutamyl transpeptidase, 58 U/L (reference range, 10-60 U/L); alanine aminotransferase, 112 IU/L (reference range, 9-50 IU/L), and aspartate aminotransferase, 50 IU/L (reference range, 15-40 IU/L).
Imaging examinations
Case 1:Due to abnormal liver enzyme levels, a plain abdominal CT scan was performed which revealed increased liver density. We measured the Hounsfield unit(HU) values in four different locations of the liver. Regions of interest were manually delineated on the liver parenchyma, avoiding major vessels, bile ducts, and liver edges. The average CT value of the liver at 120 kVp was 118 HU (the normal range for the liver is 55-70 HU).
An additional DECT scan was performed to assess the status of amiodaroneinduced iodine accumulation in the liver. The CT values of the liver were 144 HU and 104 HU at 100 kVp and 140 kVp (Figure 1A and B), respectively. No additional lesions were found. In the fused iodine image, an increased iodine concentration in the liver was observed relative to the iodine concentrations in the spleen and pancreas. The measured iodine concentration in the liver was 2.9 mg/mL (Figure 1C). In the VNC image, the liver density was normal (68 HU). The imaging findings were compatible with the diagnosis of intrahepatic iodine deposition associated with hepatic dysfunction.
Case 2:DECT imaging of the abdomen demonstrated a significant increase in the density of the liver: the mean liver attenuations were 116 HU and 83 HU at 100 kVp and 140 kVp, respectively; the iodine concentration in the liver was 2.3 mg/mL(Figure 2A-C). CT images also showed multiple liver cysts throughout the entire liver.
