多孔柿饼状BiOBr光催化剂的简易溶剂热法合成
2020-03-18杨黄根王治伟杨家添朱立刚覃利琴戴洪兴
杨黄根 陈 渊*, 王治伟 杨家添 朱立刚 覃利琴 戴洪兴*,
(1玉林师范学院化学与食品科学学院,广西农产资源化学与生物技术重点实验室,玉林 537000)
(2北京工业大学环境与能源工程学院化学化工系,绿色催化与分离北京市重点实验室,区域大气复合污染防治北京市重点实验室,先进功能材料教育部重点实验室,催化化学与纳米科学实验室,北京 100124)
0 Introduction
Photocatalysis has been an effective pathway to address the urgent energy crisis and environmental problems since Fujishima and Honda reported the photocatalytic splitting of water over a TiO2electrode in 1972[1-2].However,the wide bandgap of TiO2(ca.3.2 eV)hinders its widespread applications due to its only ultraviolet-light (4% of the solar spectrum)response[3].Therefore,it is of significance to develop novel high-efficiency,good-stability and visible-lightdriven photocatalysts for pollutant decomposition,water splitting,and carbon dioxide reduction[4-5].Bismuth oxyhalides(BiOX;X=Cl,Br,I)have attracted much attention because of their good physicochemical properties[6-7].Among the family of BiOX,BiOBr with a layered structure,in which there are an alternating arrangement of [Bi2O2]2+slabs and double Br slabs,can absorb a part of visible light owing to its narrow bandgap (ca.2.7 eV)[8-9].The unique structure endows BiOBr with high carrier mobility and efficient separation of photogenerated electron-hole pairs,which would be beneficial for enhancing its photocatlytic activity and stability[10-12].Nonetheless,it is necessary to further improve the photocatalytic activity of BiOBr for the practical applications by tailor-making its morphology and microstructure.
It is well known that controlling the particle size,surface area,and morphology of a photocatalyst is an effective strategy to optimize its photocatalytic performance.Hence,controllable synthesis of BiOBr with specific morphologies is important.In the past years,the BiOBr photocatalysts with various morphologies and structures,including nanobelts[13],nanosheets[14],and microflowers[15],and microsperes[16],have been developed.For example,Sin et al.[17]prepared the flower-like BiOBr hierarchical structures by the hydrothermal method,which exhibits much higher photocatalytic activity as compared to the commercial TiO2(P25)for the degradation of sunset yellow.The two-dimensional BiOBr ultrathin nanosheets synthesized via a hydrothermal route were used as an oxygen reduction reaction catalyst in the air electrode of aluminum-air batteries[18].Flower-structured BiOBr photocatalysts with excellent photocatalytic activity and high stability in the degradation of rhodamine B(RhB)was fabricated adopting the chemical precipitation procedures[19].The photocatalytic activity of the ultrathin BiOBr nanosheets is better than the BiOBr microspheres for the degradation of RhB due to the unique micromorphology and microstructure of the former[20].Hollow BiOBr microspheres with nanoslices were synthesized via a simple one-pot solvothermal method,which exhibits high photocatalytic activity for MB and RhB degradation under visible-light irradiation[21].Flower-like BiOBr microspheres with a controlled morphologywerefabricated in ethanol using Bi(NO3)3·5H2O and NaBr as precursors,and the obtained sample shows a high photocatalytic activity due to its abundance of exposed (110)facets for RhB degradation[22].
BiOBr with three-dimensional hierarchical structures assembled from low-dimensional nanoscale building blocks has become one of attractive materials due to peculiar structures and unique properties.As previous studies have demonstrated,BiOBr with hierarchical structures is more structurally stable and could provide a large number of surface active sites for the diffusion and mass transportation of organic molecules and reactive radicals,hence exhibiting good photocatalytic activity in photocatalytic dye degradation[23].Recently,persimmon-like nanostructures have attracted considerable attention.For example,Cao et al.[24]fabricated persimmon-like(BiO)2CO3,and found that it is able to efficiently degrade RhB and eosin sodium salt under the simulated solar-light irradiation.Dong et al.[25]synthesized persimmon-like N-doped(BiO)2CO3with a hierarchical superstructure,and observed that such samples show enhanced visiblelight-driven catalytic activity as compared to pure(BiO)2CO3and TiO2-based visible-light photocatalysts for NO removal under visible-or solar-light illumination.Nearly monodisperse YVO4with a persimmonlike architecture was synthesized on a large scale via a complexing agent-assisted solution route,the ultraviolet absorption and photoluminescence spectra of the samples showed that the optical properties of YVO4nanopersimmons are relevant to their sizes and shapes[26].Hexagonal ZnIn2S4photocatalysts with a three-dimensional (3D)-hierarchical persimmon-like shape were fabricated using the oleylamine-assisted solvothermal method, and hydrogen evolution experiments revealed that the obtained hierarchical ZnIn2S4possesses good photocatalytic activity[27].Persimmon-like SrMoO4with a uniform structure was obtained via a microwave radiation route with ethylenediamine tetraacetic acid as the chelating agent[28].Using the rapid and high-efficient microwave radiation method,CaMoO4with an interpenetrated persimmonlike structure was generated in a high yield[29].Uniform and highly hierarchical CaMoO4microstructures with a persimmon-like morphology were obtained on a large scale by the green solution-based synthesis method without adding any surfactant[30].Employing N(C4H9)4BF4as fluoride source,multilayerstacked hierarchical persimmon-shaped CeF3was prepared via a template-free hydrothermal route[31].Persimmon-like NaLa(WO4)2with a microarchitecture was obtained through a hydrothermal process using trisodium citrate as chelated reagent[32].To the best of our knowledge,however,preparation of porous nanosheet-aggregated persimmon-like BiOBr and its influence on photocatalytic activity has not yet been investigated systematically.
