Cu2+ Modified g-C3N4 Photocatalysts for Visible Light Photocatalytic Properties
2020-03-27XiaoweiLiBinWangWenxuanYinJunDiJiexiangXiaWenshuaiZhuHuamingLi
Xiaowei Li, Bin Wang, Wenxuan Yin, Jun Di, Jiexiang Xia , Wenshuai Zhu , Huaming Li
School of Chemistry and Chemical Engineering, Institute for Energy Research, Jiangsu University, Zhenjiang 212013,Jiangsu Province, P. R. China.
Abstract: Photocatalytic technology can effectively solve the problem of increasingly serious water pollution, the core of which is the design and synthesis of highly efficient photocatalytic materials. Semiconductor photocatalysts are currently the most widely used photocatalysts. Among these is graphitic carbon nitride (g-C3N4), which has great potential in environment management and the development of new energy owing to its low cost, easy availability, unique band structure, and good thermal stability. However, the photocatalytic activity of g-C3N4 remains low because of problems such as wide bandgap, weakly absorb visible light, and the high recombination rate of photogenerated carriers. Among various modification strategies, doping modification is an effective and simple method used to improve the photocatalytic performance of materials. In this work, Cu/g-C3N4 photocatalysts were successfully prepared by incorporating Cu2+ into g-C3N4 to further optimize photocatalytic performance. At the same time, the structure, morphology, and optical and photoelectric properties of Cu/g-C3N4 photocatalysts were analyzed by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy, UV-Vis diffuse reflectance spectroscopy (DRS), and photoelectric tests.XRD and XPS were used to ensure that the prepared photocatalysts were Cu/g-C3N4 and the valence state of Cu was in the form of Cu2+. Under visible light irradiation, the photocatalytic activity of Cu/g-C3N4 and pure g-C3N4 photocatalysts were investigated in terms of the degradation of RhB and CIP by comparing the amount of introduced copper ions. The experimental results showed that the degradation ability of Cu/g-C3N4 photocatalysts was stronger than that of pure g-C3N4. The N2 adsorption-desorption isotherms of g-C3N4 and Cu/g-C3N4 demonstrated that the introduction of copper had little effect on the microstructure of g-C3N4. The small difference in specific surface area indicates that the enhanced photocatalytic activity may be attributed to the effective separation of photogenerated carriers. Therefore, the enhanced photocatalytic degradation of RhB and CIP over Cu/g-C3N4 may be due to the reduction of carrier recombination rate by copper. The photoelectric test showed that the incorporation of Cu2+ into g-C3N4 could reduce the electron-hole recombination rate of g-C3N4 and accelerate the separation of electron-hole pairs, thus enhancing the photocatalytic activity of Cu/g-C3N4. Free radical trapping experiments and electron spin resonance indicated that the synergistic effect of superoxide radicals (O2·-), hydroxyl radicals (·OH) and holes could increase the photocatalytic activity of Cu/g-C3N4 materials.
Key Words: Cu/g-C3N4; Photocatalytic; Visible light; Active species; RhB; CIP

1 Introduction
The exploration and optimization of photocatalysts has aroused extensive attention due to the potential application in the domain of solar-energy conversion1-4. Among which, TiO25,6,ZnO7,8, WO39,10, and other wide bandgap semiconductor materials have been widely used in environmental remediation and clean energy. In order to ameliorate the limited light absorption of wide bandgap semiconductors, various visible light driven photocatalysts are developed. Usually, there are two main thinking of research. One way is to broaden the spectrum absorption range of traditional TiO2, ZnO and other materials to visible light, such as designing justifiable structural11, doping cation or anion12,13, deposing noble metal14and forming composites15, which all can extend the optical absorption range;another method is to develop novel visible-light-driven photocatalysts16,17, for instance, the development of black TiO2nanocrystals. At present, the photocatalysts mostly contain metallic element, so the development of photocatalysts with lowcost, excellent stability, and appropriate valance and conduction band position has been the most concern research.
