What is the gut feeling telling us about physical activity in colorectal carcinogenesis?
2020-04-07MajaCigrovskiBerkovicVjekoslavCigrovskiInesBilicCurcicAnnaMrzljak
Maja Cigrovski Berkovic, Vjekoslav Cigrovski, Ines Bilic-Curcic, Anna Mrzljak
Maja Cigrovski Berkovic, Department for Endocrinology, Diabetes and Metabolism, Clinical Hospital Dubrava, Zagreb 10000, Croatia
Maja Cigrovski Berkovic, Vjekoslav Cigrovski, Faculty of Kinesiology, University of Zagreb,Zagreb 10000, Croatia
Ines Bilic-Curcic, Department of Pharmacology, Faculty of Medicine, J J Strossmayer University Osijek, Osijek 31000, Croatia
Anna Mrzljak, Department of Medicine, Merkur University Hospital, Zagreb 10000, Croatia
Anna Mrzljak, Department of Medicine, School of Medicine, University of Zagreb, Zagreb 10000, Croatia
Abstract In the last decades, more efforts are focused on the prevention and treatment of malignant diseases, given the increase in all cancers incidence A lifestyle change,including healthy eating habits and regular physical activity, has significantly impacted colorectal cancer prevention. The effect of dose-dependent physical activity on mortality and recurrence rates of colorectal carcinoma has been unequivocally demonstrated in observational studies. However, clear recommendations are not available on the frequency, duration, and intensity of exercise in patients with colorectal cancer due to the lack of evidence in randomized clinical trials. Regarding pathophysiological mechanisms, the most plausible explanation appears to be the influence of physical activity on reducing chronic inflammation and insulin resistance with a consequent positive effect on insulin growth factor 1 signaling pathways.
Key Words: Physical activity; Colorectal cancer; Insulin signaling pathway; Inflammation;Gut microbiota
INTRODUCTION
Colorectal cancer (CRC) is the fourth most common cancer worldwide, with increasing incidence due to lifestyle, environmental changes, and aging demographics; it is estimated to rise to 2.2 million new cases and 1.1 million deaths by 2030[1,2]. Forty-four percent of new CRC diagnoses are older patients, and 20%-25% of CRC is diagnosed at the advanced stage[3]. Five-year overall survival for CRC ranges from 58.4% to 65%[4,5].CRC screening programs have led to a 16% decline in overall mortality rate without affecting incidence[6]. Thus, strategies that could prevent or decrease the burden of CRC are of major importance.
PHYSICAL ACTIVITY AND ITS RELATION TO CHRONIC DISEASES WITH A FOCUS ON CANCER
Sedentary behavior (both viewed through the prism of reduced physical activity and/or time spent on sitting) is emerging as an independent risk factor for chronic diseases such as diabetes, hypertension, obesity, and cardiovascular disease and can directly increase mortality. According to objectively assessed measurements, adults spend 50% to 60% of the day being physically inactive, in sedentary behaviors[7].
Cancer is a leading cause of death in many modern societies, although largely preventable through healthy lifestyle choices[8]. A meta-analysis of 43 observational studies recently linked the sedentary lifestyle to the development of three specific types of cancers; lung, endometrial, and colon. For the later, authors reported an 8%increase of risk in case of each 2-h increase in sitting time[9]. Another meta-analysis,which included 17 prospective studies, reported the associations between sedentary behavior and some cancer types, including CRC [relative risk, 1.30; 95% confidence interval (CI), 1.12-1.49][10].
The Reasons for Geographic and Racial Differences in Stroke study, which included a cohort of 8002 adults aged ≥ 45 years in the United States and prospectively followed them by accelerometry for a mean of 5.7 years, reported an association between greater sedentary time with greater risk of cancer mortality. Moreover, the authors found that replacing 30 min of sedentary time with physical activity (either light or moderatevigorous) can reduce the cancer mortality for 8% and 31%, respectively[11]. Analysis of cancer risk in relation to the level of leisure-time physical activity involving more than 1.44 million adults showed that a high level of activity provides protection against 13 cancers. For the cancer protection effects, the hazard rates (HR) of cancers in gastrointestinal tracts, namely esophagus, gastric cardia, colon and rectum, are shown to be reduced to 0.58, 0.78, 0.84 and 0.87 respectively with corresponding 95%CI values being 0.37-0.89, 0.64-0.95, 0.77-0.91 and 0.80-0.95. Other cancer protection by elevated physical activities pertains to liver, lungs, kidney, endometrium, breast, bladder, head and neck, and two hematological malignancies (myeloma and myeloid leukemia),with HR in a range of 0.73-0.90. It is noteworthy that these protection effects are irrespective of patients’ body mass index or previous physical fitness. Overall, the higher the physical activity level, the lower the total cancer risk[12]. Similarly, a recently published umbrella review evaluating physical activity and cancer risk showed that regular physical activity might prevent seven types of cancers (colon, breast,endometrium, lung, esophagus, pancreas, and meningioma)[13].