In this work,we report synthesis of the porous nanosheet-aggregated persimmon-like BiOBr with hierarchical microstructures for the first time utilizing a facile polyvinyl pyrrolidone (PVP)-assisted solvothermal approach.Their physicochemical properties were characterized,and their photocatalytic performance for MB degradation under visible-light irradiation was evaluated.
1 Experimental
1.1 Catalyst preparation
The persimmon-like BiOBr with hierarchical microstructures and the BiOBr nanosheets were fabricated using (or without)the PVP-assisted or solvothermal method.In a typical preparation process,5.0 mmol of Bi(NO3)3·5H2O and 7.5 mmol of cetyltriethylammnonium bromide(CTAB)were dissolved in 30 mL of ethylene glycol (EG)under stirring until a homogeneous solution was formed.Then,0,0.2,0.5,0.7,1.0 and 1.5 g of PVP was dissolved in the above mixed solution under stirring for 0.5 h,respectively.The obtained mixed solution was transferred to a 50 mL Teflon-lined stainless autoclave for the solvothermal treatment at 120℃for 12 h.The resulting product was filtered and washed with pure ethanol and deionized water several times until the pH value of the filtrate reached 7,followed by drying in an oven at 60℃for 24 h,thus obtaining the persimmonlike BiOBr with hierarchical microstructures and the BiOBr nanosheets.The preparation of the BiOBr contrast samples was same as described above except that the time was changed at a solvothermal temperature of 120℃in the presence of 0.7 g PVP.The commercial TiO2(which was denoted as P25)was purchased from Degussa company.
1.2 Catalyst characterization
The X-ray diffraction (XRD)patterns of the samples were recorded on an X-ray diffractometer(Bruker D8-advance,Germany)operated at 40 kV and 35 mA using Cu Kα radiation and nickel filter(λ=0.154 178 nm)within a range of 5°~80°(2θ).The N2adsorption of the samples were investigated at 196℃on a Quadrasorb S1-3MP analyzer with the samples being degassed at 250℃for 3 h in vacuum before measurement.The surface areas of the samples were calculated by Brunauer-Emmett-Teller(BET)method.The surface morphologies of the samples were observed by a scanning electron microscopy (SEM,Hitachi S-4800)operated at15 kV.Transmission electron microscopic (TEM)images and selected-area electron diffraction (SAED)patterns of the samples were obtained using a JEM-2100F equipment(operating at 200 kV).Ultraviolet-visible diffuse reflectance spectra(UV-Vis DRS)was recorded with a UV-Vis spectrophotometer(UV-2550,Shimadzu)using BaSO4as the reference,and the scanning range was 200~700 nm.
1.3 Photocatalytic activity evaluation
Photocatalytic activities of the samples for MB degradation were measured in a quartz reactor(AK6522,Lianyungang,Jiangsu,Guibaoda Customized Quartz Processing Sci.&Technol.Co.Ltd.)under visible-light irradiation using a 300 W Xe lamp with a 400 nm cutoff filter(PLS-SXE300UV,Beijing,Perfect Light Sci.&Technol.Co.Ltd.).0.2 g BiOBr or commercial TiO2(Degussa P25)samples were added to 100 mL of aqueous solution containing MB(initial MB concentration C0=10 mg·L-1).Prior to visible-light irradiation,the suspension was ultrasonicated for 0.5 h and magnetically stirred for 2 h in the dark to ensure that the MB could reach an adsorption-desorption equilibrium on the photocatalyst surface.Then,the suspension was magnetically stirred and exposed to visible light.The temperature of the reaction solution was kept at ca.25℃using flowing cool water.At regular intervals,5 mL of the suspension was taken out and then centrifuged to remove the photocatalyst particles for the analysis of MB concentration.The concentration (Ct)of the remaining MB was measured by its absorbance(At)at 664 nm with an Agilent Cary 5000 spectrophotometer.The MB degradation efficiency(X)was calculated according to the following equation:X=(C0-Ct)/C0×100%=(A0-At)/A0×100%.
2 Results and discussion
2.1 Crystal phase composition
Fig.1 shows the XRD patterns of the BiOBr samples prepared without and with 0.7 g PVP after solvothermal treatment at 120℃for 12 h.By referencing to the XRD pattern of the standard BiOBr sample (PDF No.09-0393),one can realize that both samples with good crystallinity contain no other impurity phases,and all of the diffraction peaks are characteristic of a tetragonal bismuth oxybromide crystal structure.Similar results were also reported by other researchers[22].According to the Scherrer formula,the broad diffraction peak in the BiOBr sample synthesized with 0.7 g PVP (Fig.1a)indicates a small size of the BiOBr crystal.As shown in Fig.1b,the XRD pattern of the BiOBr sample synthesized without PVP possessed the stronger intensity of the (102)and(110)crystal planes,indicating that this BiOBr crystal had a preferential growth in the two directions.However,the (102)crystal plane (Fig.1a)of the sample synthesized with 0.7 g PVP was significantly inhibited,and the (101), (112), (104),and(214)crystal planes were also suppressed to certain degree.This might be due to the selective adsorption of PVP on the crystal surface,which could induce generation of the samples with the anisotropic structure and morphology[33].

