An exploration for quantification of overdiagnosis and its effect for breast cancer screening
2020-03-13LeiYangShengfengWangYubeiHuang
Lei Yang,Shengfeng Wang,Yubei Huang
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Beijing Office for Cancer Prevention and Control,Peking University Cancer Hospital &Institute,Beijing 100142,China;2Department of Epidemiology &Biostatistics,School of Public Health,Peking University,Beijing 100191,China;3Department of Epidemiology and Biostatistics,Key Laboratory of Cancer Prevention and Therapy,Tianjin,Key Laboratory of Breast Cancer Prevention and Therapy,Ministry of Education,National Clinical Research Center for Cancer,Tianjin Medical University Cancer Institute and Hospital,Tianjin 300060,China
Abstract Objective:To redefine overdiagnosis and reestimate the proportion of overdiagnosis of breast cancer caused by screening based on the Surveillance,Epidemiology,and End Results (SEER,1973−2015) Program data.Methods:The breast cancer diagnosed before 1977 was defined as the no-screening cohort since America had initiated breast cancer screening from 1977.The breast cancer diagnosed in 1999 was defined as the screening cohort due to no increases in both the proportion of early-stage breast cancer until 1999 and the overall survival of early-stage breast cancer diagnosed over the three years since 1999.The magnitude of overdiagnosis was calculated as the difference in the proportions of early-stage breast cancer patients with long-time (15-year) survival to all breast cancer patients between two cohorts.Results:Over 23 years before and after widespread screening in America,the proportion of early-stage breast cancer patients increased from 52.1% (16,891/32,443) to 72.7% (16,021/22,025) (P<0.001).The 15-year survival rate of early-stage breast cancer patients increased from 51.1% to 61.5% (P<0.001),while the proportions of earlystage breast cancer patients with long-time survival to all breast cancer patients increased from 26.6%(52.1%×51.1%) to 44.7% (72.7%×61.5%).Assuming no improvements in cancer screening technology and treatment technology,18.1% (44.7%−26.6%) of breast cancer patients were overdiagnosed associated with screening.The age-specific overdiagnosis rates were 18.9%,24.7%,24.5%,20.5%,and 8.3% for breast cancer patients aged 40−49,50−59,60−69,70−74,and ≥75 years old,respectively.Conclusions:Overdiagnosis caused by mammographic screening is probably overestimated in current screening practices.Further trials with more sophisticated designs and analyses are needed to validate our findings in the future.
Keywords:Breast cancer;screening;overdiagnosis
Introduction
Cancer screening has always been considered a doubleedged sword.Females who participate in mammographic screening often expect that screening would result in a health benefit and minimal risk of harm.Moreover,physicians believe that cancers detected by screening have favorable biologic characteristics and would have better prognoses than those diagnosed in the clinic.However,the benefits of screening always coexist with the risks of screening,like two sides of a coin.Therefore,before organizing a population-based screening,the key questions under consideration for policy makers are whether the benefits outweigh the risks,and vice versa.
Several previous studies have investigated the risk of breast cancer screening,especially the magnitude of overdiagnosis.However,highly variable estimates of overdiagnosis have been reported by different studies(1-16).As reported by Welch using data from the U.S.Surveillance,Epidemiology,and End Results (SEER)program,only 30 of the 162 additional small cancers detected per 100,000 women were expected to progress to large cancers,and the remaining 132 cases were classified as overdiagnosed cancers (5).More recent studies from Denmark and the Netherlands showed that nearly one in three and half of screening-detected breast cancers represent overdiagnosis,respectively (14,15).Based on these results,distinctly different standpoints for mammographic screening can be found from different organizations.The Swiss Medical Board even suggests that mammography screening should be abolished due to overwhelming overdiagonsis (17).However,the International Agency for Research on Cancer (IARC)Handbook Working Group concluded that there is a net benefit for women aged 50−69 years who were invited to undergo screening (18).
Several reasons could lead to these highly variable estimates of overdiagnosis.The definitions and methodologies used to calculate overdiagnosis are the primary reasons (19).Even among cohort studies,selection of different proximate indicators of overdiagnosis [e.g.,the excessive incidence of smaller tumors,non-advanced cancers,or carcinomain situbefore and after widespread screening,or between no screening and screening areas(5,14,15)]could also lead to huge differences in the estimates of overdiagnosis.However,whether all these excessive early-stage cancers detected by screening should be considered overdiagnosed remains controversial.This study aimed to assess the basic conditions of overdiagnosed cancers and quantify overdiagnosis of breast cancer screening using the latest SEER data.