EXERCISE AND GASTROINTESTINAL POLYPOSIS
Physical activity offers the protection against colorectal precancerous polyps[14]. There was an exercise duration-related lower prevalence of colon adenomas and polyps in persons who exercised for ≥ 1 h per week as opposed to those who exercised less than an hour weekly[15]. Moreover, the risk for polyp development was decreased throughout the colon, regardless of its specific part. Another study reported the exercise-related decrease in the total number of intestinal polyps, and more specifically in the development of large polyps by 67%[16]. Animal studies suggest preventing of colorectal polyp formation through pro-inflammatory cytokine interleukin-6, which is decreased after treadmill running[17].
EXERCISE AND CRC
Despite the treatment advances, new therapies have a limited impact on cure rates and colon cancer patients’ survival. Therefore, adjuvant treatment strategies, among which lifestyle interventions, are needed. Several observational studies have demonstrated the anti-cancer effects of exercise and its dose-dependent effect on decreasing cancer recurrence mortality and risk. More specifically, physical activity may prevent approximately 15% of colon cancer[18]. A systematic review by Des Guetz and colleagues[19]analyzed six observational studies involving over 7500 CRC survivors and reported that higher post-diagnosis physical activity was associated with a lower risk of CRC-specific mortality (HR, 0.61; 95%CI, 0.44-0.86) and a lower risk of all-cause mortality (HR, 0.62; 95%CI, 0.54-0.71). Despite noticed benefits, no randomized controlled trials have been published exploring the beneficial association between physical activity and survival after CRC treatment. The Challenge Trial[20]is the first prospective randomized controlled trial evaluating the effects (improvements of 3-year disease-free survival, QoL) of structured physical activity on the survival of stage 2 and 3 CRC survivors.
POTENTIAL EXPLANATIONS FOR THE PREVENTIVE ROLE OF PHYSICAL ACTIVITY IN THE CRC DEVELOPMENT
Randomized exercise trials and longitudinal studies initially suggested the link between the preventive role of physical activity on cancer through primarily weight reduction and/or prevention of weight gain[21-23], but nowadays, the association seems more direct and straightforward. There is data suggesting preventive effects even when physical activity lacked the weight loss or maintenance of healthy weight[24J.Potential mechanisms might include a reduction in insulin resistance and inflammation that have been directly linked to colorectal carcinogenesis, or stimulation of digestion and intestinal motility (Figure 1). Moreover, physical activity might directly influence the molecular and genetic pathways involved in colorectal carcinogenesis and improve patient survival[25]. According to available data, physical activity increases the expression of P27 and cyclooxygenase-2 (COX-2) and decreases that of insulin receptor substrate 1 and B-catenin, which correlates to improved cancerspecific survival[26-30]. Another plausible mechanism in need of further exploration is the effect of physical activity on soluble intercellular adhesion molecule, which is known to affect early death and disease recurrence[31].
The role of exercise on inflammation
Inflammation is a well-known risk factor not only for metabolic disorders like obesity,metabolic syndrome, and diabetes but also for CRC. In animal model studies, physical activity decreased systemic inflammation through the effect on pro-inflammatory cytokines and reduced the risk of colonic polyp formation and progression to cancer[32,33]. Additionally, physical activity decreases the inflammation by affecting the expression of inducible nitric oxide synthase and COX-2 in the colon mucosa[34]. COX-2-positive CRC patients engaging in the highest intensity physical activity have a significant decrease in cancer-specific mortality compared to those who are least active[35].
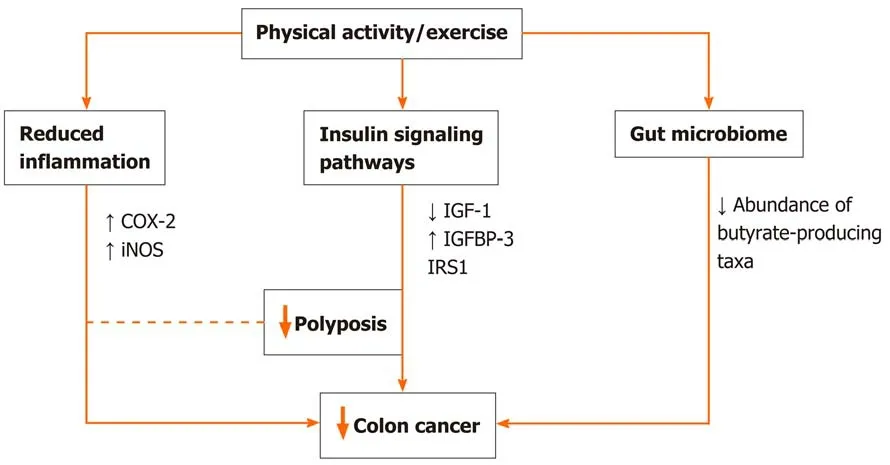
Figure 1 Potential explanatory mechanisms of the association between physical activity and prevention of colorectal cancer.