A nonmetallic material, namely g-C3N4, has been found during the study of various semiconductor photocatalysts, which can be able to respond to visible light, possess proper band edge and good stability18-21. The carbon and nitrogen atoms in g-C3N4photocatalysts are connected by strong covalent bond, which can be stabilized in water, acid and alkali solution. Therefore, g-C3N4has a great application prospect in photocatalysis22-24. However,the pure g-C3N4has a high recombination rate of photocarrier,low quantum efficiency and specific surface area, a widebandgap (≈ 2.7 eV) leading to low utilization of visible light,these inherent defects of g-C3N4limit its application. Therefore,in order to adjust the bandgap and extend the absorption range to long wavelength region, doping is applied as an effective and simple method that mental and nonmetal are usually used as dopants25-27. The results show that the doping of transition mental (TM) such as iron, cobalt, nickel, copper and vanadium may introduce defect or change crystallinity of body materials,which can act as trap for electrons and holes to extend the lifetime of photocarriers28-31. Meanwhile, doping TM can promote the red shift of optical absorption edge and reduce the recombination rate of photogenerated carriers. Zhanget al.32prepared Cu2+modified TiO2/reduced graphene oxide sheets,and confirmed that the introduction of Cu2+facilitated the charge separation, thereby producing a high photocatalytic performance in the degradation of phenol. Yanet al.33use Cu/TiO2photocatalysts to reduce CO2into CH4under UV light illumination. The Cu/TiO2photocatalysts were prepared by solgel method, and they found that the introduction of copper ions could improve the light absorption capability of TiO2, thus facilitated to the enhancement of photoreduction activity.Thence, it is deduced that the introduction of copper species into g-C3N4may improve its limitation of easy recombination of photogenerated electron hole pairs, thus providing an alternative method for the environmental purification.
In this study, Cu2+doped g-C3N4has been synthesized to improve the photocatalytic activity of g-C3N4. The structure and morphology of Cu/g-C3N4were examined by XRD and TEM,and the elemental composition and valance state of which were analyzed by XPS. Meanwhile, the effects of copper ions on the structural and degradation properties of g-C3N4were discussed in detail. The difference in photocatalytic activity of pure g-C3N4and Cu/g-C3N4materials were evaluated by using RhB and ciprofloxacin (CIP) as the degradation model. The degradation mechanism of as-prepared photocatalysts was studied according to the free radial capture experiments.
2 Experimental and computational section
2.1 The synthesis of g-C3N4 and Cu/g-C3N4 photocatalysts
All of the reagents were analytical reagent (AR) from Sinopharm Chemical Reagent Co., Ltd and no further purified.
2.1.1 The synthesis of g-C3N4
Typically, crucible with 6 g dicyandiamide (98%) was placed in a tube furnace and calcined through temperature programming, where nitrogen as the protective atmosphere. The temperature-programmed procedure as follows: dicyandiamide was heated at 350 °C for 2 h, and then calcined at 600 °C for another 2 h. The obtained samples were yellow bulk g-C3N4and ground into powders. The yellow powders were first calcined at 500 °C for 2 h in a muffle furnace and cooled down to room temperature. The 500 °C-samples were then calcined at 550 °C for 2 h and cooled down to room temperature naturally, then the white few-layered g-C3N4that we required was obtained.
2.1.2 The synthesis of Cu/g-C3N4
Firstly, 0.076 g cetyltrimethyl ammonium bromide (CTAB,99%) was dissolved in deionized water and 0.1 g ethylene diamine tetra acetic acid disodium salt (EDTA-2Na, 99%) was added subsequently for stirring at a certain time. 0.1 g g-C3N4was added into the above mixture and sonicated for 30 min to obtain a homogeneous suspension at room temperature.Afterwards, 0.012 g Cu(Ac)2(98%) was added and stirred magnetically. Then, 4 mL NaOH (0.09 mol·L-1, 96%) was dropwise slowly for stirring another 30 min, and then the same volume of ascorbic acid (AA, 0.06 mol·L-1, 99.7%) was dropwise into the solution and stirred evenly. Finally, the resulting samples, namely Cu/g-C3N4, were washed with water and alcohol several times and then dried in a vacuum drying oven.Under the same condition, changing the mass ratio of Cu(Ac)2and g-C3N4(1 : 16.7, 1 : 8.3, and 1 : 4.2) could obtain Cu/g-C3N4with different Cu contents, donated as Cu/g-C3N4-1, Cu/g-C3N4-2, and Cu/g-C3N4-3, respectively.
