植物糖基化磷脂酰肌醇锚定蛋白LORELEI家族研究进展
2020-01-16贾明生张咏雪张恒韩霞侯笑颜戴绍军
贾明生 张咏雪 张恒 韩霞 侯笑颜 戴绍军


摘 要: 植物LORELEI(LRE)蛋白家族是植物糖基化磷脂酰肌醇锚定蛋白(GPI-AP)亚家族的一种,在拟南芥中有4个成员,分别为LRE,LRE-like GPI-AP 1(LLG1),LLG2和LLG3。这些成员在植物体内的表达位置和功能不同。LRE主要在雌配子体的助细胞、卵细胞和中央细胞表达,在助细胞中表达量最高,另外在受精卵与胚乳中也有部分表达。LRE主要参与高等植物的双受精作用,介导花粉管接受并调控胚胎的早期发育。LLG1在植物各组织器官中都有表达,在营养器官(根和叶)中表达水平最高,主要调控植物生长发育(如根与根毛生长)、盐逆境应答,以及免疫应答过程。LLG2和LLG3主要在成熟花粉粒和花粉管中表达,调控花粉管生长与爆裂,释放精子完成双受精作用。该文综述了植物LRE家族成员组成、蛋白质特征,及其在植物生长发育与逆境应答过程中的作用。
关键词: 糖基化磷脂酰肌醇(GPI); LORELEI(LRE); LRE-like GPI-AP(LLG); 花粉管; 根; 免疫和盐应答
中图分类号: Q 946.1 文献标志码: A 文章编号: 1000-5137(2020)06-0603-11
Abstract: LORELEI(LRE) protein family belongs to a subfamily of glycosylphosphatidylinositol-anchored proteins(GPI-AP) in plants.In Arabidopsis thaliana,four members of LRE family proteins with various expression patterns and functions are found,which are LRE,LRE-like GPI-AP 1(LLG1),LLG2,and LLG3.LRE is mainly expressed in the synergid cell,egg cell,and central cell of the female gametophyte.The highest expression level is detected in the synergid cell.In addition,LRE is also observed in the zygote and endosperm.LRE participates in the process of double fertilization in higher plants by mediating the reception of pollen tube and regulating the early development of embryos.Expression of LLG1 is detected in all of the tissues / organs in plants,and has the highest expression level in vegetative organs,such as roots and leaves.LLG1 plays important role in regulating the plant growth and development(e.g.,root and root hair growth),salinity response,and immune response.LLG2 and LLG3 are expressed in mature pollen grains and pollen tubes.They