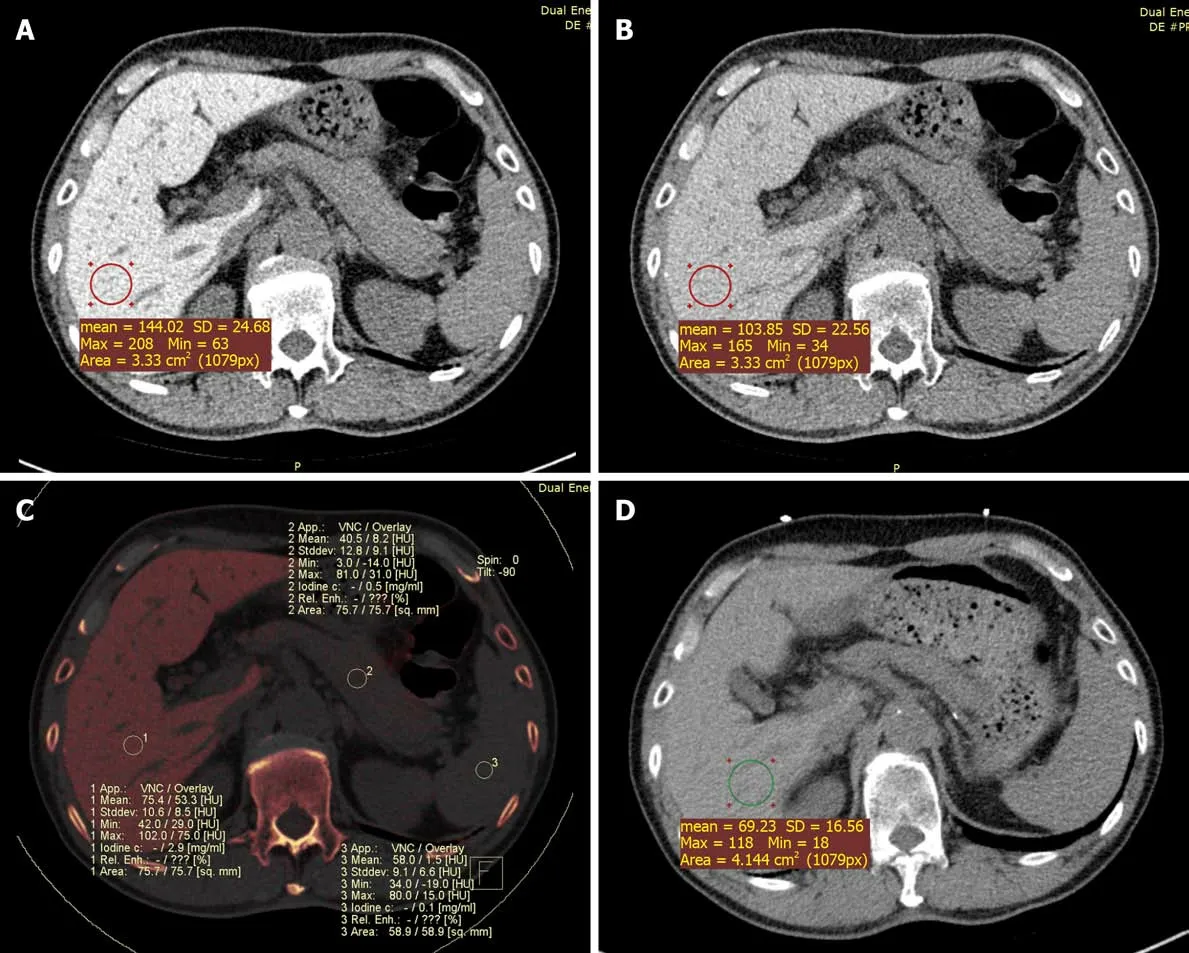
Figure 1 Dual-energy computed tomography images and fused iodine imaging. These images were obtained in a 66-year-old male with liver dysfunction after therapy with amiodarone for ten years. All regions of interest (ROIs) measurements were obtained at the same level for calculations. In this case, the liver attenuation at 100 kVp was 144 Hounsfield unit (HU) and at 140 kVp was 104 HU. The color-coded iodine map reveals high iodine depositions in the liver; iodine concentration was 2.9 mg/mL (C, ROI 1). A: The 100 kVp image attenuation; B: The 140 kVp image attenuation; C: Iodine density; D: Chest non-enhanced computed tomography showed that liver attenuation at 120 kVp was 69 HU one year later. SD: Standard deviation.
Case 3:An abdominal DECT scan showed that the liver was small, and the proportions were altered, with diffusely increased attenuation; the mean liver attenuations were 110 HU and 86 HU at 100 kVp and 140 kVp, respectively. In addition, the color-coded iodine map revealed high iodine deposition in the liver; the iodine concentration in the liver reached 1.4 mg/mL (Figure 3). These findings were determined to be attributable to the side effects of amiodarone.
FINAL DIAGNOSIS
Case 1
Paroxysmal atrial fibrillation.
Case 2
Severe pneumonia, respiratory failure and heart failure.
Case 3
Paroxysmal atrial fibrillation.
TREATMENT
Case 1
Treatment with amiodarone was discontinued. Radiofrequency ablation was performed for atrial fibrillation.
