Colonoscopic management of diverticular disease
2020-01-17PhillipFejlehJamesTabibian
M Phillip Fejleh, James H Tabibian
Abstract
Key words: Acute diverticulitis; Diverticular bleeding; Colon stricture
INTRODUCTION
Colonic diverticula are generally defined as regions of colonic mucosa and submucosa that protrude through areas of the muscularis propria. The term “diverticular disease” encompasses the range of clinical manifestations and complications that can occur with colonic diverticula, in particular diverticular bleeding and acute diverticulitis[1]. Diverticula are the most common finding during colonoscopy and increase in prevalence as patient age increases[2]. Thus, not surprisingly, diverticular disease represents a leading gastrointestinal (GI) diagnosis in the clinic, emergency department, and hospital settings and is among the top 20 causes of all GI-, liver-, and pancreas-related deaths[3].
Colonoscopy is the most useful method of determining the presence and extent of diverticulosis and can be vital to the diagnosis and management of diverticular diseases. For example, diverticular bleeding can be localized and treated with prompt bowel preparation followed by colonoscopy with hemostasis, diverticulitis-associated colonic strictures can be stented as a bridge to one-stage resection, and acute diverticulitis should be followed by diagnostic colonoscopy approximately two months afterward to rule out other entities masquerading as diverticulitis (e.g., colon cancer, Crohn's disease, lymphoma). In addition, it is imperative that providers and endoscopists recognize that severe (i.e., dense) sigmoid diverticulosis and complications of prior acute diverticulitis can make colonoscopy technically difficult and higher risk for colonic perforation, at times requiring alterations in usual technique for successful cecal intubation.
In this editorial, we discuss the current and emerging role of colonoscopic management of diverticular disease, specifically acute diverticular bleeding and complications and aftercare of acute diverticulitis.
DIVERTICULAR BLEEDING
Diverticular bleeding is a common cause of acute lower GI bleeding, accounting for 20% to 65% of such episodes[4-7]. Erosion into the vasa recta that traverse the neck and dome of diverticula can lead to brisk, dramatic bleeding[1]. Endoscopic management of diverticular bleeding can be challenging for multiple reasons including the inconvenience of rapid bowel preparation, the difficulty of carefully examining each diverticulum in the colon, identification of true stigmata of recent hemorrhage (SRH),and achieving hemostasis of such small lesions within diverticula.
Management
Urgent endoscopy:In hemodynamically stable patients with lower GI bleeding,urgent colonoscopy should be performed within 8 to 24 h of presentation (or sooner, if possible) following sufficient bowel preparation[8,9]. The likelihood of identifying SRH is higher when early colonoscopy is performed, which, in turn, increases the chance for definitive hemostasis[10]. Two meta-analyses in 2017 compared early and late colonoscopy for patients presenting with lower GI bleeding and found no reduction in rebleeding rates, transfusion requirements, and other important outcomes, but did show an increased rate of endoscopic intervention[11,12]. Other studies have also directed attention toward the possible low impact of early colonoscopy on patient outcomes in lower GI bleeding[13-18], but none of these investigations focused specifically on diverticular bleeding. In our experience, if diverticular bleeding can be identified and treated, the benefits are clear.
Visualization and identification:The SRH seen in diverticular bleeding are similar to those seen in the upper GI tract. Active bleeding, visible vessels, adherent clots, and pigmented spots can all be seen associated with diverticula. Localization of such lesions can be difficult given the potential for numerous diverticula throughout the colon that require investigation, inadequate bowel preparation, and the fact that diverticular bleeding frequently stops spontaneously or can be intermittent. Published rates of SRH identification vary widely, with one notable study listing SRH identification in 17% of cases[19]. The study by Niikura et al[19]also found factors useful in SRH identification, which included urgent colonoscopy, expert endoscopists, the use of a transparent cap, and water jet use. Based on current literature and experience,the most reasonable approach is to optimize bowel preparation with use of a nasogastric tube if the patient is unable to tolerate oral prep, and to have a team member confirm successful bowel preparation prior to attempting colonoscopy.During colonoscopy, each diverticulum should be carefully examined using a transparent cap attached to the colonoscope and with water washing and infusion into diverticula as necessary. Another technique that can be attempted is inversion of diverticula by suctioning into the distal cap attachment. Using these methods, a satisfactory examination of the colon for stigmata of recent diverticular hemorrhage should be possible.