are involved in regulation of pollen tube growth and burst,and sperm release for double fertilization.In this review,we summarize the components and protein characteristics of LRE family,and highlight the advances on their functions in the processes of plant growth,development,and stress response.
Key words: glycosylphosphatidylinositol(GPI); LORELEI (LRE); LRE-like GPI-AP (LLG); pollen tube; root; immune and salinity response
1 糖基化磷脂酰肌醇錨定蛋白的发生及结构
糖基化磷脂酰肌醇锚定蛋白(GPI-APs)是一类非常重要的膜蛋白,广泛存在于真核生物中,具有高度保守的核心结构域,一般由糖基磷脂酰肌醇(GPI)部分和蛋白部分组成。GPI由脂质和多糖组成,脂质可以是磷脂酰肌醇或肌醇磷酸神经酰胺,多糖部分由保守的核心多糖骨架和可变支链构成,核心多糖骨架包含1个磷酸乙醇胺、3个甘露糖和1个葡萄糖胺[1]。虽然GPI部分的组分是保守的,但当GPI锚定在前体蛋白上后,支链的不同修饰导致GPI结构多变[1]。GPI锚定修饰作为一种常见的蛋白质翻译后修饰,将前体蛋白C末端以共价键形式与GPI相连[1-2]。被GPI锚定修饰的前体蛋白具有特殊结构,包括N端信号肽(SP)和C端保守的GPI锚定位点ω。GPI锚定的生物合成途径从内质网胞质表面合成氨基葡萄糖磷酸肌醇开始,随后翻转到内质网腔侧,添加甘露糖,最后末端加入乙醇胺磷酸(图1(a))。在GPI锚定修饰过程中,GPI转酰胺酶在指定ω位点切割前体蛋白,并识别GPI锚,转移到前体蛋白上(图1(b))。然后,经GPI修饰的蛋白质通过膜泡运输转至高尔基体(图1(c)),在高尔基体中经过进一步修饰后,由独特的囊泡运输途径分泌至细胞膜外小叶,并定位于富含鞘磷脂和胆固醇的膜微区,调控细胞表面活动(图1(d),1(e))[1,3]。
GPI-APs在调节真核生物生长发育、形态发生和疾病免疫等过程中起重要作用。GPI-APs合成、分泌、膜定位,以及信号转导过程中出现缺陷,将导致植物死亡或生长发育异常[4-7]。在植物GPI-APs合成过程中,磷脂酰肌醇聚糖合酶家族蛋白SETH1和SETH2作为两个关键作用酶参与GPI合成的第一步,这两种蛋白的缺失导致花粉管细胞壁中胼胝质异常积累,花粉萌发率降低,花粉管生长异常[4]。在GPI合成过程中的关键酶是甘露糖基转移酶家族蛋白PEANUT(PNT),拟南芥pnt功能缺失突变体中,细胞壁纤维素含量减少,果胶、木葡聚糖和胼胝质等异常积累,严重影响细胞壁形成[5]。此外,转酰胺基酶GPI8负责将前体蛋白ω位点后的C末端切除,并识别GPI锚,连接到此位点。GPI8基因不同程度的缺失会对植物生长有不同影响,该基因点突变后会影响植物叶片气孔形成,而T-DNA插入突变体则会影响蛋白的正确定位,引起植物生殖发育缺陷[6-7]。在植物体内,GPI-APs在细胞壁合成、器官形成,以及生殖发育等过程中都发挥作用,但其作用的分子机理仍有待研究。
2 LORELEI(LRE)家族蛋白序列特征及进化关系
