Gut microbiota and obesity:Impact of antibiotics and prebiotics and potential for musculoskeletal health
2020-03-14TejKlncicRyleneReimer
Tej Klncic,Rylene A.Reimer,b,*
a Faculty of Kinesiology,University of Calgary,Calgary,AB T2N 1N4,Canada
b Department of Biochemistry&Molecular Biology,Cumming School of Medicine,University of Calgary,Calgary,AB T2N 4N1,Canada
Abstract Obesity is a complex disease with multiple contributing factors.One of the most intensely studied factors during the past decade has been the gut microbiota,which is the community of all microbes in the intestinal tract.The gut microbiota,via energy extraction,inflammation,and other actions,is now recognized as an important player in the pathogenesis of obesity.Dysbiosis,or an imbalance in the microbial community,can initiate a cascade of metabolic disturbances in the host.Early life is a particularly important period for the development of the gut microbiota,and perturbations such as with antibiotic exposure can have long-lasting consequences for host health.In early life and throughout the life span,diet is one of the most important factors that shape the gut microbiota.Although diets high in fat and sugar have been shown to contribute to dysbiosis and disease,dietary fiber is recognized as an important fermentative fuel for the gut microbiota and results in the production of short-chain fatty acids that can act as signaling molecules in the host.One particular type of fiber,prebiotic fiber,contributes to changes in the gut microbiota,the most notable of which is an increase in the abundance of Bifidobacterium. This review highlights our current understanding of the role of gut microbiota in obesity development and the ways in which manipulating the microbiota through dietary means,specifically prebiotics,could contribute to improved health in the host,including musculoskeletal health.2095-2546/© 2020 Published by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords: Gut microbiota;Musculoskeletal health;Obesity;Prebiotics
1. Introduction
According to the World Health Organization, worldwide obesity has more than tripled since 1975, with more than 650 million adults living with obesity and more than 41 million children under the age of 5 considered to be overweight or obese.1Obesity is associated with metabolic disorders affecting multiple organs and systems2and is recognized as a major risk factor for the development of type 2 diabetes (T2D), cardiovascular diseases(heart disease and stroke),musculoskeletal disorders(osteoarthritis(OA)),and certain forms of cancer(endometrial, breast, ovarian, prostate, liver, gallbladder, kidney,and colon).1,3
Reduced to its most simplistic nature, obesity is the consequence of greater energy intake than expenditure; however,intensive research over the past decades has uncovered obesity's extremely complex etiology, which encompasses a dynamic interplay between host genetic and environmental factors.3One of the most recent factors to be identified as playing a critical role in obesity development is the gut microbiota. Through its role in energy harvest, metabolic signaling, and inflammation,the gut microbiota is now recognized as an important player in body weight regulation.4,5Strategies aimed at shifting the gut microbiota back to a “healthy state” are providing new therapeutic targets for interventions that might help to reduce the burden of obesity and its comorbidities.
2. Gut microbiota
The intestinal tract contains the human body's most densely colonized ecosystem, consisting of bacteria, archaea, viruses,and unicellular eukaryotes—the so-called gut microbiota.6The number of microbes in the intestinal tract is approximately 100 trillion cells,7which is estimated to be in the same order of magnitude as human cells.8The number of bacteria increases along the length of the gut to approximately 108bacteria per gram of content in the distal ileum and 1011bacteria per gram in the colon.9Bacteria are classified according to their taxonomical rank (Fig. 1). At the division level (phylum), Firmicutes (gram-positive, anaerobic, spore-forming bacteria,mainly represented by the genera Clostridium, Faecalibacterium, Blautia, Ruminococcus, and Lactobacillus10) and Bacteroidetes (gram-negative, anaerobic, non-spore-forming bacteria, mainly represented by Bacteroides and Prevotella10)are dominant and can constitute over 90%of the bacteria present in the large and small intestine.11Even though other phyla such as Actinobacteria (Bifidobacterium), Proteobacteria(Gammaproteobacteria with Enterobacteriaceae),or Verrucomicrobia(Akkermansia)are low in numbers,they have a major impact on health.12,13It is clear that individuals share similar core microbiota; nevertheless, all individuals have numerous differences in their microbiota, including proportions, diversity, species, and gene functions.14Turnbaugh et al.11suggested that instead of sharing a core human microbiome definable by a set of abundant microbial lineages, we might share a core gut microbiome at the level of metabolic functions.The gene pool of our gut microbiota(gut microbiome)is at least 150 times larger than our own, providing us with a range of otherwise inaccessible metabolic capabilities.15Despite the fact that a definition of a healthy microbiota remains elusive,16it has been established that the microbiota develops and matures over the course of infancy and childhood and reaches its adult form at 3 years of life.8

Fig. 1. Bacterial taxonomy. Scientific classification of bacteria by rank or level.