Fig.1 XRD patterns of persimmon-like BiOBr(a)and BiOBr nanosheets(b)
2.2 Morphology,pore structure and surface area
Morphologies and nanostructures of the BiOBr samples were analyzed using SEM and HRTEM techniques,as shown in Fig.2.The sample prepared with 0.7 g PVP after solvothermal treatment at 120℃for 12 h was composed of a large number of persimmonlike structures with a diameter of 15~20 μm(Fig.2a),and these persimmon-like microstructures were formed by the aggregation of a number of nanosheets(Fig.2b and 2c).These nanosheets were intersected and grown together to form a persimmon-like shape that would facilitate separation of the photogenerated electronhole pairs and hence improve the photocatalytic performance for dye degradation[24-25].Two complete persimmon-like BiOBr micronstructures displayed a nearly circular morphology with a serrated edge and a layered structure (Fig.2d and 2e).The central part of the persimmon-like BiOBr was lighter than the marginal part,suggesting that the actual thickness of the middle section is smaller than the edge part.Formation of such a morphology(Fig.2d)might be a cause of the persimmon-like BiOBr shaping hierarchical structure with cavity.This void made the central portion of the persimmon-like BiOBr hierarchical structure loose,forming a structure similar to a hollow sphere.The recording of multiple bright electron diffraction rings in the SAED patterns (inset of Fig.2d)of the BiOBr sample suggests that the obtained persimmon-like BiOBr is polycrystalline.The persimmon-like BiOBr micronstructures were composed of many small square nanosheets(Fig.2e).The intraplanar spacings(d values)were measured to be ca.0.254 8 and 0.257 1 nm(Fig.2f),in good consistence with those of the (200)and(113)crystal planes of standard BiOBr(PDF No.09-0393),respectively.It can be seen from Fig.2g that the persimmon-like BiOBr sample was mainly composed of Bi,O and Br.Most of the sample derived without PVP after solvothermal treatment at 120℃for 12 h were irregular sheet particles with poor dispersibility and serious agglomeration (Fig.2h and 2i).This result indicates the importance of PVP for the formation of persimmon-like BiOBr hierarchical microstructures.
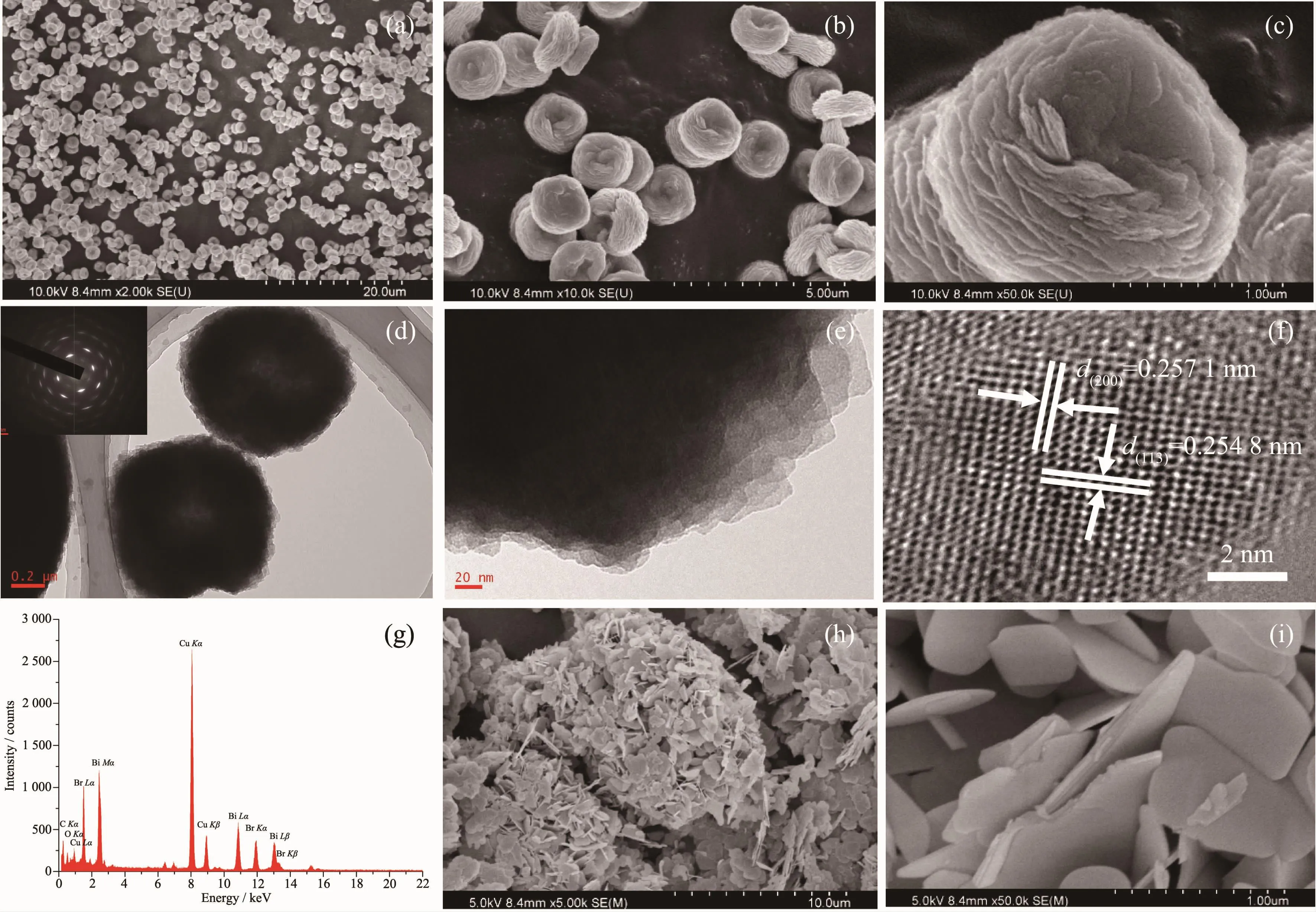
Fig.2 SEM(a~c,h,i)of persimmon-like BiOBr(a~f)and BiOBr nanosheets(h,i);TEM(d~f)images and EDS spectra(g)of persimmon-like BiOBr
Fig.3 shows the N2adsorption-desorption isotherms and pore-size distributions of the persimmon-like and nanosheet-like BiOBr samples.According to the BDDT classification[34],the isotherms were type Ⅳ.In the range (P/P0=0.8~1.0)of high relative pressures,there was a hysteresis loop for the BiOBr nanosheets, indicating formation of a mesoporous slit structure.Compared with the BiOBr nonasheets,the hysteresis loop of the persimmon-like BiOBr sample moved to a lower relative pressure(P/P0=0.1),suggesting existence of a bimodal mesoporous structure.This was reflected more clearly in the corresponding pore-size distributions (inset),which contained small mesopores of ca.3.9 and 9.8 nm in diameter and big mesopores of ca.39.2 nm in diameter.The small mesopores are formed within the nanosheets[35],while the big mesopores are generated between two-dimensional nanolayers.BET surface areas of the persimmon-and nanosheet-like BiOBr samples were 4 and 2 m2·g-1,and the pore volumes were 0.011 and 0.004 mL·g-1,respectively.Obviously,such a 3D hierarchical structure could be conducive to increasing the adsorption efficiency of reactants[36].