Endoscopic treatment:Current American College of Gastroenterology practice guidelines recommend the use of through-the-scope (TTS) clips for the treatment of diverticular bleeding due to the safety and ease of use compared to other modalities like band ligation[9]. Current American Society of Gastrointestinal Endoscopy practice guidelines suggest the use of a heater probe or bipolar coagulation alone or in combination with epinephrine followed by tattooing near the lesion for future identification in the case of rebleeding. TTS clips and endoscopic band ligation (EBL)are alternatives to thermal coagulation and endoscopic band ligation (EBL) is also discussed. Clip placement near culprit diverticula can also serve as a radiographic landmark during angiography (were it to become necessary)[8].
TTS clips (Figure 1) are frequently used to treat diverticular hemorrhage, and they have been shown to provide satisfactory primary and rebleeding hemostasis after initial clipping[20]. Being mindful of the anatomy of diverticula and nearby arteries is important during hemostasis attempts. Clips can be used in multiple ways, for example clipping a vessel in the neck or dome of a diverticulum or clipping the mouth of a diverticulum closed. If SRH are not readily visible or accessible, we recommend the use of a clear cap (i.e., distal attachment) for improved navigation and examination of diverticula and facilitatation of treatment[21].
EBL is another available treatment modality. If SRH can be identified, then a TTS clip is placed to mark the site, and the colonoscope is withdrawn for placement of a band ligator device. After return to the bleeding site, the diverticulum is inverted into the cap of the ligator and a band is deployed. This technique may be limited or infeasible in the case of diverticula with narrow orifices or with diverticula in the right colon.
A recent meta-analysis comparing coagulation, EBL, and TTS clips in the treatment of diverticular bleeding demonstrated comparable rates of initial hemostasis and prevention of early rebleeding between the three treatment modalities, and that EBL may be more likely to prevent IR or surgery than TTS clip use[22]. A prospective trial comparing TTS clips to EBL showed lower 1 year recurrent bleeding in the EBL group, but no statistically significant difference in early rebleeding[23].
A newer technique that has been described is the use of a detachable endoscopic snare (Endoloop, HX-20Q-1; Olympus), most commonly used for reducing bleeding during resection of large, pedunculated colon polyps[24]. The bleeding site is located with a colonoscope equipped with a transparent cap, and then a clip is used to mark the site. The SRH is placed at the location of the instrument channel, suction is used to invert the diverticulum into the cap, and then the detachable snare is deployed over the base of the diverticulum to achieve hemostasis. In a large, multicenter prospective study, detachable snare deployment was successful in 82% of the 123 included patients[25]. The most common reason for failed treatment was insufficient suction.Early recurrent bleeding occurred in 7.9% of patients treated with detachable snare ligation. There was one episode of diverticulitis related to the treatment.