2.2 Characterization
X-ray powder diffraction (XRD) was collected on a Shimadzu XRD-6100 Lab X-ray diffractometer by a scan speed of 7° per minute form the 2θrange of 10° to 80°. FT-IR spectra was measured on a Nicolet Nexus 470 FT-IR spectrophotometer at room temperature. The morphology of the samples was obtained by transmission electron microscopy (TEM, JEOL-JEM-2010)and scanning electron microscopy (SEM, JEOL-JSM-7001F)equipped with an energy-dispersive X-ray spectroscope (EDS).X-ray photoelectron spectroscopy (XPS) of Cu/g-C3N4was carried on a VG MultiLab 2000 system (Mg-Kαsource, 20 kV).The PL spectra of as-prepared materials was recorded by the Varian Cary Eclipse spectrometer. The optical absorption of Cu/g-C3N4was investigated by UV-Vis diffuse reflection spectroscopy (DRS, Shimadzu UV-2450 spectrophotometer).The N2adsorption-desorption isotherms were collected at 77 KviaTriStar II 3020 analyzer.
2.3 Photocatalytic experiments
The degradation efficiency of Cu/g-C3N4with different Cu contents on RhB and CIP was in accordance with the photocatalytic performance. The light source was a 300 W Xenon lamp with 400 nm filter. Typically, 30 mg catalysts were added to the photoreactor flask containing 100 mL RhB (10 mg·L-1) or 100 mL CIP (10 mg·L-1) and ultrasonic for a period of time. During the experiment, the reaction temperature of this system was maintained at 30 °C by a circulating water bath to eliminate the thermal effect. Before visible light illumination, the solution was stirring for 30 min in dark so that the reaction system could reach adsorption-desorption equilibrium. After 30 min, the light was turned on for the visible light irradiation.Approximately 4 mL solution was extracted out at certain time and centrifuged to separate the catalysts and RhB or CIP for analyzing. The absorbance of RhB and CIP was measured at 553 nm and 276 nm, respectively, and the degradation efficiency was calculated by equation: Degradation (%) = (C0-C)/C0× 100%,in which,C0andCwere the initial and time-dependent concentrations of RhB and CIP under visible light irradiation,respectively.
2.4 Photoelectrochemical measurements
The photocurrent and electrochemical impedance spectroscopy (EIS) were measured by a standard three-electrode system on CHI 660B electrochemical work-station. 0.1 mol·L-1phosphate buffer saline (PBS, pH = 7) solution was used as electrolyte for the photocurrent measurement, and the working electrode was catalysts loaded ITO glass. EIS was carried out in 0.1 mol·L-1KCl solution containing 5 mmol·L-1
3 Results and discussion
3.1 XRD and EDS analysis

Fig. 1 XRD patterns of pure g-C3N4 and Cu/g-C3N4 photocatalysts.

Fig. 2 FT-IR spectra of pure g-C3N4 and Cu/g-C3N4 samples.
XRD patterns are used to analyze the crystallinity and crystalline phases of the pure g-C3N4and Cu/g-C3N4samples.As shown in Fig. 1, the pure g-C3N4has a strong diffraction peak at 2θ= 27.8°, indicating the stacked conjugated aromatic system,which is indexed to (002) plane34. In addition, there is a relative weak peak at 2θ= 13.1°, suggesting the existing of melon substances, which is related to the (100) plane35. The diffraction peak position of Cu/g-C3N4is in line with that of pure g-C3N4,illustrating that the introduced Cu does not change the crystal structure of g-C3N4. Moreover, the presence of copper species(such as metallic copper, copper oxides, etc.) is not found in the XRD pattern of Cu/g-C3N4, indicating that Cu may be exist in the form of ions. The diffraction peak intensity at 2θ= 27.8° is enhanced by the introduction of Cu. Meanwhile, it can be seen from the XRD spectrum that the samples have a good crystallinity.