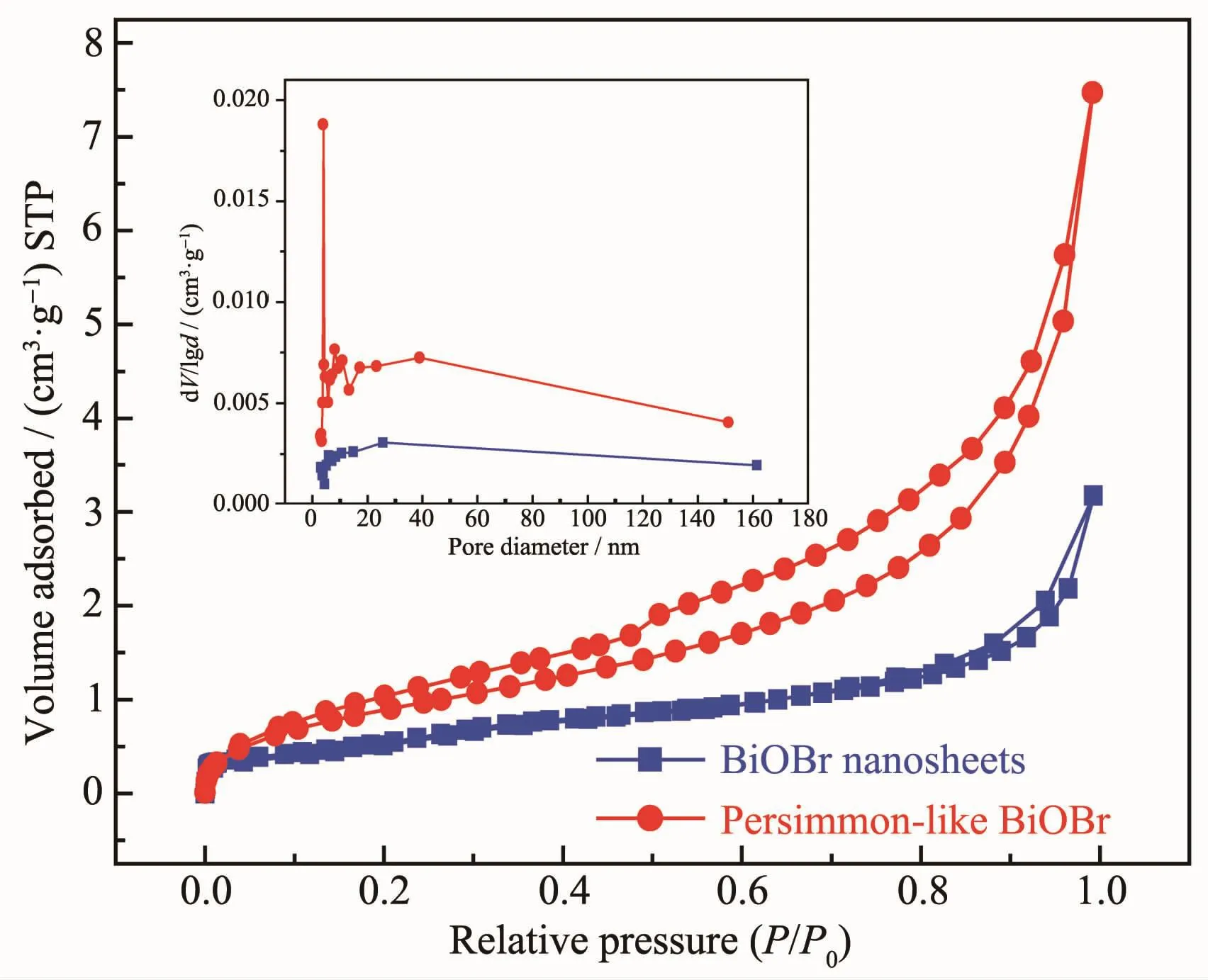
Fig.3 Nitrogen adsorption-desorption isotherms and poresize distributions(inset)of persimmon-like BiOBr and BiOBr nanosheets
2.3 Optical absorption behavior
Fig.4 shows the UV-Vis DRS and (αhν)1/2versus hν plots(inset)of the persimmon-and nanosheet-like BiOBr samples.Both samples displayed a sharp increase in absorption below 450 nm.Compared with the BiOBr nonasheets,absorption of the persimmonlike BiOBr sample was significantly enhanced in the ultraviolet-and visible-light regions.As a semiconductor material,BiOBr obeys the following formula:αhν=A(hν-Eg)n/2,where A is the optical absorption coefficient,ν is the optical frequency,h is Planck constant,n is related to carrier transition,and Egis the bandgap energy.Since BiOBr is the indirect transitive semiconductor[37-38],n is equal to 4.According to the above formula,we can draw the (αhν)1/2versus hν plots of the samples.When αhν=0,the corresponding tangent value is the Egof the sample.The Egvalues of the persimmon-and nonasheet-like BiOBr samples were 2.64 and 2.73 eV,respectively.The narrower bandgap energy can be excited by the lowerenergy visible-light to produce electron-hole pairs.Therefore,the persimmon-like BiOBr hierarchical microstructures would more effectively absorb the incident light,thus improving the catalytic activity.