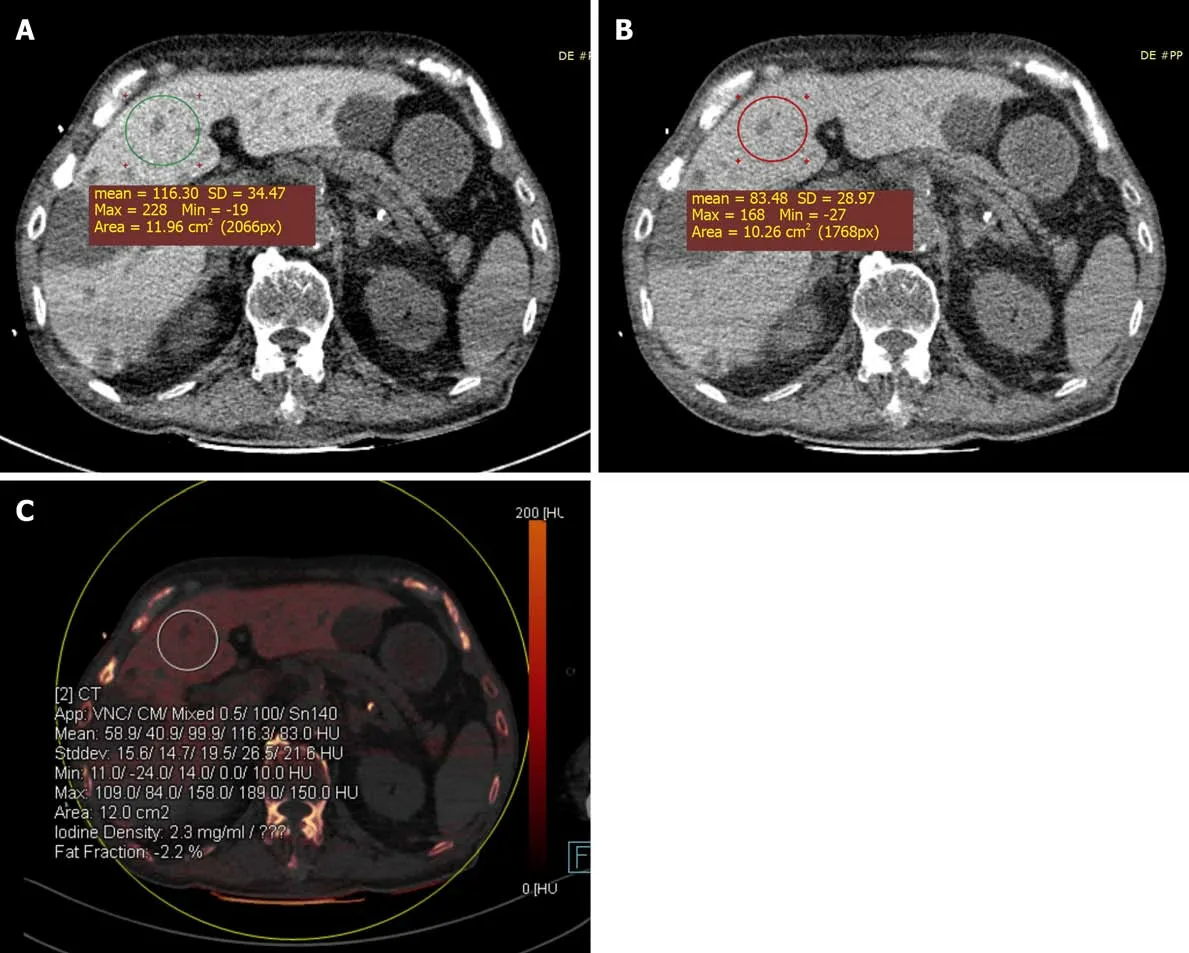
Figure 2 Color-coded iodine map was obtained in an 85-year-old male treated with amiodarone for 6 yr. A: The liver attenuation at 100 kVp was 116 Hounsfield unit (HU); B: The liver attenuation at 140 kVp was 83 HU; C: The color-coded iodine map shows that the iodine density was 2.3 mg/mL in the liver(region of interest 2), the liver attenuation at 100 kVp was 116 HU and at 140 kVp was 83 HU. SD: Standard deviation.