The endoscopic treatment modality choice should be based on endoscopist expertise, but efforts for early hemostasis should be made. In cases of rebleeding after initial hemostasis attempts, repeat colonoscopy should be performed for another careful examination of the entire colon and terminal ileum. If a bleeding site can be identified, then treatment should be performed.
LARGE BOWEL OBSTRUCTION FROM ACUTE DIVERTICULITIS
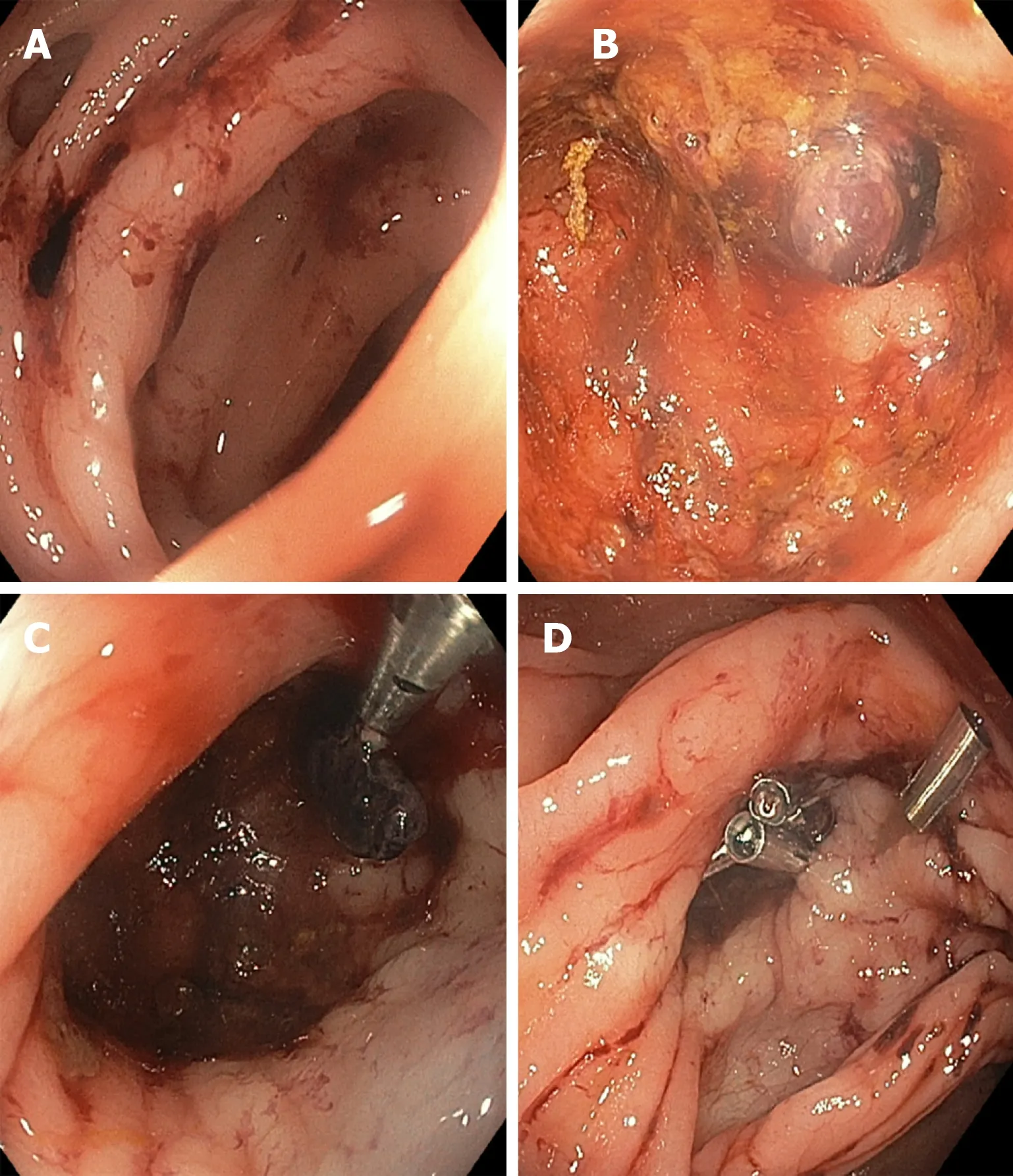
Figure 1 Endoscopic treatment of diverticular bleeding. A: Diverticula with fresh blood nearby; B: Interrogation of the diverticula reveals a visible vessel within a diverticulum; C: Through-the-scope clipping of the visible vessel following submucosal 1:10000 epinephrine injection; D: Additional clipping performed to secure more durable hemostasis.
Diverticular disease can cause colonic stenoses and/or strictures leading to partial or complete bowel obstruction with a clinical presentation similar to other etiologies of obstruction, such as malignancy, volvulus, and inflammatory bowel disease. Large bowel obstruction is thought to be caused by diverticular disease in approximately 10% of cases, though this figure can vary considerably depending on the practice environment[26,27]. The mechanism by which diverticular disease causes large bowel obstruction is most likely via recurrent episodes of acute diverticulitis triggering fibrosis of a segment of colon, most frequently the sigmoid colon. It should be mentioned that in a subset of patients, there will not be a preceding clinical diagnosis of acute diverticulitis; however, upon further questioning, patients may report a history of left lower quadrant pain compatible with diverticulitis.
Medical management of diverticulitis-associated strictures includes nasogastric tube placement, bowel rest, antibiotics if acute diverticulitis is suspected, and a multidisciplinary discussion involving gastroenterology and surgery teams.Diagnostic colonoscopy may be considered if the diagnosis is uncertain and there is a need to rule out malignant obstruction (e.g., for surgical planning). This typically reveals nonspecific inflammatory changes, including friability, and purulent fluid may also be seen. Definitive treatment historically involved urgent surgery to relieve acute bowel obstruction, but frequently resulted in colostomy creation due to the inability to prep the colon prior to surgery.