Several factors influence the microbial colonization of the infant gut,such as gestational age(term vs.preterm),mode of delivery (vaginal delivery vs. caesarean section), infant diet(breast milk vs. formula), breast-feeding patterns,17maternal diet, genetics, sanitation, smoking during pregnancy, familial environment (rural vs. urban), home structure (large vs. small families), geography, and antibiotic treatment.18Given the breadth of factors that influence the development of the infant's gut microbiota in the first year of life, interindividual differences in gut microbiota are significantly greater among children than among adults, even though the infant's gut microbiota is dominated by fewer bacterial genera.14The sequence of bacterial species appearing in the first months of life is complex, and many transient species emerge owing to changes in the gut environment.11This normal maturation can be disrupted,leading to an imbalance in the microbial community or “dysbiosis”, which can ultimately affect obesity risk5and several other diseases(Fig.2).19
2.1. Gut microbiota disruption and obesity risk
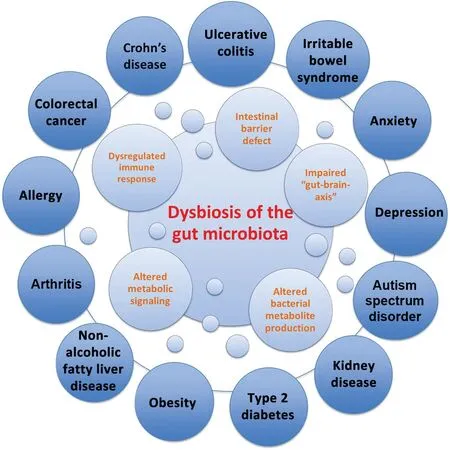
Fig.2. Dysbiosis of the gut microbiota in disease.Dysbiosis of the gut microbiota impairs the intestinal barrier, immune system, metabolic functions, and bacterial metabolite production (i.e., short-chain fatty acids), as well as function/development of the central nervous system. Dysbiosis has been linked to several intestinal disorders such as inflammatory bowel disease (i.e., Crohn's disease,ulcerative colitis),irritable bowel syndrome and colorectal cancer,as well as extraintestinal disorders (i.e., obesity, type 2 diabetes, arthritis, and depression).19
The gut microbiota of an individual with obesity may promote more efficient extraction and/or storage of energy from a certain diet, compared with gut microbiota of a lean individual. The earliest evidence supporting this hypothesis was the observation that germ-free (GF) mice are leaner when compared with conventionally raised animals and that the transplantation of gut microbiota into adult GF mice substantially increased their body fat mass despite reduced food intake.20In addition to more efficient energy extraction from the diet,obesogenic gut microbiota also leads to intestinal inflammation contributing to the obese phenotype.21-24Specifically,proinflammatory tumor necrosis factor-α (TNF-α) messenger RNA levels in the ileum show strong correlation with the degree of weight gain,increased fat mass,and plasma glucose and insulin upon exposure to an high-fat diet (HFD).21Furthermore, studies showed that only conventionally raised animals developed inflammation, whereas GF animals had no upregulation of TNF-α messenger RNA levels,suggesting that an HFD requires enteric bacteria to trigger intestinal inflammation.Interestingly,only obesity-prone Sprague Dawley rats and not obesity-resistant rats had increased ileal inflammation,neutrophil infiltration and innate immune Toll-like receptor 4(TLR-4) activation once challenged with an HFD.23In addition, obesity-prone Sprague Dawley rats displayed increased intestinal permeability, favoring increased leakage of gutderived bacterial lipopolysaccharides (LPS) into the systemic circulation, which contributes to the chronic, low-grade inflammation associated with obesity.23,24It is well-established that LPS (component of the outer membrane of Gramnegative bacteria)25and saturated fatty acids (Western diet)26are ligands for TLR4 and can, therefore, activate the innate immune system. Upon activation of TLR4 in several tissues(intestinal epithelial cells, adipose tissue, muscle, and liver),immune cells such as proinflammatory M1 macrophages are activated and secrete proinflammatory cytokines (i.e., TNF-α and Interleukin-6).27Proinflammatory cytokines further recruit/attract additional proinflammatory immune cells while inhibiting anti-inflammatory cells such as M2 macrophages and/or regulatory T cells.27Chronic immune system activation and excessive production of proinflammatory cytokines in the tissues interfere with insulin signaling as demonstrated by the inhibition of insulin-stimulated glucose uptake when insulin and TNF-α were coinjected into humans.28When mice were fed a normal diet and infused subcutaneously with LPS for 4 weeks, increased weight (whole body, liver, and adipose tissue) and inflammation (i.e., TNF-α, Interleukin-1, Interleukin-6) were seen, and the phenotype was similar in many respects to 4 weeks of high-fat feeding.29While acute inflammation is necessary to start the healing process, there is now compelling evidence that chronic bacteria/diet-induced inflammation can contribute to obesity and the metabolic syndrome.