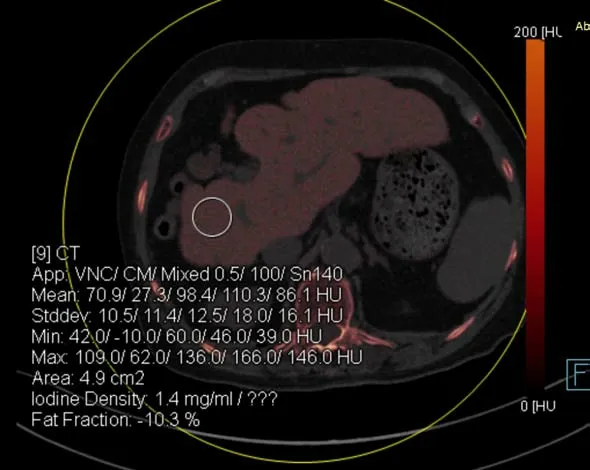
Figure 3 Color-coded iodine map was obtained in a 73-year-old female treated with amiodarone for 6 yr. The color-coded iodine map shows that the iodine density was 1.4 mg/mL in the liver (region of interest 9), the liver attenuation at 100 kVp was 110 Hounsfield unit (HU) and at 140 kVp was 86 HU. Iodine map also showed liver cirrhosis.
Case 2
Amiodarone was discontinued.
Case 3
The patient refused radiofrequency ablation for atrial fibrillation. The treating physician and patient decided to continue amiodarone to control atrial fibrillation. She was also informed of the potential hazard to the liver; routine liver function tests were required every 3-6 mo in this patient.
OUTCOME AND FOLLOW-UP
Case 1
A chest non-enhanced CT scan performed 1 year later showed a significant decrease in hepatic attenuation with 69 HU at 120 kVp (Figure 1D). Furthermore, the patient’s liver enzyme activities returned to reference ranges.
Case 2
Over the next 5 wk, the patient suffered from rapidly progressive pneumonia.Eventually, he died in the intensive care unit of multiple organ failure.
Case 3
The patient was discharged with further cardiology and hepatology follow-up, but refused an abdominal DECT scan follow-up.
DISCUSSION
Amiodarone is the most commonly used antiarrhythmic drug. Due to severe and potentially life-threatening adverse drug reactions, careful use is essential to derive optimal benefits from the drug with the least risk[1].
Kimet al[3]reported a case of amiodarone toxicity in a long-term amiodarone user who showed an abnormally high liver density on a non-enhanced CT scan but normal liver enzyme results and serum amiodarone levels. Previous reports have also shown that plasma amiodarone levels may be poorly correlated to amiodarone toxicity[4]. In light of the present data, it is unclear if amiodarone-induced hepatotoxicity may occur without any abnormalities in liver enzymes[5]. In a previous case report of a patient who was administered the low dose of 200 mg amiodarone once daily for 2 years, the symptoms progressed to hepatic cirrhosis[6]. In conclusion, for those cases that show normal liver function test results and amiodarone plasma levels but symptoms of nausea, vomiting and tremor, which correspond to the side effects of amiodarone,clinicians should be reminded that amiodarone toxicity can be a cause of marked hyper-density in the liver on CT scan. To date, there are no evidence-based standards for monitoring amiodarone-induced hepatotoxicity.
Amiodarone has two iodine atoms that make up 37.3% of its molecular weight. This drug is metabolized in the liver to produce the active metabolite desethylamiodarone.Iodine is highly attenuated on CT; therefore, high attenuation on non-enhanced CT might represent iodine accumulation in the liver of chronic amiodarone users[7]. The levels of amiodarone and its major metabolite desethylamiodarone are correlated with liver CT density[8]. Studies have shown that high liver density observed on CT, which is secondary to increased iodine content due to amiodarone and reflects the tissue levels of amiodarone, may be useful in detecting amiodarone toxicity, but the radiological appearance of these effects is nonspecific[3].
Liver histology shows amiodarone-induced nonalcoholic steatohepatitis in patients who are long-term users of amiodarone[9]. Moreover, conventional, single-energy CT fails to detect and quantify iodine concentration in the liver in the presence of coexisting fat, which has an inverse effect on attenuation by lowering the CT values;the values of single-energy CT are also influenced by different tube voltages, beam hardening artifacts, and other factors. We speculated that the conventional CT appearance of amiodarone hepatotoxicity may not yet be accurate enough to provide useful results.