3.2 FT-IR analysis
The chemical bonds and composition of pure g-C3N4and Cu/g-C3N4samples are analyzed by FT-IR spectra. As can be seen from Fig. 2, the strong and wide absorption band in the range of 1200-1650 cm-1can be observed in pure g-C3N4, which are seated at 1240, 1320, 1410, 1570, and 1640 cm-1,respectively, belonging to the vibration mode of 3-s-triazine heterocyclic units (C=N and C―N). In virtue of the incomplete polymerization of dicyandiamide, the peak appeared at 3180 cm-1is the stretching vibration of N―H, indicating the existence of uncondensed amino group at the edge of layered structure. A consistent one-to-one match of the characteristic peaks is observed between the Cu/g-C3N4and pure g-C3N4photocatalysts. No other impurity peaks are found and the characteristic peaks of Cu/g-C3N4are not shift, suggesting that the introduction of copper species does not change the initial network structure of pure g-C3N4materials.
3.3 UV-Vis diffuse reflectance spectra (DRS) analysis
The optical adsorption spectra are identified to investigate the effect of Cu incorporation on the electronic structure of g-C3N4.As shown in Fig. 3, for the pure g-C3N4, the valance band and conduction band are formed by N 2pand C 2porbitals,respectively36, and between which the transition of charge carriers rendered the typical absorption mode of the semiconductor. The adsorption threshold of pure g-C3N4is 460 nm. When copper was introduced into the g-C3N4, the obvious red shift of adsorption band was observed, noting the reduced bandgap energies. And with an increasing content of Cu, the Cu/g-C3N4materials displayed a boost light absorption in the visible-light region. According to the DRS analysis, the incorporation of Cu can affect the electronic structure of g-C3N4,thus improve the optical properties of g-C3N4.

Fig. 3 DRS of pure g-C3N4 and Cu/g-C3N4 samples.
3.4 X-ray photoelectron spectroscopy analysis
XPS analysis is used to elucidate the structure and chemical state of Cu/g-C3N4. It can be seen from Fig. 4a that the C, N and Cu elements with sharp electron peaks at 932 eV (Cu 2p), 398 eV (N 1s), and 288 eV (C 1s) in Cu/g-C3N4are investigated. The corresponding C 1s, N 1sand Cu 2phigh-resolution XPS spectra are shown in Fig. 4b-d. The binding energy at 288.1 eV in the C 1sspectrum indicates the existence of thesp2N―C=N bond in g-C3N4. Additionally, the weaker and broad peak at 284.9 eV is usually observed as the calibration peak. Fig. 4c shows the highresolution N 1sspectrum being deconvoluted into four peaks at 398.7, 400.1, 401, and 404.4 eV and are ascribed tosp2-hybridized nitrogen (C =N―C) in triazine rings, tertiary nitrogen N―(C)3groups, the amino functions carrying hydrogen that are related to the structural defects and incomplete polymerization of the catalyst, and theπ-excitations of CN heterocycles, respectively37. The Cu 2pin Fig. 4d displayed two weak peaks at 933.1 and 953.1 eV which are corresponded to the electron energies of Cu 2p3/2and Cu 2p1/2, respectively, and belong to the characteristic peak of Cu2+. The appearance of shake-up satellites at a little higher binding energy than 933.1 eV also indicates the presence of Cu2+in the catalyst, and suggesting the unfilled Cu 3dshell38. As shown in Fig. 4e, f, compared with the XPS spectrum of g-C3N4, the peaks shift of N 1sin Cu/g-C3N4to low binding energy were observed, while the peak position of C1sorbital does not change. This may be due to the high electronegativity of the N atom, which forms a strong interaction with the doped Cu. The electrons transfer from Cu to N lead to the increase of electron cloud density of N, which reduce the binding ability of nucleus to extranuclear electrons,thus decreasing the binding energy. In addition, g-C3N4is a covalent compound and Cu2+cannot be substituted for doping in the ionic state. Therefore, Cu2+may be doped into the lattice gap of g-C3N4.
3.5 N2 adsorption-desorption isotherms analysis

Fig. 4 XPS analysis of (a) the survey; (b) C, (c) N and (d) the oxidation state of Cu included in the g-C3N4; and (e) C, (f) N for g-C3N4.