Although many of the initial studies linking the gut microbiota to obesity centered around adulthood, it is now recognized that long-term metabolic perturbations could already be initiated in early life if an obesogenic gut microbiota from mothers is transferred to the infant and/or is altered in the first years of life when microbial colonization is still in progress(e.g., from antibiotic exposure or formula feeding).5When mothers were given antibiotics during pregnancy, newborns had higher birth weights30and children were 84%more likely to be obese at 7 years of age.31Similarly,several other studies,including 3 large cohorts involving 28,000 mother-child pairs,3210,000 children,33and 6114 boys and 5948 girls,34all reported an increased risk of being overweight when children were exposed to antibiotics in the first 12 months of life.Mechanistically, treating mice with low doses of penicillin(LDP) increased adiposity through altered gut microbiota,increased short-chain fatty acid (SCFA) levels, and altered hepatic metabolism of lipids and cholesterol.35Cox et al.36demonstrated that LDP enhanced the effect of HFD-induced obesity and,even though the microbial communities recovered after termination of LDP, the metabolic phenotype persisted.Microarray gene expression analysis revealed that early life exposure to broad-spectrum amoxicillin-based antibiotic delayed the maturation process of the intestine in 10%-30% of genes, downregulated the genes involved in the immune system (antimicrobial products and antigen presentation), and consequently interfered with gut barrier function.37The weight gain observed in this study and others after early life antibiotic treatment was more pronounced in males/boys34-36and was a consequence of reduced abundance of metabolically protective bacteria,increased availability of microbiota-derived energy, and altered hepatic metabolic signaling and/or intestinal defenses.38
In addition to antibiotics,caesarean-section(C-section)also alters early microbiota development as it bypasses exposure to vaginal microbiota during labor and exposes the child to skin and environmental microbes instead. For example, 72% of newborns' microbiota (vaginal delivery) matched species found in the stool of their mother, whereas only 41% of these species were detected in C-section newborns, as shown by B¨ackhed et al.17To assess the associations of a C-section with body mass from birth to adolescence, 10,219 children (of which 9.06% were delivered by a C-section) were investigated.39By 6 weeks of age, children born by C-section had a greater weight-for-length z-score, a phenotype that persisted until 15 years of age.39Similarly, in 7-year-old children, a 46% higher obesity risk was observed in children born by Csection when compared with children delivered vaginally.31Unlike in human C-section studies where perinatal antibiotics are used during a C-section and confound the independent effects of birth mode, Martinez et al.40performed a study in mice to investigate the impact of antibiotic-free C-section on early life microbiota and obesity risk.Mice born via C-section gained 33%more weight at 15 weeks of age and female mice showed an even stronger phenotype(70%higher weight gain),a finding also reported in 1 birth cohort in humans.41In addition to increased fat and body mass, microbiota development was altered in C-section mice.40Under-represented taxa in Csection animals included Bacteroides, Ruminococcaceae,Lachnospiraceae,and Clostridiales (associated with lean phenotypes in mice36), and overrepresented taxa included S24-7,Lactobacillus,and Erysipelotrichaceae.40
2.2. Gut microbiota composition in obesity
After more than a decade of research describing the link between gut microbiota and obesity,many important questions about the host-microbiota relationship remain.42Initially,animal studies demonstrated that obesity is associated with a change in the relative abundance of the 2 dominant bacterial phyla with a reduction in the abundance of Bacteroidetes and a proportional increase in Firmicutes.43,44Similar gut microbiota changes have been seen in adults45and children with obesity,46but some studies did not support these findings,11,47including 2 meta-analyses.48,49Accordingly, phylum-level changes in individuals with obesity are less clear, mostly because of large interpersonal variation, insufficient sample sizes, and the different methods used for the sequencing and quantifying of the taxa.49