Materials and methods
Redefinition of overdiagnosed cancer
As defined by Welch,an overdiagnosed cancer is a cancer that would never present clinically or from which the patient would not die during his or her lifetime in the absence of screening (20).According to this definition,we identified two basic conditions of overdiagnosed cancers.First,overdiagnosed cancers must be early-stage cancers detected by screening due to occult symptoms and signs,whereas most advanced-stage cancers,even in the absence of screening,would also be diagnosed in clinics due to obvious lumps or other clinical symptoms.Therefore,any advanced-stage cancers should not be classified as overdiagnosed.In contrast,should all early-stage cancers detected by screening be considered overdiagnosed because of the lack of obvious clinical symptoms and signs?According to Welch’s definition,if early-stage breast cancer patients die of cancer itself or other diseases (such as cardiovascular diseases) in their life expectancy,these earlystage cancers should not always be classified as overdiagnosed.Therefore,the second condition is that an overdiagnosed breast cancer patients would not die early in their life expectancy.Namely,overdiagnosed breast cancer patients should survive longer than clinic-diagnosed breast cancer patients within the same life expectancy.In summary,if we classify all cancers into three categories:symptomatic advanced-stage cancer,asymptomatic earlystage cancer with short-term survival,and asymptomatic early-stage cancer with long-term survival,the asymptomatic early-stage cancer with long-term survival should be considered as overdiagnosed cancers.
Based on the two basic conditions,overdiagnosed cancers caused by screening should be redefined as asymptomatic early-stage cancers that are excessively detected by screening and have a longer survival time than asymptomatic early-stage cancers in the absence of screening.
Data source
All analyses were based on the latest incidence data of breast cancer from the SEER database (SEER9) (21).The SEER program is a population-based registry program for incident cancers in the United States,and SEER9,which include 9 registries,represents approximately 10% of the population of the United States (21).SEER-based estimates of breast-cancer mortality are virtually identical to those ascertained from U.S.mortality data (22).Moreover,the SEER program has had virtually complete case ascertainment and reporting for decades (23).More importantly,the study period (1973−2015) spans the periods before and after the widespread dissemination of mammographic screening.
The analyses were performed in 756,636 female breast cancer patients with complete information of age at diagnosis [≥40 years old,which was the youngest starting age for mammographic screening suggested by the American Cancer Society (ACS) and U.S.Preventive Services Task Force (USPSTF),Supplementary Table S1,S2],year of diagnosis,SEER historic stage (in situ,localized,regional or distant),vital status (alive or dead),and survival months.Although American Joint Committee on Cancer (AJCC) TNM stage is a better predictor of cancer prognosis,data of AJCC TNM stage were only available since 1988.Tumor size,as suggested by Welch(5),could be considered as a direct proximate indicator of screening effect.However,the SEER historic stage is a more comprehensive and direct proximate indicator of screening effect and includes comprehensive information of the involvement in the limits of the original organ,lymphnode involvement,and metastasis.Moreover,SEER historic stage has been collected since 1973,and only 2.7%of patients in the database lack this information.Therefore,SEER historic stage,rather than AJCC TNM stage or tumor size,was used in our final analyses.Moreover,in this study,early-stage breast cancers were defined as carcinomasin situand localized carcinomas,and the remaining cancers (regional and distant carcinomas) were defined as advanced-stage cancers.
Screening and no screening cancer cohorts
Guidelines for mammographic screening were first suggested by the ACS in 1977 (24),and the use of mammographic screening was rare before 1977.Therefore,the breast cancer patients diagnosed before 1977 were defined as the no-screening cohort.Due to the limited number of early-stage breast cancers diagnosed before 1977,breast cancer patients diagnosed from 1973 to 1976(32,443 cancers;16,891 early-stage cancers and 15,552 advanced-stage cancers) were incorporated into the noscreening cohort.