Fig. 5 N2 adsorption-desorption isotherms of g-C3N4 and Cu/g-C3N4-2 samples.
The N2adsorption-desorption isotherms of g-C3N4and Cu/g-C3N4were performed to investigate the specific surface areas(Fig. 5). It can be seen that both photocatalysts exhibit typical type IV isotherms with H3 hysteresis loops, indicating the existence of mesoporous structure. The corresponding specific surface areas of which are calculated to be 136.14 m2·g-1and 144.02 m2·g-1, respectively. Obviously, the introduction of copper has a small effect on the microstructure of g-C3N4, as the little difference in specific surface area. Therefore, the enhanced photocatalytic degradation of RhB and CIP over Cu/g-C3N4may be due to the reduction of carriers’ recombination rate by copper.
3.6 SEM and TEM analysis
The morphology and microstructure of Cu/g-C3N4and pure g-C3N4materials are analyzed by SEM and TEM. From Fig. 6a, b,the morphology of pure g-C3N4and Cu/g-C3N4showed similarly layered structure with multitudinous nanopores, mainly due to the condensation of dicyanamide contributing the formation of layered structure and nanopores during the calcination process.According to the TEM images (Fig. 6c, d), the pure g-C3N4materials are composed of thin nanosheets with curled edges,and the sheets are almost transparent, as well as Cu/g-C3N4.Simultaneously, no solid particles attached onto the surface of nanosheets are observed from the TEM images, indicating that Cu may be existed in the form of ions. The results are in fairly coincide with the XRD analysis. Fig. 6e is the energy dispersive X-ray spectrum of the Cu/g-C3N4materials. It can be observed the existing of C, N and Cu elements in Cu/g-C3N4, indicating that the material contains Cu element, which can be further certified by XPS. Moreover, the EDS mappings of Cu/g-C3N4materials in Fig. 6e further show the C, N, and Cu elements are homogeneously distributed throughout the layered materials,illustrating that Cu is successfully incorporated into the framework of g-C3N4and the elements are highly dispersed.
3.7 Photocatalysis activity
The photocatalytic activity of pure g-C3N4and Cu/g-C3N4materials was evaluated by the degradation of RhB under visible light irradiation. The results of which are shown in Fig. 7a. The blank experiment displayed the slightly degradation of RhB in the absence of photocatalyst, suggesting the photolysis of RhB can be neglected under visible light. After 45 min of visible light irradiation, the degradation efficiency of pure g-C3N4was 24.4%. When Cu2+was introduced into the g-C3N4material, the photocatalytic activity of Cu/g-C3N4materials increased significantly. Moreover, the photocatalytic degradation efficiency of Cu/g-C3N4-1, Cu/g-C3N4-2, and Cu/g-C3N4-3 were 46%, 58.9%, and 35.5%, respectively, at the same illumination time. The Cu/g-C3N4-2 showed the highest photocatalytic activity, and the RhB removal efficiency reached 93.6% after 105 min illumination.

Fig. 6 SEM (a, b) and TEM (c, d) images of pure g-C3N4 and Cu/g-C3N4-2, respectively; (e) EDS spectrum of Cu/g-C3N4 material and the corresponding mappings of C, N, and Cu.
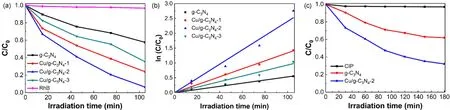
Fig. 7 Photocatalytic degradation of (a) RhB and (c) CIP in the presence of pure g-C3N4, Cu/g-C3N4 materials; (b) kinetic fit for the degradation of RhB with the pure g-C3N4 and Cu/g-C3N4 samples.
The photocatalytic degradation kinetics were determined to understand the photocatalytic performance of Cu/g-C3N4materials more clearly, and was shown in Fig. 7b. It can be seen that the photocatalytic degradation of RhB by Cu/g-C3N4materials fitted well with pseudo-first-order kinetics model via the equation of -ln(C/C0) =kt, in whichkis the apparent rate constant,C0is the initial concentration of RhB, andCis the RhB concentration at timet. Meanwhile, the Cu/g-C3N4material has a larger rate constant than pure g-C3N4. The maximum rate constant for Cu/g-C3N4-2 is 0.0262 min-1, which is as high as 5 times than that of g-C3N4, indicating the efficient influence of the introduction of Cu2+on the photocatalytic activity of g-C3N4.