The most consistent finding in humans appears to be a higher abundance of Escherichia coli(E.coli)and Lactobacillus in individuals with obesity.50,51Interestingly, there are many pathogenic strains of E.coli(in addition to the majority of harmless E. coli), whereas certain strains of Lactobacillus are commonly used as probiotics owing to their health benefits.3This seeming discrepancy was clarified in part by Drissi et al.,52who review evidence that the effects of Lactobacillus are age dependent and strain specific.With more than 150 Lactobacillus species identified to date, this represents a diverse group of bacteria.52Similarly, bifidobacteria are also wellknown probiotics, and lower abundance has been shown in people with a higher body mass index (BMI)53,54and a negative correlation was observed between Bifidobacterium and visceral adiposity.53Likewise, lower levels of Akkermansia have been observed in individuals with a high body mass index;55,56however, individuals with T2D from Asia showed an increased Akkermansia muciniphila abundance.57The authors concluded that Akkermansia could have a beneficial role in metabolic profiles depending on the environment in the gut.Since Akkermansia is a mucin-degrading bacteria,it could make the intestinal barrier thinner, thereby allowing bacterial translocation and pathogenesis of T2D.57In line with this finding, a study in rodents showed that dietary fiber deficiency allows the mucin-degrading bacteria such as Akkermansia muciniphila to grow, express mucin-degrading enzymes, and enhance disease susceptibility.58
Regardless of inconsistencies in the precise obesogenic microbiota composition, it is clear that obesity is associated with a lower diversity and richness of the gut microbiota,which might compromise microbial function and lead to disease.3It has been suggested that obese microbiomes can utilize a more diverse set of energy sources, resulting in greater energy harvest.59To better understand changes in metabolism in obesity, analysis of microbial metabolites such as SCFA and bile acids can provide further insight given their role in activating signals that control appetite.60
2.2.1. Bile acids
Primary bile acids are synthesized from cholesterol by the liver and secreted into the small intestine, where Gram-positive bacteria(mostly lactobacilli and Clostridium species)convert them into secondary bile acids that can act as signaling molecules.3,61Insulin sensitivity, energy expenditure, lipid accumulation, and glucose homeostasis have all been shown to be modified by secondary bile acids,which act in large part via binding to receptors such as farnesoid X receptor and the G protein-coupled bile acid receptor.62For example, secondary bile acids can bind to ileal farnesoid X receptor receptors,which in turn stimulate production of fibroblast growth factor 19 that can cross the blood-brain barrier63and suppress activity of hypothalamic agouti-related peptide/neuropeptide Y neurons to improve energy homeostasis and glucose metabolism.60
2.2.2. SCFAs
SCFAs are the end products of bacterial polysaccharide fermentation that can be used as an energy source by the host and can, therefore, influence body weight.3The most prominent SCFAs are butyrate, propionate, and acetate; butyrate serves as the energy substrate for the colonocytes,and propionate and acetate act as substrates for gluconeogenesis and lipogenesis in the gut and liver.64Higher levels of SCFAs are found in the feces of obese children and adults when compared to normal weight individuals.47,65These higher levels likely result from increased colonic energy harvest66rather than from reduced intestinal absorption.66,67The higher fecal SCFA seen in obesity appear to be at odds with the known beneficial effects of SCFA acting as signaling molecules that improve insulin sensitivity,increase satiety,and reduce inflammation in the pancreas,muscle,and adipose tissue.3,64Many of these benefits occur via the G protein-coupled receptors,free fatty acid receptor 2(FFAR 2)and FFAR 3.64For example,SCFA stimulation of FFAR 2 receptors in the gut stimulates the release of the satiety hormone glucagon-like peptide-1,while in neutrophils it suppresses inflammation.64Given the limitations of interpreting higher concentrations of SCFA in feces in isolation from overall turnover and metabolism,64the balance of evidence to date favors a beneficial metabolic effect for SCFA,particularly when produced from the fermentation of dietary fiber.