拟南芥有248个GPI-APs[8]。根据其保守结构域,分为COBRA,ENODL和LORELEI共3个亚家族,分别调节细胞壁纤维素生物合成、花粉管接受和双受精作用[9-11]。拟南芥LORELEI家族有LORELEI,LRE-like GPI-AP 1(LLG1),LLG2和LLG3共4个成员,定位于细胞质膜外表面,作为长春花类受体激酶(Catharanthus roseus receptor kinase 1-like,CrRLK1L)家族的分子伴侣,参与CrRLK1L的转运和胞外信号转导[12]。
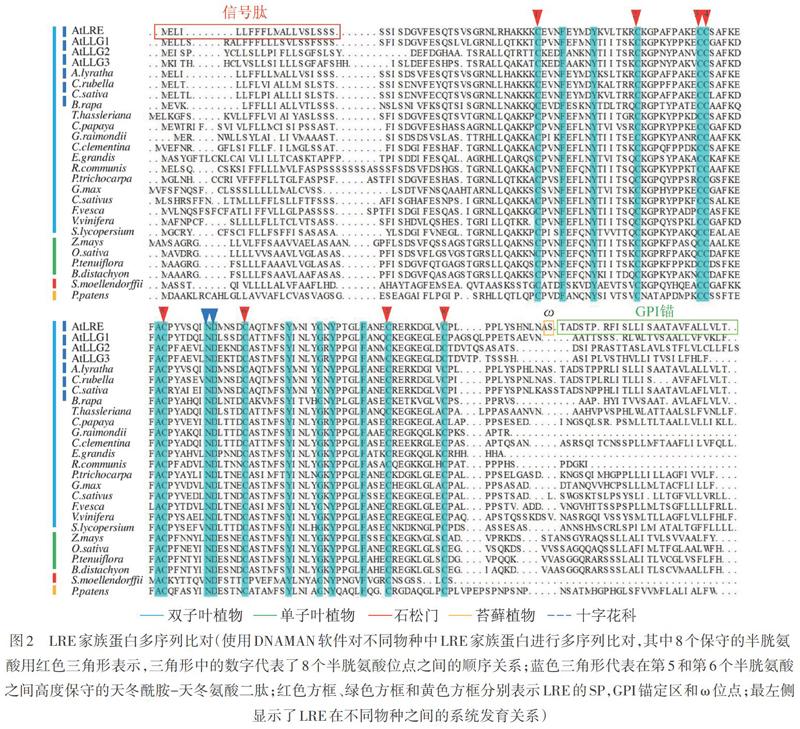
拟南芥LRE的氨基酸序列包括N端SP、中央区域、构象可变区,以及C端GPI锚定结构域,保守的GPI锚定位点ω紧邻C端GPI锚定区(图2)。中央区域内有8个高度保守的半胱氨酸(Cys),可以形成4对二硫键,参与维持蛋白3D结构(图2)[7,13-14]。在以拟南芥等十字花科(Brassicaceae)植物为代表的双子叶植物,如水稻(Oryza sativa)、玉米(Zea mays)、小花碱茅(Puccinellia tenuiflora)等单子叶植物,以及石松和苔藓植物中,LRE成员都高度保守。LRE第5个和第6个Cys之间的12个氨基酸中存在一个高度保守的天冬酰胺-天冬氨酸(Asn-Asp)二肽(图2),这12个氨基酸形成的结构域对于LRE在花粉管接受过程中发挥作用十分重要[7]。
对拟南芥LRE家族成员的开放阅读框进行聚类分析表明:LLG1与LLG2/3属一个分支,但LLG2和LLG3同源性最高,而LLG1与LRE同源关系较为密切。其蛋白同源性基本反映了成员的生物学功能(图3)。LRE和LLG1/2/3密切相关,通过在不同组织器官的差异表达调控不同的生物学过程[12,15-17]。
3 LRE介导高等植物花粉管接受
在被子植物中,花粉管中的两个精细胞在助细胞作用下被转运到胚珠中,分别与卵细胞和中央细胞融合,完成双受精作用。在受精之前LRE主要介导花粉管接受过程。LRE在雌配子体的助细胞、卵细胞和中央细胞中高丰度表达,在受精卵和胚乳中也有表达,但不在花粉或花粉管中表达[18]。因此,lre突变体仅在雌配子体中表现出功能缺陷[15,18]。预测的SP是LRE在助细胞中表达所必需的,当SP被破坏时,错误定位的LRE前体蛋白会被降解,直接导致LRE在助细胞中不能正确定位[7]。保守的GPI锚定位点ω是影响LRE功能的关键位点,最初认为Ser-139为LRE中的ω位点[19],但是Ser-139缺失时并不影响其定位;后来发现Ala-141是LRE隐藏的ω位点,同时缺失Ser-139和Ala-141两个ω位点,会使蛋白不能进行正确的GPI修饰,导致LRE不能被有效地分泌到细胞质膜中,中断了LRE向助细胞运输的可能性[7,20]。缺失GPI锚定结构域中靠近ω位点的部分序列也会导致相似的结果[7]。虽然ω位点和蛋白C端部分序列影响其定位,但LRE仍可以诱导花粉管接受,这表明LRE GPI锚定的前端区域可能并不影响LRE的功能。LRE中第5和第6个Cys之间的12个氨基酸,尤其是高度保守的Asn-Asp二肽结构域,对其功能至关重要(图2)。将这12个氨基酸突变成1个亮氨酸(Leu),回补至lre突变体中,发现其花粉管接受仍有缺陷,说明12个氨基酸的缺失影响LRE功能。同时,将高度保守的Asn-Asp二肽突变成2个丙氨酸(Ala)时,LRE的定位不会改变,但其花粉管接受的功能丧失[7]。這表明LRE功能发生异常影响花粉管正常接受,雌配子体中花粉管过度生长,直至盘曲,导致双受精作用失败(图4)。在拟南芥雌配子体中,也有其他基因调控花粉管接受,其突变体与ler表型类似,如Feronia(FER),Nortia(Nta),Scylla(Syl),evan和turan等[15,18,21-23]。LRE可在内质网中与FER类受体激酶胞外区的exJM区直接相互作用,作为伴侣蛋白协助FER从内质网合成后经高尔基体加工转运至细胞质膜,共同作用于助细胞和花粉管交界处,并作为FER的共受体感知胞外信号,引起Ca2+变化,激活下游活性氧(ROS)信号通路,调控花粉管极性生长[7,24-27]。由此可见,FER发挥其功能需要依赖于LORELEI家族成员的参与。