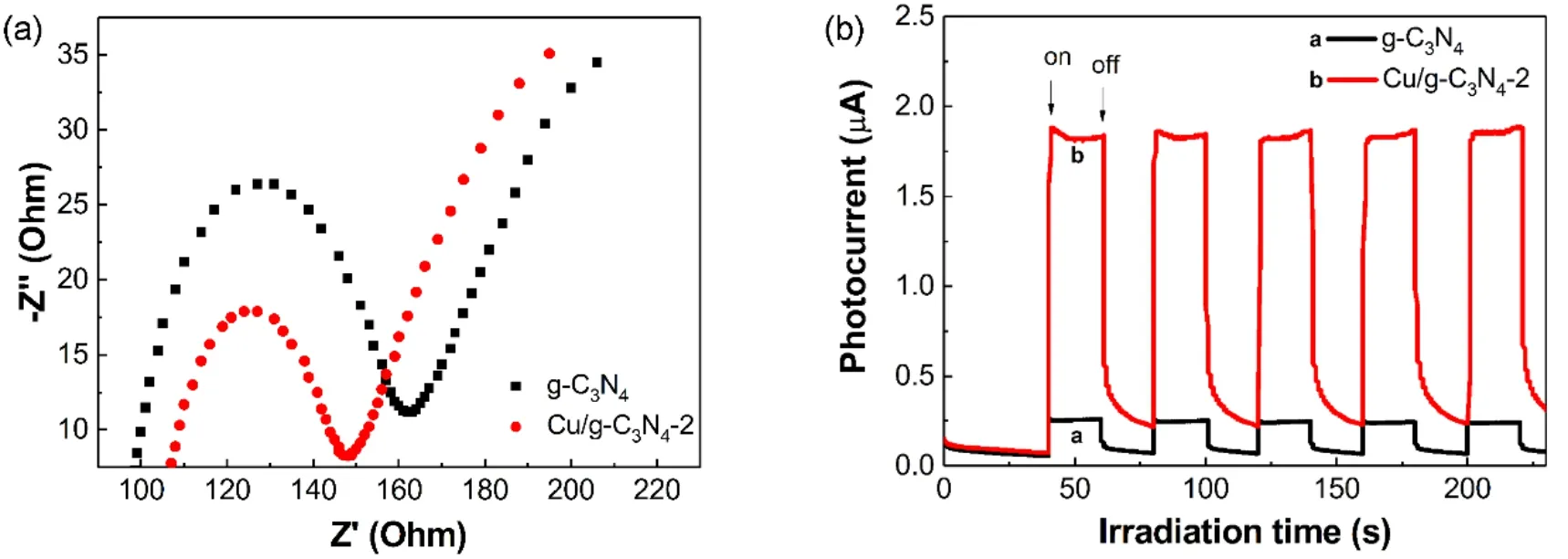
Fig. 8 (a) Electrochemical impedance spectroscopy and (b) transient photocurrent response of the as-prepared g-C3N4 and Cu/g-C3N4-2 materials.
In addition, the colorless CIP was selected as model substance without absorption of visible light to further evaluate the photocatalytic performance of the materials under visible light,and the results are shown in Fig. 7c. The direct photolysis of CIP has almost no change. It is seen that Cu/g-C3N4-2 showed higher photocatalytic activity for CIP than pure g-C3N4.
3.8 Electrochemical analysis
As we all know, photon absorption efficiency, photogenerated carrier separation efficiency, adsorption, and other factors often affect the activity of the material. Based on UV-Vis DRS analysis, the photon absorption efficiency of pure g-C3N4and Cu/g-C3N4materials are almost at the same level. Therefore,the enhanced photocatalytic activity should depend on the separation efficiency of photo-generated carriers. To prove this point, the electrochemical impedance and photocurrent response of the materials was carried out. Fig. 8a shows the electrochemical impedance spectroscopy of the pure g-C3N4and Cu/g-C3N4-2 materials, where the semicircles in the high frequency represent the electron transport constraints, and the diameters correspond to the electron transfer resistance (Ret)39.The smaller the arc radius, the lower the resistance of the materials to charge. It can be seen from Fig. 8a that the arc radius of Cu/g-C3N4-2 material is smaller than g-C3N4, indicating a higher charge transfer efficiency for Cu/g-C3N4-2. The results of electrochemical impedance spectroscopy showed that the conductivity of g-C3N4increases after the introduction of Cu2+into g-C3N4, thereby accelerating the separation and migration of photo-generated carriers.