3. Modulation of gut microbiota in obesity with diet(specifically prebiotics)and exercise
While our individual host genome does not change over time,many environmental and lifestyle factors can profoundly change our gut microbiome throughout our lives.68One of the characteristics of the gut microbiota that make it an opportune target for new obesity treatments is the relative ease by which it can be manipulated with dietary agents. Interestingly, some gut microbes can remember past diets and exhibit a so-called hysteresis that reflects those prior diets.69For example, when mice were put on a chow diet between 2 bouts of a high-fat lard-based diet, accelerated weight regain was seen after the second exposure to the Western diet.70The authors were able to identify a gut microbiome signature that persisted after successful dieting in the obese mice and contributed to faster weight regain upon re-exposure to the HFD.70Experiments in so-called “humanized mice” (GF mice colonized with human fecal samples)also provide similar evidence in that the dietary history of the human donor determines the response to the diet intervention in mice.71This effect is transmittable across generations.When“humanized mice”were exposed to a low-fiber diet, reduced microbial diversity/function was seen and the effects were transmitted to future generations.72Microbiota diversity loss was greater with each subsequent generation (4 in total) with an additional loss of microbial fiber-degrading capacity.72Exposing the 4th generation of mice to a high-fiber diet could not correct the loss of diversity and function.Recapturing this function could only be achieved through the reintroduction of lost bacteria with a fecal microbiota transplant from control mice.72After the fecal transplant and a switch to a high-fiber diet, 110 taxa were restored and the differences between the low-fiber and high-fiber diet groups were no longer detectable.72These studies demonstrate the importance of a high-fiber diet to prevent the loss of microbial taxa and function seen with consumption of a low-fiber Western diet.73
3.1. Prebiotics
When Gibson and Roberfroid74first defined prebiotics in 1995,only a few compounds fit the definition,including shortand long-chain β-fructans(fructo-oligosaccharides and inulin),galacto-oligosaccharides, and lactulose. The most recent definition of prebiotics is that they are a substrate that is selectively utilized by host microorganisms conferring a health benefit.75Changes in the definition from its inception have enabled more compounds, such as resistant starches, pectin,arabinoxylan, whole grains, and noncarbohydrate compounds(polyphenols),to be considered as candidate or confirmed prebiotics.75,76Interestingly,not all dietary fibers can be classified as prebiotics since consumption of prebiotics must result in a health benefit for the host.76For example, soluble dextrin fibers from corn failed to be classified as prebiotics even though microbial changes in the gut were detected along with a lower secretion of proinflammatory and immunoregulatory cytokines.77Nevertheless, no improvement in histological colonic inflammation was seen.77It might be that the dose administered was too low to improve health, since a dosedependent effect of prebiotics on disease risk has been described,with higher doses displaying more health benefits.78
Intake of prebiotics has been associated with improvements in metabolic health that have included lower body weight and fat mass, improved glucose control, a reduction in inflammation, and an increase in health-promoting bacteria.75,79For example,in infants,breast milk is a rich source of human milk oligosaccharides (candidate prebiotics), which stimulate the growth of commensal bacteria (Bifidobacterium and Bacteroides spp.) and restrict the adhesion of pathogens such as E.coli, Campylobacter jejuni, and Helicobacter pylori.80As early as 1935, a report from Massachusetts General Hospital convincingly showed benefits of breast-feeding.81In an analysis of 20,000 patients,breast-fed infants had a lower incidence of mortality and morbidity,especially of enteric disease,otitis media, and respiratory infection, when compared to exclusively formula-fed infants.81It is plausible that the microbiota,at least in part,is involved in these improved infant outcomes.