Several studies have touched upon the use of colonic stents for diverticulitisassociated strictures either as a bridge to surgery or for palliation in patients who are poor surgical candidates. The majority of literature on this topic has combined strictures associated with diverticular disease with a larger cohort of patients with other benign and often also malignant etiologies of strictures, thus confounding interpretability. A meta-analysis examining technical and clinical success of selfexpanding metal stents (SEMS) for benign etiologies of colorectal obstruction concluded that the complication rate for stenting of benign obstruction is too high to warrant its use. The study included a large number of patients with diverticulitisassociated strictures (66 of 122, 54%)[28]. Based on this study, the use of SEMS for diverticulitis-associated strictures should not be deemed to be standard therapy;rather, it should be considered in select cases, and more studies should be conducted on this subject to determine if SEMS can be used (and in which patients) for short periods of time as a bridge to surgery or for palliation in patients who will not be surgical candidates.
Our experience with colonic stenting of diverticulitis-associated strictures has been largely very favorable; in > 75% of patients, it has permitted colonic decompression and prepping followed by 1-stage segmental resection in lieu of emergency colostomy followed by stoma takedown and colo-colonic anastomosis (Figure 2). It should be noted, however, that these strictures tend to be relatively rigid, and that despite the radial forces of SEMS, full expansion is typically not seen. Placing a SEMS within the existing SEMS may be considered (though of considerable cost and unclear benefit), as may cautious balloon dilation within the SEMS, though the effect is often only temporary, and the stricture/SEMS has a tendency to return to the pre-dilation diameter. In addition, given there is essentially no mucosal abnormality (i.e.,neoplasm) onto which the stent can readily grip, despite use of uncovered SEMS,there can be “yo-yoing” of a stent distally or proximally depending on anatomic as well as other factors. Thus, TTS clipping of the distal end with multiple clips is advisable so as to mitigate the risk of stent migration in either direction.
COLONOSCOPIC AFTERCARE FOLLOWING AN ACUTE DIVERTICULITIS EPISODE
Current treatment guidelines recommend diagnostic colonoscopy after resolution of acute diverticulitis if no recent colonoscopy has been completed[29,30]. Colon wall thickening seen on imaging may also be from processes other than diverticulitis, such as ischemia, inflammatory bowel disease, or malignancy. The rationale behind a follow-up colonoscopy is to avoid missing a diagnosis of any of these conditions,particularly malignancy. This is generally done 6-8 weeks following improvement of an episode of acute diverticulitis in patients who have not had a high-quality colonoscopy in the antecedent 1-2 years, though the optimal timing of colonoscopy is as yet not clear. In addition, as the yield of post-acute diverticulitis colonoscopy is generally low, methods to risk stratify and identify those patients in whom diagnostic colonoscopy is useful should be considered and are a topic of our current research.
GENERAL TENETS PERTAINING TO COLONOSCOPY IN PATIENTS WITH DIVERTICULOSIS
Diverticular disease can increase the difficulty of colonoscopy due to luminal narrowing, angulations, colon spasm, and difficulty with insufflation. Standard technique with an adult colonoscope using air insufflation may not be sufficient for achieving cecal intubation. Use of a pediatric colonoscope, which is suitable for sharper angulation, may be beneficial. The addition of a transparent cap to the end of the colonoscope can also be helpful. Instead of using air insufflation, water infusion during intubation can help facilitate movement through a narrow sigmoid colon. In more challenging cases, fluoroscopy can be used to help navigate the colonoscope. Of course, patient positioning and directed abdominal pressure are always useful adjunct maneuvers.
The distribution and severity of diverticulosis and related colonic changes are different in each patient; therefore, we suggest using detailed descriptions of the location and extent of diverticulosis when writing each colonoscopy report. This will provide richer information to physicians caring for the patient's diverticular bleed,diverticulosis, or diverticulitis in the future.
CONCLUSION
In this editorial, we have summarized the current state of colonoscopic management of diverticular disease, with an emphasis on the treatment of diverticular bleeding and stenting for strictures related to prior acute diverticulitis. We are encouraged by the refinement of techniques for treating diverticular bleeding; for diverticulitisrelated strictures, emerging data appear promising for the use of colonic stents as a temporizing measure and bridge to surgery, but further research remains needed with regard to utility and optimal application/patient selection. Follow-up colonoscopy after an episode of acute diverticulitis continues to be recommended in those who have not had a high-quality colonoscopy in the antecedent 1-2 years,though the optimal timing of and true yield of colonoscopy in this context remains uncertain and may benefit from further study. Lastly, we have outlined a number of practical steps which can be implemented to maximize procedural safety and success in patients with dense diverticulosis undergoing colonoscopy.