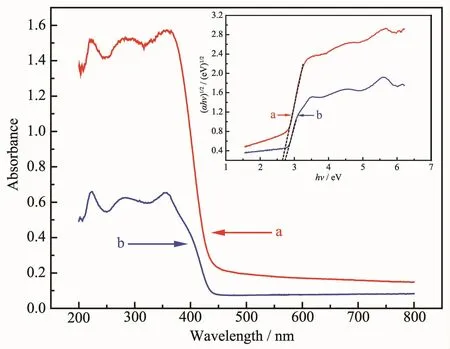
Fig.4 UV-Vis DRS and plots of the(αhν)1/2vs hν(inset)of persimmon-like BiOBr(a)and BiOBr nanosheets(b)
2.4 Formation mechanism of persimmon-like BiOBr

Fig.5 SEM images of BiOBr samples synthesized after solvothermal treatment for 1 h(a),4 h(b),6 h(c),8 h(d),and 18 h(e,f)
In order to explore the formation mechanism and evolution process of the persimmon-like BiOBr,we synthesized the BiOBr samples by changing the solvothermal time at 120℃in the presence of 0.7 g PVP,and their SEM images are shown in Fig.5.As can be seen from Fig.5a,a large number of square nanosheet entities were formed after 1 h of solvothermal treatment,and a few square nanosheets were stacked together.This result suggests that the initial stage of the reaction leads to first generation of small square nanoparticles and then assembling to larger ones.After 4 h of solvothermal treatment,the flaky particles gradually decreased,and the persimmon-like structure began to appear (Fig.5b).After 6 h of solvothermal treatment,accumulation of the thinnanosheet particles basically disappeared,and the skeleton structure of primary persimmon-like microstructures were formed (Fig.5c).After 8 h of solvothermal treatment,a skeletal structure of the persimmon-like stratified BiOBr sample was generated(Fig.5d).As shown in Fig.2a~c,the perfect porous sheet-aggregated persimmon-like BiOBr sample were obtained after 12 h of solvothermal treatment.When the solvothermal time was 18 h,part of the persimmon-like structure was damaged(Fig.5e and f).
To further investigate the crystallization process of BiOBr,we recorded the XRD patterns of the BiOBr samples derived from different solvothermal time,as shown in Fig.6.When the solvothermal time was 1 h,the product was amorphous.With the extension of solvothermal time to 4 h,crystallinity of the product increased.When the solvothermal time was 6,8,12 and 18 h,the diffraction peaks became sharper and increased in intensity.Therefore,formation of the persimmon-like BiOBr phase is a slow crystallization process,and the oriented growth of BiOBr is in agreement with the SEM observations.

Fig.6 XRD patterns of BiOBr samples synthesized after solvothermal treatment for different times
Fig.7 shows SEM photographs of the BiOBr samples fabricated with different amounts of PVP after solvothermal treatment at 120℃for 12 h.When 0.2 g of PVP was used,some persimmon-like particles of BiOBr were preliminarily generated (Fig.7a).When the amount of PVP was 0.5 g,the persimmon-like morphological BiOBr particles were basically formed(Fig.7b).Addition of 0.7 g PVP led to formation of a microstructure of BiOBr with a diameter of 15~20 μm(Fig.2a~c).When the amount of PVP increased to 1.0 g,the morphology of the product did not change significantly(Fig.7c).Further rise in amount of PVP to 1.5 g obtained the BiOBr sample with a ball-shaped structure and a diameter of ca.25 μm (Fig.7d).The above results indicate that an appropriate amount(0.7 g)of PVP could allow the nanosheets to form a persimmon-like BiOBr hierarchical nanostructure.Fig.8 shows the XRD patterns of the BiOBr samples derived with different amounts of PVP.The crystallinity of the sample first increased and then decreased with the rise in amount of PVP,confirming the presence of an appropriate amount of PVP.

Fig.7 SEM images of BiOBr samples synthesized with PVP addition of 0.2 g(a),0.5 g(b),1.0 g(c)and 1.5 g(d)
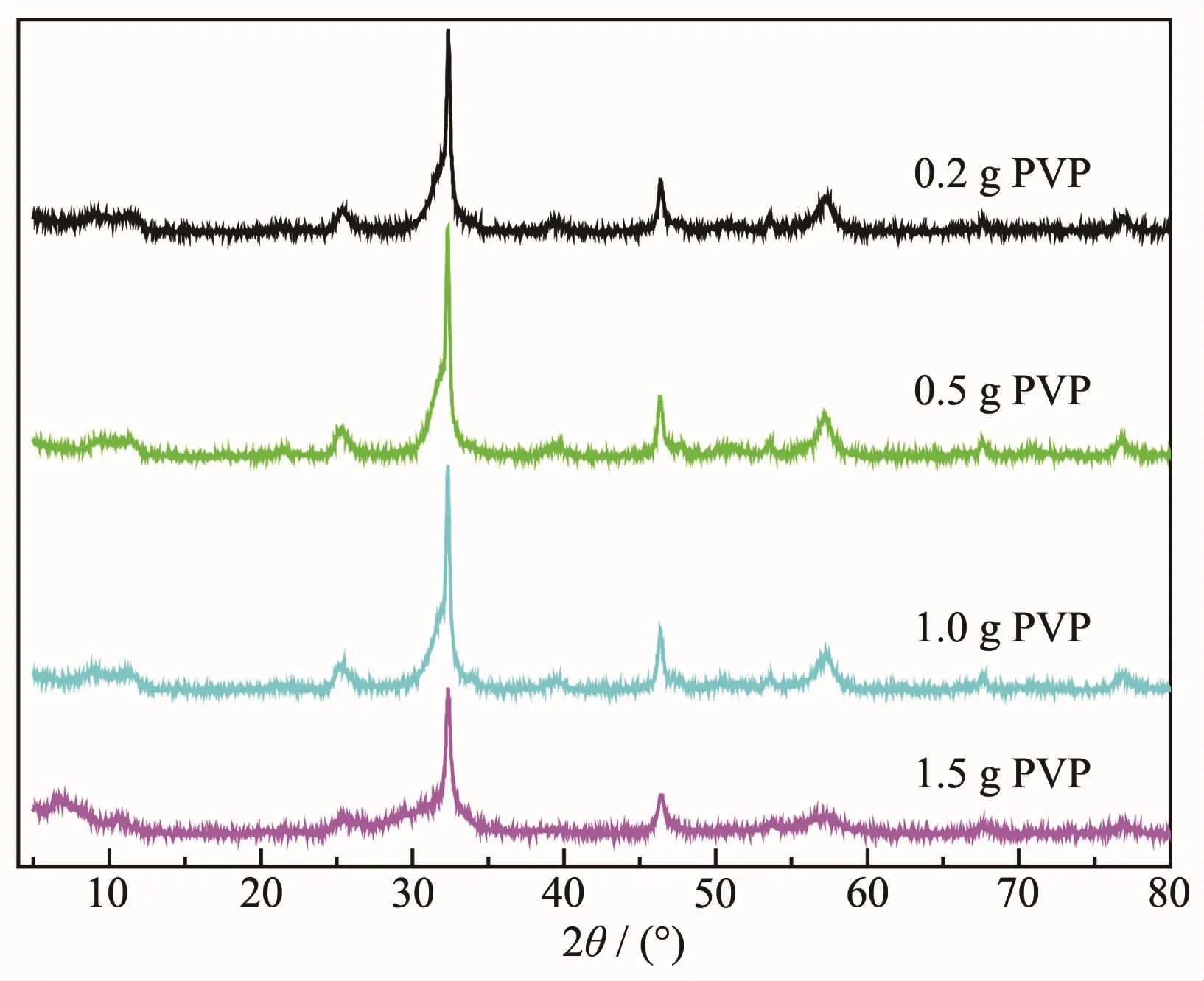
Fig.8 XRD patterns of BiOBr samples synthesized with different added PVP amounts
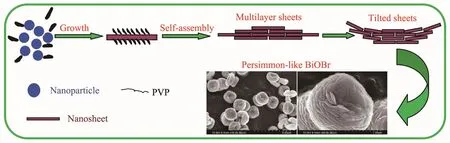
Fig.9 Schematic illustration of morphological evolution process of persimmon-like BiOBr