4 LRE介导种子早期发育
被子植物雌配子体不仅调节双受精,还调控种子发育。TSUKAMOTO等[18]在筛选育性降低的拟南芥突变体时鉴定到一种突变体,表型为大量胚珠不发育,仅有很少种子可以萌发。他们通过TAIL-PCR发现了LRE的新等位基因,并命名为lre-5。少数lre-5雌配子体可以成功接受花粉管,但种子萌发后发育延迟,这表明LRE参与调控种子发育[18]。在lre-5/lre-5突变体的雌蕊中出现两种类型的雌配子体,一种含有2个或不含有助细胞,另一种可能是由于花粉管过度生长导致助细胞不规则。与野生型相比,lre-5雌配子体中的助细胞不退化[18]。lre-5/lre-5突变体雌配子体缺陷,仅完成中央细胞受精,未受精的卵细胞在授粉后退化,导致种子败育。在野生型拟南芥雌蕊中,几乎每个发育中的种子都有胚和胚乳,而突变体败育主要是由于胚和/或胚乳发育异常导致[28-29]。研究发现:自花授粉的lre-5/lre-5雌蕊有两种发育中的种子,一种(约90%)含有胚和胚乳,另一种只含有增殖的极核但没有胚[18]。lre-5/lre-5突变体雌蕊中没有胚,但当中央细胞受精后,胚乳开始发育,种子早期发育被延迟,最终败育[18]。
5 LLG调控植物发育与逆境应答
5.1 LLG1调控根与根毛生长
LLG1在植物各器官中均有表达,在根和叶中表达量最高。LLG1同LRE一样,也与FER跨膜结构域N末端的胞外近膜区exJM结合,将FER从内质网转移到细胞质膜[27]。虽然LLG1与LRE同源性较高,但是两者在功能上没有冗余[18]。LLG1可以帮助FER定位到根部细胞质膜,作为共受体感知细胞外部信号或配体,调节下游信号控制根与根毛生长[27,30]。
llg1突变体早期生长发育缺陷表型与fer的表型相似,表现为对快速碱化因子1(RALF1)的敏感性降低、表皮细胞形状改变,以及根毛生长缺陷等[27]。fer突变体的营养生长[31]、根毛生长[30,32],以及下胚轴伸长都受到抑制[33-34],根毛出现卷曲或异常分枝[27,30]。分别将LLG1和FER回补到llg1突变体和fer突变体中,可以恢复其野生型表型[27,30]。在llg1突变体中,FER-GFP会滞留在内质网和细胞质中,而在llg1突变体中回补LLG1可以减少FER-GFP在细胞质的滞留,恢复FER的质膜定位[27,30]。
llg1与fer-4突变体根中的ROS水平显著降低[35],导致细胞壁完整性丧失、细胞质外渗、细胞塌陷,影响细胞极性生长[27]。同时,fer突变体与几种rac/rop突变体表型相似,这表明FER和RAC/ROP具有调控关系。LLG1和FER相互作用共同感受RALF1,形成的LLG1-RALF1-FER复合物可以激活FER,激活后的FER可以与RopGEFs相互作用,促进RAC/ROP转换为与GTP结合的活化状态,调节NADPH氧化酶(RBOH)产生ROS,调控根生长(图5)[27,30]。激活的FER激酶结构域(FERKD)调控质子ATP酶2(AHA2)磷酸化并失活,导致质外体中pH值升高,引起细胞壁硬化,抑制根部细胞伸长和根毛生长[26,36-37];与之相反,低pH值会导致细胞因膨胀紊乱而爆裂[38]。RALF-LLG1-FER复合物“精细调控”根部下游ROPGEF-ROP-RBOH通路,诱导ROS的产生,但其分子机制仍有待研究。
5.2 LLG1调控植物盐逆境应答