Several studies have reported a correlation between a low abundance of Bifidobacterium spp. and obesity,82,83along with an increased capacity of obesogenic gut microbiota to produce SCFAs47,67,84; however, both studies were modified with a prebiotic approach. One study showed that a 3-month supplementation with oligofructose-enriched inulin(16 g/day)increased the abundance of health-promoting Bifidobacterium spp. and decreased total fecal SCFA concentration in 44 women with obesity; however, no significant reduction in BMI was observed.85Oligofructose-enriched inulin provides a blend of long-chain (inulin) and short-chain (oligofructose)fructans that ferment at different rates in the colon; oligofructose ferments more rapidly. Intervention studies that exposed normal-weight, healthy adolescents to oligofructose-enriched inulin(8 g/day)for 1 year86and adults with overweight or obesity to oligofructose (21 g/day) for 3 months87reported decreased body weight gain and fat mass. The study in adults also reported a decrease in energy intake and an increase in satiety hormones, thus showing additional positive effects of prebiotics relevant to obesity management.Similarly,a sample of children (7-12 years of age) who were administered 8 g/day of oligofructose-enriched inulin for 16 weeks had reduced body fat88and improved appetite control89compared to children given a placebo.Prebiotic consumption normalized childhood weight gain, reduced total and trunk body fat,altered primary fecal bile acids,and changed microbiota composition by increasing Bifidobacterium species.88Mechanistically, several animal studies have provided insight into prebiotic-mediated outcomes noted in human studies. In rodents, prebiotic intake led to the following positive outcomes: increased number/activity of enteroendocrine L-cells responsible for the production of satiety hormones and improved glucose homeostasis,90,91recovery of gut barrier function through increased Bifidobacterium spp.,91,92and expression/activity of tight junction proteins with a subsequent decrease in circulatory LPS levels,92,93reduced hepatic accumulation of triglycerides and cholesterol,94,95and improved weight maintenance and weight loss.90,91Thus, the positive effects of prebiotic use likely go beyond weight loss,since all of the benefits described also contribute to the improvement of overall host health.
3.2. Exercise
Diet and exercise are often prescribed together,and in combination form the cornerstone for lifestyle modifications aimed at maintaining health and managing chronic diseases. Given the profound role that diet plays in shaping the gut microbiota,96the question soon emerged whether or not exercise also influenced gut microbiota composition. One of the first indications that exercise might influence gut microbiota composition came from Clarke et al.97in 2014,when they showed that, compared to more sedentary control subjects, professional rugby athletes had a higher diversity of gut microorganisms, a characteristic often associated with a “healthy” gut microbiota.More recently,this same group has shown that the differences observed between the elite athletes and more sedentary controls at the microbial composition level are even greater when considered at the functional and metabolic levels(using metagenomics to examine the microbial genes and metabolomics to examine metabolites).98Importantly,the elite athletes had higher levels of SCFAs than the controls, which can influence such important actions as intestinal barrier integrity, brain function, and immunity.98This finding is consistent the findings of Estaki et al.,99who also observed increased butyrate-producing bacteria and higher microbiota diversity in healthy participants with higher cardiorespiratory fitness (measured with peak oxygen uptake) compared with those who were less fit. Although our understanding of the mechanisms by which exercise and the gut microbiota interact to provide health benefits is still in its infancy,the mechanisms may include the aforementioned SCFAs production, microbiota-mediated changes in immune function,and enhanced gut barrier function.100
Some of the first animal work to examine the effect of exercise on gut microbiota used a 6-week,low-intensity treadmill running protocol in normal and diabetic(db/db)mice,with running occurring 5 days/week.101After adjusting for the mice's body weight and blood glucose,the exercise protocol reduced Bacteroides/Prevotella spp.and Methanobrevibacter spp.and increased Clostridium cluster I in all animals;however,the abundance of the healthpromoting Bifidobacterium increased only in nondiabetic mice,suggesting that exercise may not exert the same effects on gut microbiota in a healthy host versus a host with diabetes.101In another study, nondiabetic rats also showed an increase in the health-promoting Bifidobacterium following exercise.102In contrast to these studies,Evans et al.103saw a reduction in Bifidobacteriaceae in mice that were fed a low-fat diet and used a non load-bearing hamster wheel for 12 weeks. The discrepancy between the study by Evans et al.103and other work requires further investigation, but it is possible that in metabolically challenged states(diabetes/HFD),a typical increase in Bifidobacterium with exercise is overridden by the disease or precipitating diet,given that an HFD is known to have a suppressive effect on Bifidobacterium.92Interestingly,exercise was able to alter the gut microbial composition and lean mass to a greater extent in juvenile versus adult rats,highlighting the importance of also considering the developmental stage and vulnerability/instability of earlylife microbiota in future investigations.104
4. Potential for gut microbiota in musculoskeletal disorders
OA is a highly prevalent, debilitating joint disorder commonly associated with obesity.105The risk imposed by obesity is not just due to the mechanical burden on joints,but also due to the metabolic and inflammatory derangements associated with obesity.106Given that microbial dysbiosis is associated with obesity,there is growing interest in determining if modifying the gut microbiota signature could in turn improve pain and physical function in patients with OA and obesity.Indications that this might be possible come from 2 large-cohort studies investigating knee OA in the United States.107,108Using data from the Osteoarthritis Initiative and the Framingham Offspring Osteoarthritis Study, Dai et al.107found that total dietary fiber was inversely associated with the risk of symptomatic knee OA.Using data from only the Osteoarthritis Initiative,Dai et al.108also showed that a higher intake of total dietary fiber or cereal grain fiber(e.g., whole-grain wheat and bran cereals) was inversely associated with the likelihood of developing moderate to severe knee pain over an 8-year time course.Dietary fiber is one of the most important fuels for the gut microbiota.109Therefore,although no randomized clinical trials examining the effect of microbiota-altering diets (e.g.,high fiber or high prebiotic) on knee OA have been published to date, there is good reason to initiate these trials in the near future.Additional support for such trials also comes from very promising studies in rodents.