Based on the above results and combined with the crystal structure and morphology analyses,formation processes of the persimmon-like BiOBr hierarchical microstructures are illustrated in Fig.9.In the initial stage of the persimmon-like BiOBr growth,Bi(NO3)3·5H2O and CTAB reacted to form the BiOBr nanoparticles in the ethylene glycol solution,and PVP molecules were adsorbed on the surface of the nanoparticles[39].When the solution was thermally treated for 1 h,the surface of the particles adsorbed by PVP molecules began to crystallize,forming the tiny square nanosheet particles.As the solvothermal treatment time was prolonged,rectangular nanoparticles were aligned to generate big two-dimensional nanosheets via the side-side binding.With the aid of PVP,these two-dimensional nanosheets were arranged in a certain way to form a three-dimensional microstructure.Formation of the primary BiOBr square nanosheets was a typical Ostwald ripening process.The crystal nuclei of BiOBr were formed and grown in the supersaturated solution.According to Gibbs-Thomson′s law,the solubility of big and small particles is different,and the big nanoparticles are grown from the small nanoparticles.Further growth of BiOBr crystals into two-dimensional nanostructures is directly related to the intrinsic crystal structure of the substance itself.BiOBr(200)and(113)crystal facets have higher chemical activity than other crystal facets.In this way,the growth rate of(200)and(113)crystal facets is faster than that of other crystal facets.Therefore,the growth of crystals is preferentially carried out along the layered plane,and the square nanosheet BiOBr was formed after 1 h of reaction.In the subsequent self-assembly process,PVP played an important role in forming big layered nanosheets and multilayered structures[40].Selective adsorption of PVP on multiple crystal planes of square nanosheet BiOBr was an important process in the initial stage of crystal growth.TEM results show that self-assembled layered nanosheet BiOBr is composed of a large number of small square nanosheet BiOBr,and these primary small nanosheets tend to gradually expand their twodimensional surface areas via the side-by-side selfassembly.With the help of PVP,these small nanosheets rotated to make the two-dimensional crystal surface of the adjacent nanosheets aligned,and then these small nanosheets fused to form a complete layered nanosheet.As observed in the SEM image(Fig.5c),newly formed layered nanosheets contained flat and multilayered disks.This process could further reduce the surface energy of the layered nanosheets,and the two-dimensional geometry was particularly conducive to such an accumulation.As the reaction time increased,the layered nanosheets did not continue to accumulate into a cylindrical structure,but was slightly warped to form a concave surface around the flat disks,possibly due to lattice tension or surface interaction at the edge of the layered nanosheets.After the assembly process was carried out in this concave range,more warped layered nanosheets were added to this concave surface,causing the edges of the entire structure to gradually thicken and become denser,while the central part of the disk was almost stagnant in the process[41].In this way,BiOBr evolved from a flat two-dimensional disk into a three-dimensional persimmon-like BiOBr hierarchical microstructure with small cavities in the middle.Therefore,the phenomenon that the edge of BiOBr hierarchical microstructure was thicker than the central part is observed in the TEM image(Fig.2d).
2.5 Photocatalytic activity and reaction mechanism
Fig.10 shows the photocatalytic activities of the samples for MB degradation under visible-light illumination.The MB solution was relatively stable in the absence of a catalyst and only under visible-light irradiation,since almost no decolorization (about 1%degradation efficiency)was observed after 50 min of visible-light illumination.When the MB solution was irradiated by visible light for 10~15 min,MB degradation efficiencies of P25 and BiOBr nonasheets were 11%~15%and 49%~61%,respectively.However,when the MB solution with persimmon-like BiOBr was exposed to visible light for 5 min,MB degradation efficiency reached 99%.After 15 min of visible-light irradiation,the MB solution was almost completely decolorized (ca.100%MB degradation efficiency),probably because the concentration of MB is lower and more inactive molecules or ions are adsorbed on the photocatalyst surface as the reaction proceeds[42].The results indicate that the persimmon-like BiOBr sample shows excellent photocatalytic performance under visible-light irradiation.