Role of exercise on insulin signaling pathways
By decreasing insulin resistance and circulating insulin levels, physical activity through its effect on IGF pathway can decrease the risk of initial CRC development and its recurrence, and influence cancer mortality[35,36]. Decreased insulin-like growth factor-1 levels and increased insulin-like growth factor-binding protein 3 levels may be a reasonable mechanism underlying the inverse correlation between CRC and physical activity[37,38]. Study by Lee and coauthors[39]including CRC survivors showed that engagement in postoperative physical activity decreases both insulin levels and insulin resistance, and increases insulin-like growth factor-1 and insulin-like growth factorbinding protein 3 levels. Additionally, insulin receptor substrate 1 involved in the development of insulin resistance can be used as a marker to identify patients who will benefit most from exercise in terms of cancer-related survival[30].
A contracting muscle,viadifferent myokines, enhances insulin sensitivity and, at the same time, decreases the production of pro-inflammatory cytokines, which is a potential mechanism of anti-cancer effect. Recently published studies have shown that specific myokine, named the secretory protein that is acidic and rich in cysteine(SPARC protein) involved in intercellular interaction and cell differentiation, might be important for preventing CRC development by increasing tumor cell apoptosis[39-41].
The role of exercise on gut microbiota
Changes in the intestinal microbiota composition disrupt gut integrity and induce a systemic inflammatory response, contributing to establishing a chronic low-grade inflammation state with repercussions in the gut and beyond[42]. Therefore targeting microbiota seems like a promising prevention and/or treatment concept. Emerging evidence from animal[43-45]and human studies[46-48]demonstrate that the exercise influences the diversity, composition, and functionality of gut microbiota. In this regard, the exercise increases butyrate-producing taxa and fecal butyrate concentrations and reduces pro-inflammatory cytokines and oxidative stress in the gut[42,46]. There are several potential mechanisms by which physical activity might alter the gut microbiota: Altering the gene expression of lymphocyte (down-regulating proinflammatory and up-regulating anti-inflammatory cytokines), alerting temperature,intestinal blood flow, gut motility, the activity of the enteric nervous system,enterohepatic circulation of bile acids or metabolic flux[42]. In the context of CRC,animal studies show that spontaneous wheel activity reduces the incidence of CRC[49].
Furthermore, exercise training reduces tumor multiplicity and the number of large tumors in mice genetically predisposed to intestinal adenomas, substantiating it with a correlation between fecal butyrate concentrations and tumor number[50]. In CRC patients, alternations in gut microbiota are characterized by a reduced abundance of butyrate-producing taxa[51]. Therefore exercise-induced modifications of the intestinal microbiota are likely to have implications in the prevention and treatment of CRC patients.
CONCLUSION
According to the American Cancer Society guidelines, it is important not only to avoid inactivity but also to be engaged in minimally 150 min cumulative moderate or 75 min vigorous-intensity aerobic exercise weekly, and perform at least two days per week of strength exercises in order to prevent the development of CRC or to have a favorable prognosis[52,53]. The recommendation is to increase the amount of exercise to achieve 300 min of aerobic exercise weekly if possible. Generally, no specific precautions need to be taken if participation in physical activity is incorporated slowly, when time and intensity slowly upgrade. In older individuals or those with known cardiovascular disease, sometimes a medical clearance before engaging in physical activity is needed.Similarly, if patients are on cardiotoxic chemotherapy, a cardiologist’s consult might be prudent[25]. Finally, physical activity improves the patients’ quality of life, functional status, muscle strength, depression, and decreases the risk of CRC recurrence and cancer-specific and overall mortality[54,55].
杂志排行
World Journal of Clinical Cases的其它文章
- Understanding the immunopathogenesis of COVID-19: Its implication for therapeutic strategy
- Latest developments in chronic intestinal pseudo-obstruction
- Correlation between ductus venosus spectrum and right ventricular diastolic function in isolated single-umbilical-artery foetus and normal foetus in third trimester
- Clinical efficacy of integral theory–guided laparoscopic integral pelvic floor/ligament repair in the treatment of internal rectal prolapse in females
- Treatment of Kümmell’s disease with sequential infusion of bone cement: A retrospective study
- Application value analysis of magnetic resonance imaging and computed tomography in the diagnosis of intracranial infection after craniocerebral surgery