Photocurrent is an effective way to reflect the generation,separation and migration of photo-generated carriers. Fig. 8b shows the photocurrent-time curves for the pure g-C3N4and Cu/g-C3N4-2 materials. According to the results of electrochemical impedance spectroscopy, the resistance of Cu/g-C3N4-2 electrode is smaller than that of pure g-C3N4electrode,and thus the photocurrent intensity of Cu/g-C3N4-2 is higher than pure g-C3N4. The photocurrent of Cu/g-C3N4-2 electrode was as high as about 8 times than pure g-C3N4. The enhancement of which in Cu/g-C3N4-2 material is mainly due to the improvement of separation efficiency of electron-hole pairs.
Photocurrent and electrochemical impedance analysis further indicate that the insertion of Cu2+into g-C3N4can enhance its conductivity, thus contributing to improve the photocatalytic degradation activity upon visible light.
3.9 Free radical trapping experiment analysis
The degradation of organic pollutants is carried out by oxidation and reduction reactions, and the degradation rate is mainly determined by the number of oxidized species, which can be analyzed by free radical trapping experiments. Typically,holes, hydroxyl radicals (·OH), and superoxide radicals (O2·-)can be trapped by disodium ethylenediamine tetraacetate(EDTA-2Na), tert-butanol (TBA), and benzoquinone (BQ),respectively. When the capture agents were added to the system,the effect of the photocatalytic degradation of RhB was investigated to determine the role of holes, ·OH, and O2·-. As can be seen from Fig. 9, with the addition of EDTA-2Na and TBA,the efficiency of photocatalytic degradation of RhB was 63%and 66%, respectively, which was significantly reduced compared to the non-trapped agent (94%). When BQ was added,the most significant inhibitory effect on RhB removal can be observed over the Cu/g-C3N4photocatalysts. The above analysis results show that O2·-plays a major role in the process of photocatalytic degradation of RhB, while ·OH and holes play an auxiliary role on that.
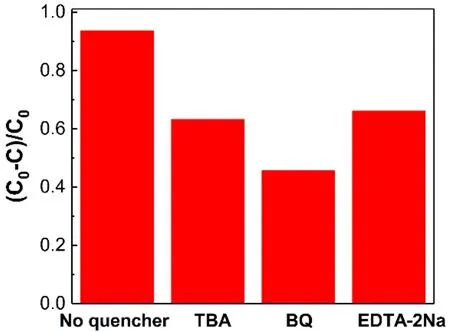
Fig. 9 Photocatalytic activities comparison of Cu/g-C3N4 materials with or without adding EDTA-2Na, BQ and TBA to degrade RhB under visible light irradiation.
The major active species in the photocatalytic degradation process can also be determined by electron spin resonance (ESR)spin-trapping techniques, and the results are shown in Fig. 10.As shown in Fig. 10a, b, the signal of DMPO-O2·-is distinctly detected by g-C3N4under visible light, which indicates that O2·-can be generated and have major role in the photocatalysis process. While the characteristic peaks of DMPO-·OH can be neglected, suggesting the ·OH is not the main active species during the reaction system. After adding Cu/g-C3N4photocatalysts, when the light was off, no signal peaks can be produced as DMPO adduct did not capture free radicals. When visible light was irradiated, two types of signal peaks can be observed. One is the peaks of DMPO-O2·-with a peak height ratio of 1 : 1 : 1 : 1 and another is the characteristic peaks of DMPO-·OH with height ratio of 1 : 2 : 2 : 1. Therefore, during the photocatalytic degradation of RhB process, there is a synergistic effect between O2·-and ·OH, and the results of ESR analysis are consistent with free radical trapping experiments.