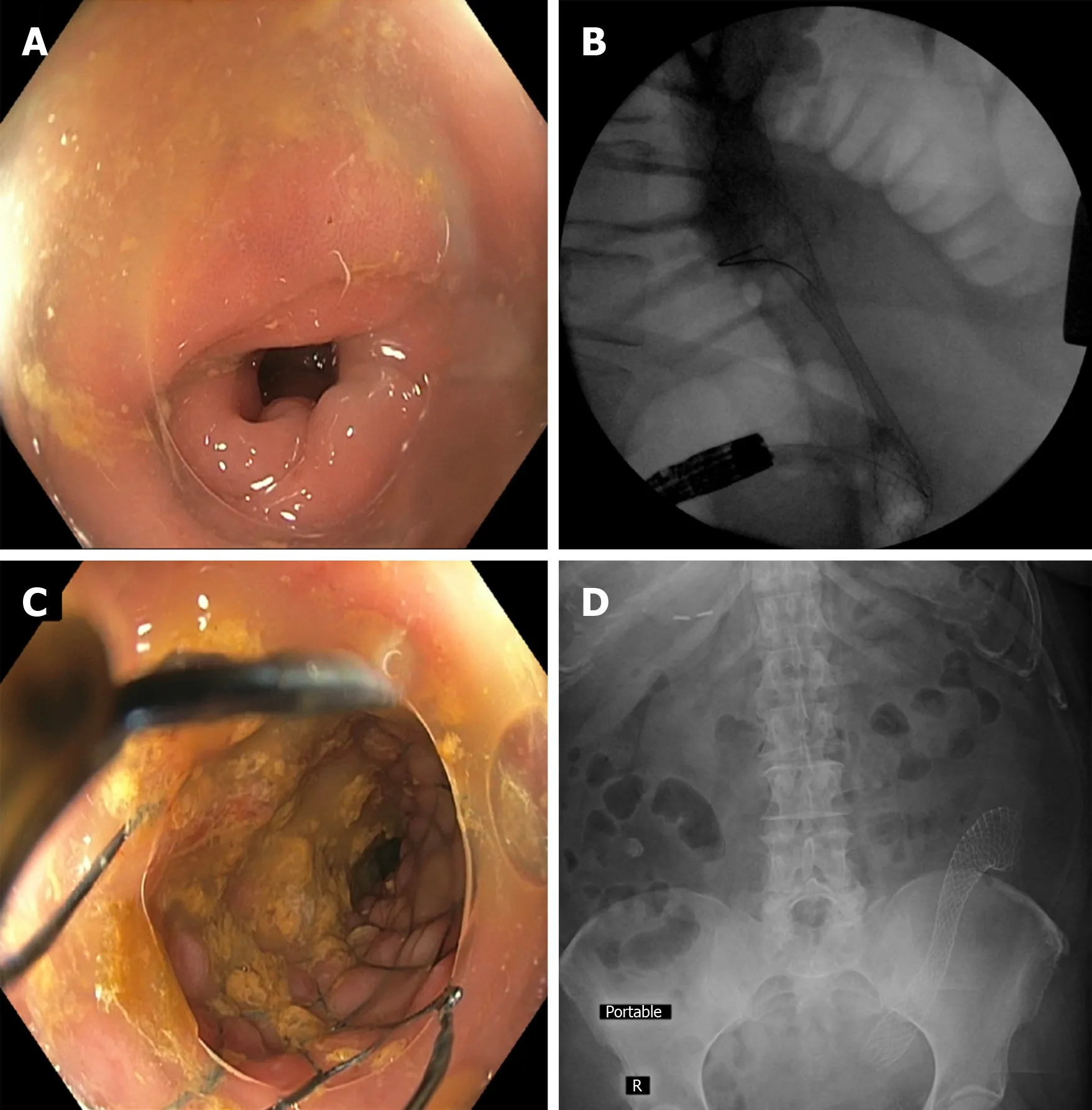
Figure 2 Colonic stent placement within a diverticulitis-associated strictures. A: Sigmoid colon luminal narrowing due to diverticulitis-associatedfibroinflammatory stricture; B: Fluoroscopic view of colonic self-expanding metal stent deployment with appreciable waist; C: Luminal view immediately after stent deployment within the stricture; D: Post-procedure abdominal x-ray showing stent in good position and with notable expansion. This management approach can allow for successful bowel preparation and 1-stage segmental resection instead of emergent partial colectomy with temporary colostomy.