Fig.10 Photocatalytic activities of the samples for MB degradation under visible-light illumination
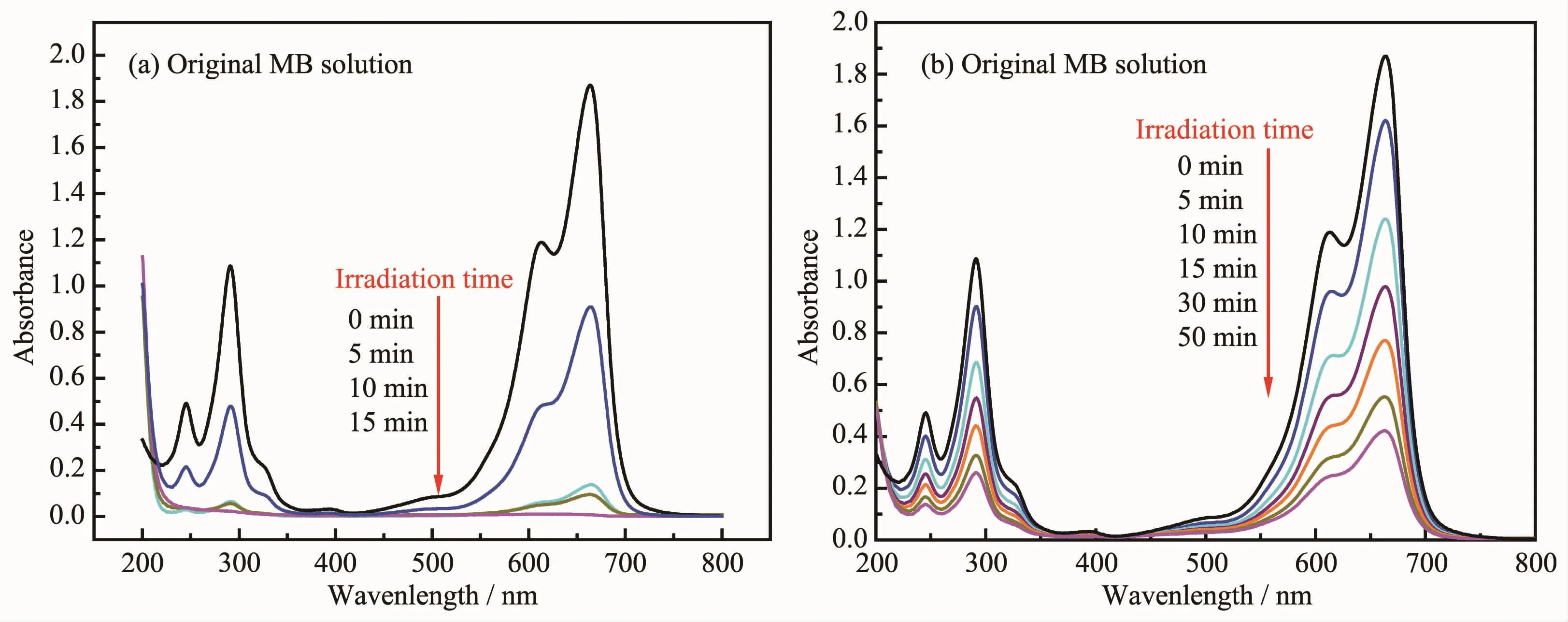
Fig.11 Temporal absorption spectra of MB during the photodegradation process over persimmon-like BiOBr(a)and BiOBr nanosheets(b)
Fig.11 shows the temporal absorption spectra of MB during the photodegradation processes over persimmon-and nanosheet-like BiOBr.As can be seen from Fig.11a,the intensity of the MB absorption peaks at 246,290 and 664 nm continuously decreased under the irradiation of visible light,and the color of the solution became lighter.After 15 min of visiblelight illumination,the MB absorption peaks almost disappeared.Over the nanosheet-like BiOBr sample,however,the intensity of the MB absorption peaks at 246,290 and 664 nm decreased with the light illumination time,but the absorbance at 664 nm was still ca.0.4 after 50 min of visible-light illumination(Fig.11b),indicating thatMB solution was not completely degraded.Therefore,the visible-lightdriven catalytic activity of the persimmon-like BiOBr sample was much higher than that of the nanosheetlike BiOBr sample.