As shown inFigure 1,the proportion of early-stage breast cancer seemed to remain steady at a high plateau since 1999,which may suggest that the effectiveness of screening also reached a potential high plateau in 1999.In order to validate whether the screening effectiveness reached a stable high plateau since 1999,a series of Cox regression models were conducted to compare the longterm cumulative survival rates of cancer cohorts diagnosed in different years.To reduce the potential bias,age at diagnosis was adjusted in all Cox regression analyses.As shown inSupplementary Table S3,there was a significant increasing trend in the 15-year cumulative survival rates for the early-stage breast cancer cohort diagnosed from 1973 to 1999 (P value for trend <0.001).Similar increasing trends were also observed for survival rates associated with less than 15 years’ follow-up among early-stage breast cancer diagnosed before 1999.However,no significant improvement in survival rates was found in the 3 years since 1999.
If there were any improvements in cancer survival,these improvements may come from screening or treatment.Conversely,if no improvements in cancer survival were found after an increasing trend,it meant that cancer screening technology and treatment technology had not changed significantly during this period.It also meant that the impact of cancer screening and treatment technology on cancer survival had arrived at a relatively stable highplateau period in 1999.Therefore,breast cancer patients diagnosed at 1999 (22,025 cancers;16,021 early-stage cancers and 6,004 advanced-stage cancers) were defined as the screening cohort.
Statistical analysis
In order to estimate the magnitude of overdiagnosis,we first calculated the proportions of early-stage breast cancer patients to all breast cancer patients and the proportions of early-stage breast cancer patients with long-term (15 years)survival to all early-stage breast cancer patients in the screening and no screening cohorts,respectively.Second,we calculated the proportions of early-stage breast cancer patients with long-term survival to all breast cancer patients in the two cohorts,which were calculated as the proportion of early-stage breast cancer patients multiplied by the proportion of early-stage breast cancer patients with longterm survival.Finally,assuming no obvious improvements in cancer screening and treatment skills from 1977 to 1999 in America,the magnitude of overdiagnosis is the difference in the proportions of early-stage breast cancers patients with long-time survival to all cancer patients between the two cohorts.
In theory,the magnitudes of overdiagnosis would differ among patients diagnosed at different ages.Based on starting and ending ages for mammographic screening suggested by the ACS and USPSTF at different periods(Supplementary Table S1,S2) (24-32),subgroup analyses on the magnitudes of overdiagnosis were conducted according to five age groups:40−49,50−59,60−69,70−74,and ≥75 years old.Moreover,both the ACS and USPSTF guidelines recommend regular screening in females aged 50−74 years old until now.Therefore,further sensitivity analyses were conducted among breast cancer patients diagnosed at 50−74 years old to investigate the magnitude of overdiagnosis associated with regular screening.
We used Pearson’s Chi-square test to compare the proportion of early-stage cancers between the screening and no screening cohorts.Kaplan-Meier analysis was used to plot survival curves and estimate cumulative survival at specific follow-up times.The Cox regression analysis was used to assess whether there were significant differences in the long-term cumulative survival rates at specific time points between screening cohort and no screening cohort after adjusting for age at diagnosis.All reported P values were two-sided,and P<0.05 was considered statistically significant.
Results
Trends in early-stage breast cancer diagnosis
The shift in the SEER historic stage of breast cancers is shown inFigure 1.From 1973 to 2015,the proportion of early-stage breast cancers increased from 49.5% to 74.8%.There seemed to be three distinct periods:a low-plateau period before 1977,a continuous-climb period from 1977 to 1999,and a new high-plateau period after 1999 (Figure 1A).A similar three-period trend was also observed in the five age subgroups (Figure 1B).These three periods may correspond to three different periods for mammographic screening in the United States:before,during,and after the implementation of widespread mammographic screening.
Mammographic screening led to an increase in early-stage cancer diagnosis
As shown inTable 1,the overall proportion of early-stage breast cancer increased from 52.1% (16,891/32,443) in the 1973−1976 cohort (no screening) to 72.7% (16,021/22,025)in the 1999 cohort (screening),with 20.7% excessive proportion of breast cancer patients being diagnosed at an early stage in the screening cohort.The age-specific excessive proportion of early-stage breast cancers after the implementation of widespread screening was lowest for patients aged 40−49 years old [13.3% (54.6%vs.67.9%)]and highest for patients aged 70−74 years old [24.9%(52.4%vs.77.3%)],then decreased to 22.4% (54.1%vs.76.4%) for patients aged ≥75 years old (all P<0.001).These results suggested not only that mammographic screening caused obvious stage shifts,but also that the stage shifts were different for patients diagnosed at different ages.Sensitivity analyses showed that the excessive proportion of early-stage breast cancers among patients aged 50−74 years old was 22.2% (50.7%vs.72.9%) after regular screening(P<0.001).