3.10 Photocatalytic mechanisms
The band gap of Cu/g-C3N4material is narrower than that of pure g-C3N4, which indicates that the Cu/g-C3N4materials can excite more photogenerated electrons and holes under visible light irradiation. According to DRS analysis, the optical absorption threshold of g-C3N4is about 470 nm, and the bandgap of which can be estimated as 2.64 eV. Fig. 11a is the XPS valence band spectrum, and the valence band (VB) of g-C3N4is 1.89 eV.Thus, the conduction band (CB) position of the materials,according to the empirical formula:ECB=EVB-Eg, is calculated to be -0.75 eV. The redox potentials of ·OH/OH-and O2/O2·-are+1.99 eV and -0.33 eV, respectively40. Therefore, the electrons on CB of g-C3N4(-0.75 eV) can reduce O2to O2·-. However, the VB position is not positive enough to oxidize OH-to ·OH, which is accordance with ESR results. The optical absorption threshold of the Cu/g-C3N4materials is about 490 nm, and the bandgap of which can be estimated as 2.53 eV. Fig. 11b is the XPS valence band spectrum, and the VB of the materials is 1.99 eV. Thus, the CB position of the materials, according to the empirical formula:ECB=EVB-Eg, is calculated to be -0.54 eV. Consequently,based on the above experimental results, a possible mechanism of Cu/g-C3N4photocatalytic activity enhancement was proposed, as shown in Fig. 11c. Doped Cu2+can serve as a temporary electron-trapping site, contributing to the separation of photogenerated electron-hole pairs, which in turn promotes interfacial charge transport. This process is mainly due to the Cu2+/Cu+reduction potential (+0.135 VvsNHE) between the VB and CB of g-C3N441. Under visible light irradiation, the electrons on CB are excited and captured by Cu2+. Trapped electrons can react with the electron acceptor (O2) and reduce it to O2·-. At the same time, the holes on the VB and Cu2+can oxidize OH-or water molecules to ·OH. The doping Cu2+into g-C3N4not only enhances the carrier transport rate, but also suppresses the recombination rate of photogenerated electrons and holes. Moreover, the tendency of charge transfer from Cu+to regenerated Cu2+can be observed because of the higher stability of Cu2+. Therefore, the synergistic effect between O2·-,·OH and holes enhance the photocatalytic activity of the Cu/g-C3N4materials.

Fig. 10 ESR spectra of radical adducts trapped by DMPO in g-C3N4 (a, b) and Cu/g-C3N4 (c, d) dispersion in the dark and under visible light irradiation.

Fig. 11 (a, b) Valence band XPS spectra of the g-C3N4 and Cu/g-C3N4 nanosheets; and (c) mechanism of the photocatalytic degradation of RhB by Cu/g-C3N4 nanosheets under visible light irradiation.
4 Conclusions
Cu/g-C3N4photocatalysts was obtain by incorporating copper into few-layered g-C3N4nanosheets. The valence of copper in Cu/g-C3N4material was determined to be +2 by XPS analysis. It was found that Cu/g-C3N4-2 had the best photocatalytic performance on degrading RhB and CIP under visible light irradiation. The incorporation of Cu2+into g-C3N4could reduce the recombination rate and improve the separation efficiency of electron-hole pairs by narrowing the bandgap, thus enhancing the photocatalytic activity. The free radical trapping experiments showed that the synergistic effect of O2·-, ·OH and the holes improved the photocatalytic activity of Cu/g-C3N4materials.
杂志排行
物理化学学报的其它文章
- 魅力光催化剂
- Rod-Shaped Metal Organic Framework Structured PCN-222(Cu)/TiO2 Composites for Efficient Photocatalytic CO2 Reduction
- Controlling Self-Assembly of 3D In2O3 Nanostructures for Boosting Photocatalytic Hydrogen Production
- 硫化镉反蛋白石光子晶体制备及光解水制氢
- Fabrication of Z-Scheme Heterojunction of SiC/Pt/Cds Nanorod for Efficient Photocatalytic H2 Evolution
- 光催化甲烷转化研究进展