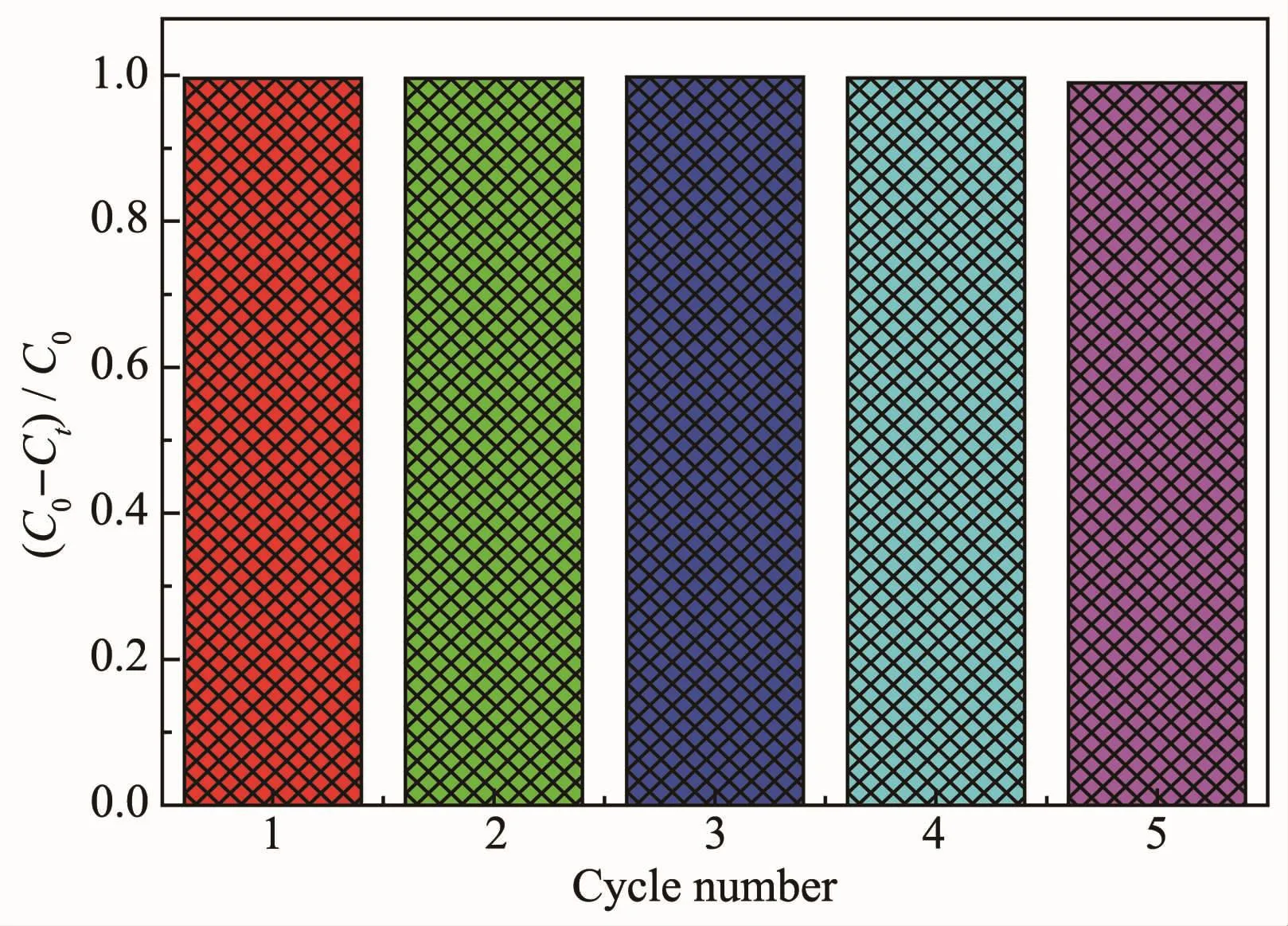
Fig.12 Recycling tests for the potocatalytic degradation of MB over persimmon-like BiOBr under visible light irradiation
Fig.12 shows the reusability of the persimmonlike BiOBr sample.It can be clearly seen that the photocatalytic activity of this sample remained basically unchanged after 5 recycles.This result demonstrates that the persimmon-like BiOBr sample is highly catalytically stable under the irradiation of visible light.
It is well known that a photocatalytic process involves the reductive and oxidative reactions of the photo-induced charge carries(e-/h+pairs)in the degradation of organic pollutants,in which the·OH radicals and active oxygen species participate[43].Under visiblelight irradiation, the porous sheet-aggregated persimmon-like BiOBr is inspired to generate photoinduced e-/h+pairs.The electrons from the valence band (VB)are transferred to the conduction band(CB),leaving lots of holes in the VB[44].The photoinduced holes react with H2O on the surface of persimmon-like BiOBr to form·OH radicals.The photo-induced electrons react with adsorbed O2on the surface of persimmon-like BiOBr or dissolved O2in water to generate·O2-radicals.Therefore,the surface of persimmon-like BiOBr could generate a large number of·OH and·O2-radicals with strong oxidation ability which would be beneficial for the degradation of MB[42].
In general,the photocatalytic activity of a semiconductor oxide is mainly determined by the factors,such as crystal structure,bandgap energy,crystallinity,grain size,specific surface area,and pore structure[4].The main reason for the higher visiblelight-driven catalytic activity of the persimmon-like BiOBr sample might be due to the following factors:firstly,the crystallinity of persimmon-like BiOBr is good and this sample possesses a special layered structure,which leads to its wider absorption band edges (corresponding to the forbidden band width of less than BiOBr nonasheets).The improvements in light absorption range and light utilization efficiency of the persimmon-like BiOBr sample render it to show enhanced photocatalytic activity.Secondly, the persimmon-like BiOBr sample possesses a larger surface area,which would be helpful for the adsorption of MB molecules and transfer between different active sites.Consequently,the MB molecules adsorbed on the surface of the material could easily undergo redox reactions with the photogenerated electrons or holes,thus showing higher photocatalytic performance[42].Thirdly,the three-dimensional hierarchical structures have a large number of threedimensional channels,which allow MB and degradation intermediates to be diffused in the channels.These structures would favor the interactions of MB and intermediates with photogenerated electrons or holes,hence enabling more effective and thorough degradation of the organic dyes[39-40].
3 Conclusions
With bismuth nitrate as Bi source,CTAB as Br source,and EG as solvent in the presence of PVP,a novel sheet-aggregated persimmon-like BiOBr photocatalyst was successfully synthesized via a facile solvothermal route.The solvothermal time and PVP addition greatly influence the hierarchical microstructure,porous structure,and crystallinity of the BiOBr product.After solvothermal treatment at 120℃for 12 h in the presence of 0.7 g PVP,a porous sheetaggregated persimmon-like BiOBr microstructure(Diameter=15 ~20 μm)was obtained.The porous persimmon-like BiOBr sample with a surface area of 4 m2·g-1and a bandgap energy of 2.64 eV exhibited good optical absorption behaviors in visible-light regions and excellent photocatalytic activity and stability for the removal of MB under visible-light illumination.It is concluded that the factors,such as high surface area,low bandgap energy,porous structure and unique morphology,are responsible for the excellent photocatalytic performance of porous persimmon-like BiOBr for MB degradation under visible-light irradiation.