Mammographic screening led to an increase in survival of early-stage cancer patients
As shown inTable 2,Figure 2,after 15-year follow-up,the overall cumulative survival rate of early-stage breast cancer patients increased from 51.1% in the 1973−1976 cohort(no screening) to 61.5% in the 1999 cohort (screening),with an overall 10.4% improvement in survival (P<0.001).Moreover,the age-specific improvement in survival of early-stage breast cancer patients gradually increased from 13.0% (75.4%vs.88.4%) for patients aged 40−49 years old to 17.4% (50.4%vs.67.8%) for patients aged 60−69 years old,and then gradually decreased to 7.7% (10.9%vs.18.6%) for patients aged ≥75 years old (all P<0.001).Sensitivity analyses showed that the improvement in survival of early-stage cancer patients aged 50−74 years old was 15.3% (55.0%vs.70.2%,P<0.001) after regular screening.
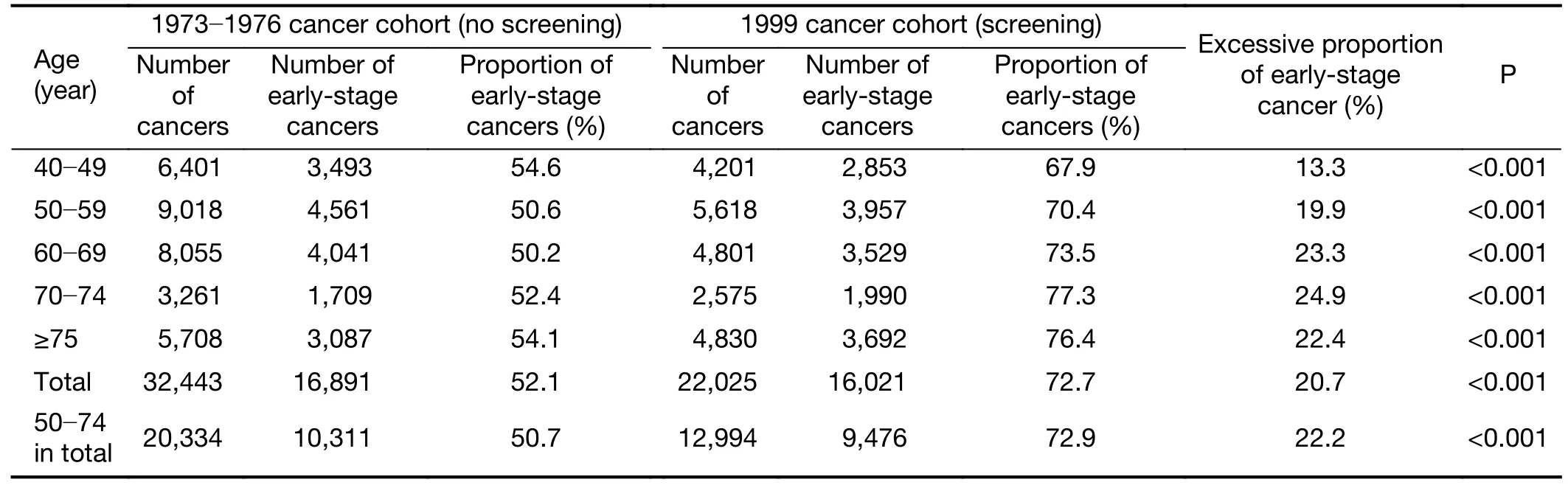
Table 1 Comparison on proportions of early-stage breast cancer between cancer cohorts with and without mammographic screening
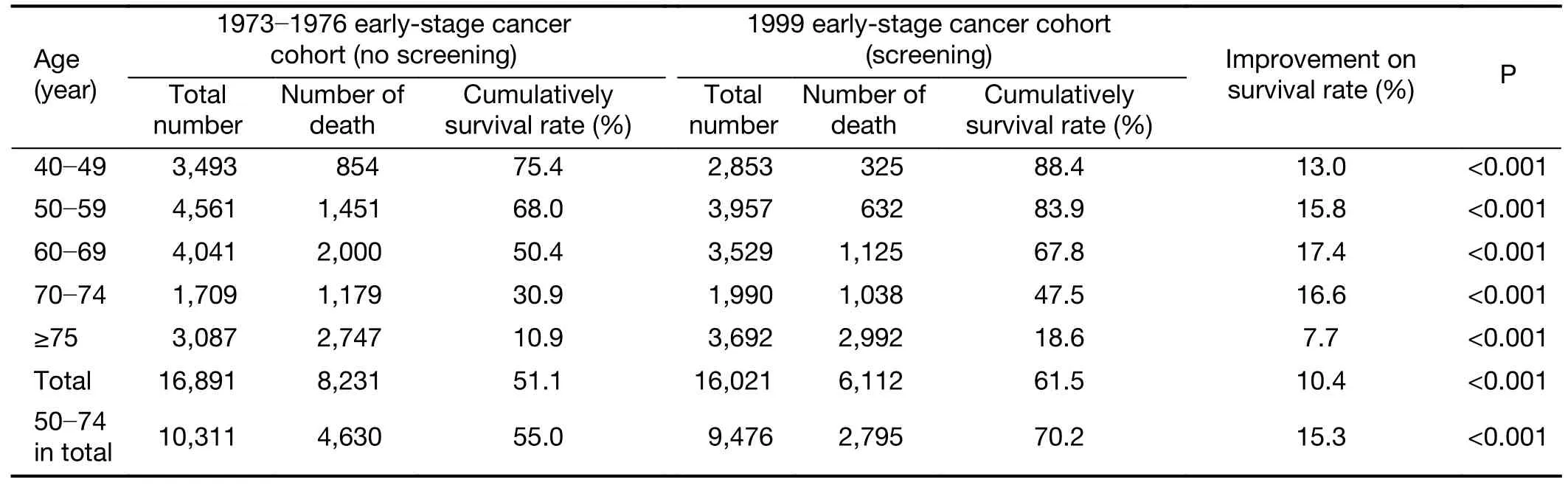
Table 2 Comparison on 15-year cumulative survival rates between early-stage breast cancer cohorts with and without mammographic screening
Magnitudes of overdiagnosis
As shown inFigure 3,based on the proportion of earlystage breast cancers and the proportion of early-stage breast cancer with long-time survival,the proportions of early-stage cancer patients with long-time survival to all breast cancer in the no screening and screening cohorts were 26.6% and 44.7%,respectively.Under the assumption that there were no obvious improvements in the cancer screening and treatment skills before and after widespread screening,18.1% of breast cancers were overdiagnosed,which would be associated with mammographic screening.The proportions of age-specific overdiagnosis