高浓度盐离子会对植物造成渗透胁迫和离子胁迫,导致细胞壁软化,细胞膜稳定性降低,严重时引起细胞死亡[39]。FER的胞外区含有串联的malectin-like domain A(MLDA)和MLDB,两者可以与细胞壁多糖相互作用,感知因高盐引起的细胞壁软化。在fer突变体中,由于FER功能缺失,根细胞在生长恢复期间会急剧爆裂,导致根部细胞呈放射状扩张[40]。LLG1与FER直接相互作用,是FER定位于质膜和信号传导所必需的(图5)[27]。llg1突变体与fer突变体都具有对离子敏感、对渗透胁迫不敏感,以及细胞壁完整性丧失的表型。与fer和llg1突变体类似,salinity overly sensitives(sos)突变体对盐逆境高度敏感,而对渗透胁迫不敏感,但fer和sos突变体在不同离子胁迫下的表型不同。fer突变体对K+敏感,盐诱导下fer突变体仅在根部延伸区的细胞活力丧失;而sos1和sos2突变体根对Na+和Li+表现出超敏感,但对K+不敏感。同时,sos1和sos2突变体在50 mmol?L-1 NaCl胁迫下根生长严重受阻,物质的量浓度超过100 mmol?L-1的NaCl才能诱发类似于fer突变体的缺陷表型,并且sos突变体从根部延伸区到根尖(除根冠外)出现大范围细胞死亡[40]。這表明LLG和FER调控的盐应答途径可能与SOS调控途径不同。
细胞壁信号的感知和传递对于植物调节生长和逆境应答至关重要。细胞壁富含亮氨酸的重复延伸蛋白(LRX)具有N端富含亮氨酸的重复序列(LRR)结构域和C端延伸蛋白结构域[41-42]。LRR结构域识别并结合RALF22/23配体,而C端高度糖基化的延伸蛋白结构域可能参与细胞壁成分(果胶质)的交联[43]。拟南芥盐胁迫条件下,细胞壁交联变化被LRX3/4/5感知,促进RALF22/23的释放。同时,盐胁迫诱导SITE-1肽酶(S1P)积累成熟的RALF22。盐诱导条件下RALF22/23的增加,促进了其与FER的互作,导致FER内化[42]。拟南芥llg1突变体、lrx3/4/5三突变体、fer-4突变体,以及RALF22/23的过表达体表现出相似的表型,如生长迟缓和对盐逆境敏感性增加等[42]。此外,拟南芥FER与G蛋白β亚基(AGB1)相互作用,形成G蛋白偶联受体(GPCR),调节胞内ROS水平,或者通过与ROP11互作调控胞内ABA信号通路,从而调控根部细胞盐逆境应答(图5)[40,42,44]。LLG1通过与FER互作参与对细胞壁信号的感知及其下游信号转导过程。
5.3 LLG1调控植物免疫应答
植物中的类受体激酶作为质膜模式识别受体(PRRs),识别与病原体相关的分子模式(PAMPs),激活下游免疫应答。PRRs一般包含用于配体识别的胞外结构域、跨膜结构域和胞质激酶结构域。LLG1作为PRRs的分子伴侣,帮助调节其质膜定位。
当植物受到病原体侵害时,富含亮氨酸重复序列受体激酶flagellin sensing 2(FLS2)和EF-TU receptor(EFR),迅速与brassinosteroid insensitive1-associated receptor kinase 1(BAK1)形成复合物,激活下游免疫反应,调控防御基因表达,促进水杨酸(SA)等防御激素的积累,提高植株抗病性(图5)[45]。植物MAPK级联信号通路中的enhanced disease resistance 1(EDR1)可以通过调节MKK4/5-MAPK3/6通路负调控植物免疫(图5)[46]。llg1-2和llg1-3突变体都对多种病原体表现出敏感性,并抑制edr1抗病性,但llg1-2突变体有明显的生长缺陷表型,而llg1-3突变体生长发育正常[14,27]。双突变体edr1/llg1-2和edr1/llg1-3都显示出对Golovinomyces chichoracearum的敏感性,llg1-3通过抑制edr1突变体中免疫标记基因PR1表达和SA积累,削弱edr1对G.chichoracearum的抗性。在llg1-2和llg1-3突变体中转入LLG1可以恢复其野生型表型,这表明LLG1在植物免疫中具有重要作用[14]。llg1-2具有与fer相同的生长缺陷表型[27],而llg1-3(LLG1G114R)与野生型表型相似,LLG1仍作为FER的共受体参与其转运及定位,这表明llg1-3仅在免疫功能方面受到影响[14]。