Traditionally, liver biopsy has been the gold standard for monitoring the side effects of amiodarone, although this type of measure is difficult to obtain. Overall,amiodarone hepatotoxicity is underrecognized and is often diagnosed too late. Hepatic disease may progress despite discontinuation of the drug; this progression may be due to the slow elimination of amiodarone[10]. However, in our first case, the return of liver enzyme activities and hepatic CT density to within the reference ranges after amiodarone discontinuation supports that it is the cumulative dose of amiodarone that has liver toxicity effects in patients with continual low-dose therapy.
In contrast to single-energy CT, DECT using an iodine-specific, three-tissue decomposition algorithm enables accurate quantification of the absolute liver iodine concentration, irrespective of the confounding effects of normal variation in liver attenuation and coexisting fat[11]. Many studies have been conducted to examine the accuracy of DECT-derived iodine concentrations[12]. A DECT scan without contrast media showed an increased iodine concentration in the liver, which provided information to analyze the extent of liver damage. In our cases, the diagnosis of hepatotoxicity was made based on liver enzyme elevations and high iodine deposition in the liver, and these patients had no history of transfusion.
Several studies have shown that CT attenuation of the liver is significantly correlated with the plasma levels of amiodarone and desethylamiodarone and can be used to estimate their deposition in the liver[7,13]. Researchers have suggested that serum amiodarone levels less than 1.5 mg/L are associated with a minimal risk of hepatotoxicity, whereas a level above 2.5 mg/L may be associated with a risk of up to 6% of hepatotoxicity[5]. Amiodarone concentrations in the liver may be up to 500-fold higher than serum levels[14]. Additional experiments have confirmed that amiodarone levels in the liver were 175- to 500-fold higher than the corresponding serum levels[15].Amiodarone contains 37.3% inorganic iodine, after conversion to liver iodine concentration, liver function damage occurs in 6% of patients when the concentration is greater than 0.47 mg/mL. We used DECT to quantitatively measure the liver iodine concentrations in our patients; the iodine concentrations ranged from 1.4-2.9 mg/mL,far exceeding the iodine concentration that can cause liver damage. According to our research, iodine is evenly distributed in the liver. In the future, DECT examination of the liver in a small field of view will determine the iodine concentration in the liver,reduce radiation exposure and improve the clinical operability.
Although two related side effects of amiodarone therapy, increased CT liver density and secondary phospholipidosis, were reported 34 years ago[16], the relationship between iodine concentration in the liver and the severity of liver damage remains unclear. The threshold of hepatic iodine concentration that leads to hepatotoxicity is not well defined. Further studies are necessary to determine the relationship between histologic progression and hepatic iodine concentration in patients treated with amiodarone.
There are some limitations in our case series. First, we do not have histopathology or biopsy results. Second, the patient sample was small; it is not clear whether the degree of elevation in liver enzymes is correlated with liver iodine concentration; this finding requires further investigation with a larger sample size in the future.
CONCLUSION
Based on the preliminary findings described above, DECT is an accurate, noninvasive diagnostic tool for quantifying iodine concentration in the liver, and may be more suitable for monitoring adverse reactions to amiodarone. Iodine quantification with liver function tests might be advantageous to establish an early diagnosis of amiodarone-induced liver toxicity before an irreversible stage is reached and to differentiate amiodarone-induced liver toxicity from other common diseases, such as hemochromatosis and hemosiderosis.
杂志排行
World Journal of Clinical Cases的其它文章
- Relationship between non-alcoholic fatty liver disease and coronary heart disease
- Remission of hepatotoxicity in chronic pulmonary aspergillosis patients after lowering trough concentration of voriconazole
- Endoscopic submucosal dissection as alternative to surgery for complicated gastric heterotopic pancreas
- Observation of the effects of three methods for reducing perineal swelling in children with developmental hip dislocation
- Predictive value of serum cystatin C for risk of mortality in severe and critically ill patients with COVID-19
- Sleep quality of patients with postoperative glioma at home