were 18.9%,24.7%,24.5%,20.5%,and 8.3% for breast cancer patients aged 40−49,50−59,60−69,70−74,and ≥75 years old,respectively.Further analyses showed that 23.3% of breast cancer patients diagnosed at 50−74 years old were estimated to be overdiagnosed cancers associated with regular screening.
Discussion
Our analysis shows that,over 23 years (from 1977 to 1999)after implementation of widespread mammographic screening in the United States,less than 18.1% of breast cancers were overdiagnosed,since the improvement of screening and therapy skills cannot be ignored.This result was similar to that reported by the Independent UK Panel on Breast Cancer Screening (33).However,it was obviously lower than those reported by recent studies in Denmark and the Netherlands.Overdiagnosis is probably overestimated in the current screening practice.
The major argument against screening mammography is that excessive detection of early-stage cancers (or smaller tumors/non-advanced cancers/carcinomasin situ) through screening does not reduce the incidence of advanced-stage cancers (or larger tumors/advanced cancers/invasive tumors) (5,14-16).Therefore,almost all excessive earlystage cancers detected through screening should be considered as overdiagnosed cancers.However,most of these excessive early-stage cancers have very similar prognoses as early-stage cancers diagnosed without screening,and more than half of these patients diagnosed before 1977 died within 15 years after diagnosis.Therefore,according to Welch’s definition,these patients with early-stage cancers who died early after diagnosis due to the natural progression or potentially fatal conditions of the cancer should not be classified as overdiagnosed.Moreover,if we extended the follow-up time,we would have observed more patients with early-stage cancers who died early.