In mice, the prebiotic oligofructose was protective against the detrimental effect of obesity induced by an HFD on trauma-induced OA.110Importantly,obesity markedly reduced beneficial Bifidobacterium microbes that coincided with increased macrophage presence in the knee capsule and accelerated joint degeneration, including cartilage loss.110Improving the composition of gut microbiota with dietary oligofructose was,in fact,able to completely rescue these obesity-associated detriments. The joint damage seen in mice is consistent with the effects of a high-fat or high-sucrose diet on knee and shoulder joints in rats.111Somewhat surprisingly,the derangements associated with a high-fat or high-sucrose diet appear to very rapidly(in as few as 3 days)alter muscle integrity, inflammation, and the gut microbiota in rats.112Early changes in muscle integrity due to obesity or poor diet,including muscle loss, intramuscular lipid accumulation, or deposition of connective tissue, may precipitate further downstream damage to tendons, bone, cartilage, and joints.113For a full review of the role of inflammation and muscle integrity on musculoskeletal-related conditions (e.g., osteoporosis, OA,tendinopathy), see Collins et al.113Important for designing future translational studies in humans is our recent demonstration that prebiotic oligofructose supplementation, aerobic exercise, and the combination of the 2 completely prevent knee damages associated with obesity induced through highfat or high-sucrose diets in rats.114Normalization of insulin resistance,dyslipidemia and endotoxemia(LPS)accompanied the protection of the knee joint.114
5. Conclusion
The environment determines bacterial growth; therefore, it is not surprising that external factors such as diet and physical activity drive our gut microbial composition and function.Diet has the potential to outweigh the effect of host genetics,immunity,and early-life disruptors(antibiotics and C-section).Unfortunately, a Western diet, with an abundance of highly processed foods that are low in fiber and rich in fat and sugar,is a major threat to our gut microbial community. This threat may not be strictly confined to the generation that consumes it,but could perpetuate dysbiosis across multiple generations.The hope of researchers in the field is that we will be able to identify personalized effective dietary strategies, such as prebiotics and other targeted interventions, that will positively modify the gut microbiota from early life onwards and ultimately reduce the burden of obesity worldwide.
Acknowledgments
This work was supported by a research grant from the Canadian Institutes of Health Research (PJT-159626). Teja Klancic is supported by a Vanier Canada Graduate Scholarship,Alberta Innovates Health Solutions Doctoral Scholarship and Eye's High Doctoral Scholarship. Author contributions
Both authors contributed to the writing and editing of the manuscript.Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
Raylene A.Reimer reports that she received honoraria from Beneo GmbH for work related to the subject of this article(i.e., prebiotics). Teja Klancic has not received any financial payments or other benefits from any commercial entity related to the subject of this article.
杂志排行
Journal of Sport and Health Science的其它文章
- Should,and how can,exercise be done during a coronavirus outbreak?An interview with Dr.Jeffrey A.Woods
- Correlation network analysis shows divergent effects of a long-term,high-fat diet and exercise on early stage osteoarthritis phenotypes in mice
- Impact of age on host responses to diet-induced obesity:Development of joint damage and metabolic set points
- Analysis of doping control test results in individual and team sports from 2003 to 2015
- Effects of obesity on breast size,thoracic spine structure and function,upper torso musculoskeletal pain and physical activity in women
- Effects of blood flow restriction without additional exercise on strength reductions and muscular atrophy following immobilization:A systematic review