Another concern regarding screening mammography is that the incidence of early-stage cancers (or smaller tumors/non-advanced cancers/carcinomasin situ) will continue to increase as screening programs continue (5,14-16).Therefore,the magnitude of overdiagnosed cancers will also increase whereas the incidence of metastatic breast cancer is essentially unchanged in the United States (34).However,as shown inFigure 1A,the proportion of earlystage breast cancers seemed to plateau in 1999.Based on this high-plateau period,it is rational to assume that it will become increasingly more difficult to achieve an additional increase in the proportion of early-stage breast cancers,even though the number of newly diagnosed early-stage cancers continue to increase.Therefore,although screening programs continue,it appears that the proportion of early-stage cancers will not continue to increase in the future,but will remain at a high plateau,as observed inFigure 1A.
In addition,as argued by Welch (5),cancer stage may be subject to“upstaging”over time as technology and practice change.Therefore,tumor size would be a better proximate indicator of screening effect than cancer stage because of its relative stability over time.However,the most needed improvement in screening technology is the ability to detect more occult and smaller tumors.If the largest dimension of a newly diagnosed tumor was 20 mm by new technology and 19 mm by old technology,and there was no finding of lymph-node involvement or metastasis by either technology.The tumor would be reclassified from a small tumor (<20 mm) into a large tumor (≥20 mm) according to Welch’s classification.However,according to the SEER historic stage standard,if the tumor was confined entirely to the organ of origin,it is classified as localized cancer,regardless of tumor size.Namely,although new technology would bring more improvements in the classifications of the SEER summary stage or modern staging (such as the AJCC TNM stage) (35),the overall simplified four-item SEER historic stage classifications (in situ,localized,regional,and distant) are more stable than tumor size over time.
Moreover,we should emphasize that the magnitude of overdiagnosis in our study is likely to be overestimated,since the improvement in treatment skills between 1977 and 1999 cannot be ignored.However,in fact,if there was no improvement in treatment skills,more early-stage breast cancers would die early during their life expectancy,which would lead to less number of early-stage breast cancer with long-term survival.Furthermore,as mentioned by Welch(5),it is not rational to ignore the possibility that women would seek medical care earlier in the course of their disease.Both increased breast cancer awareness and support from family members would promote women to seek medical service earlier than they did in the past(36,37).As shown inSupplementary Figure S1,the therapy pattern for early-stage breast cancer patients changed during the past two decades,especially during the period before 1999.Therefore,the magnitude of overdiagnosis would be no more than 18.1% after excluding the influences of improvement of therapy and earlier treatment seeking.
Our findings are limited for several reasons.First,the underlying true contribution of therapy to the survival of early-stage cancer patients is unobservable.However,as mentioned above,even though this contribution of therapy has been ignored,the magnitude of overdiagnosis would be no more than 18.1% after the implementation of widespread mammographic screening in the United States.Second,the SEER historic stage is a crude stage classification method than the AJCC TNM stage standard;therefore,the estimated magnitude of overdiagnosis would be biased in this study.Third,we have not considered the proportion of the people who received mammography screening actively during the follow-up period among no screening group,which would underestimate our results.Fourth,we did not investigate the influences of the change in risk factors (including environmental,behavioral,and dietary factors),advances in screening methods,or the lead-time bias on the stage shift in the distribution of earlystage breast cancers before and after widespread screening.However,ignorance of these influences would also bias the final estimate of overdiagnosis.
Conclusions
Over 23 years (from 1977 to 1999) after implementation of widespread mammographic screening in the United States,less than 18.1% of breast cancers were overdiagnosed,since survival improvement of early-stage cancer patients because of therapy cannot be ignored.Moreover,overdiagnosis is probably overestimated in the current screening practice.Further RCTs with more sophisticated design and analyses are needed to validate our findings in the future.
Acknowledgements
This work was supported by the Natural Science Foundation of Tianjin (No.18JCQNJC80300);Chinese National Key Research and Development Project (No.2018YFC1315600);National Natural Science Foundation of China (No.81502476) and the Beijing Young Talent Program (No.2016000021469G189).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Incidence and mortality of oral and oropharyngeal cancer in China,2015
- Incidence and mortality of laryngeal cancer in China,2015
- Updates on larynx cancer epidemiology
- Effects of neoadjuvant chemotherapy on respiratory function in patients with breast cancer
- Factors associated with metastasis in superior mesenteric vein lymph node in subtotal gastrectomy for gastric cancer:Retrospective case control study
- Prognostic impact of D2-plus lymphadenectomy and optimal extent of lymphadenectomy in advanced gastric antral carcinoma:Propensity score matching analysis