酵母雙杂交和Co-IP实验证明LLG1与EDR1不互作,这表明LLG1对免疫的调节可能与EDR1信号通路无关。LLG1与FLS2和EFR的互作不受flg22处理和LLG1中G114R点突变的影响,这暗示着LLG1的分子伴侣功能与信号转导功能可能是分开的[14]。此外,LLG1与FLS2和EFR形成的复合体调控胞内botrytis-induced kinase 1(BIK1)磷酸化,使RbohD直接被磷酸化,促进PAMP诱导的ROS产生,介导下游免疫反应。在flg22处理后,llg1-2和llg1-3突变体中BIK1的磷酸化水平降低,ROS积累受到破坏,这表明LLG1在植物先天免疫中发挥重要作用[47]。
5.4 LLG2/3调控花粉管顶端生长与爆裂
花粉管的快速生长是被子植物成功受精的关键步骤,该过程受到精细调控。花粉管在花柱道中生长需要RLKs,胞质Ca2+和ROS等多种信号因子的协同调控[48-49]。ANXUR 1/2(ANX1/2)和Buddhas paper seal 1/2(BUPS1/2)是定位于花粉管顶端的RLKs,两者可以形成受体激酶复合物,响应花粉管分泌的RALF4/19,调节花粉管的生长和花粉管细胞壁的完整性。当花粉管顶端到达胚囊附近时,胚珠分泌的RALF34会同RALF4/19竞争性结合ANX/BUPS受体激酶复合体,控制花粉管破裂并释放精细胞(图5)[49-52]。LLG2/3参与调控花粉管的极性生长。在拟南芥llg2/3敲低突变体中,花粉管生长受到阻碍,并在体外生长过程中发生破裂。这种萌发后立即破裂的表型与anx1anx2,bups1bups2和ralf4ralf9突变体极其相似[11,30,50,53]。这表明LLG2/3与ANX/BUPS-RALF调控花粉管生长的信号通路相关。LLG2/3作为分子伴侣,与ANX1/2和BUPS1/2的exJM区互作,协助ANX1/2-BUPS1/2受体激酶的内质网合成、高尔基体加工、膜泡运输,以及花粉管顶端质膜定位,两者形成受体-共受体复合物,共同感受胞外RALF信号[48-49]。LLG2/3-ANX1/2-BUPS1/2复合体在感受到胞外的RALF4/19后,会与下游GDP-ROP1相互作用,将其激活为GTP-ROP1,进而激活下游花粉特异表达的NADPH氧化酶RbohH/J产生ROS。ROS在花粉管顶端的积累,可以调节花粉管生长,防止花粉管提前爆裂[11,54-55]。LLG2/3的表达水平受到抑制会导致花粉管中ROS含量降低(图5)[52]。
LLG2/3-RNAi干扰株系的花粉管细胞壁组分改变,甲酯化果胶质在花粉管顶端区域积累,去甲酯化果胶质在花粉管亚顶端等积累,花粉管中的胼胝质含量降低,这表明LLG2/3参与花粉管生长过程中细胞壁的形成[11]。此外,RALF4/19可以与LRX互作调控花粉管生长[56]。RALF4/19-RNAi植株与LLG2/3-RNAi干扰植株的花粉管细胞壁缺陷型表型相似[56],RALF4的C端区域与LLG2/3结合,其N端(包括YISY motif)与LLG2/3微弱互作[49]。这表明LLG2/3通过协同RALF4/19与LRX相互作用,参与调控花粉管细胞壁组分,影响花粉管生长。
6 结论与展望
LRE家族是GPI-APs的重要亚家族,作为CrRLK1L(如FER和FLS2)的分子伴侣协助其转运并正确定位,响应胞外信号转导,从而参与调控植物的生长、发育、繁殖、逆境应答,以及免疫等多种生物学过程[14,27,53,57-58]。人们已对RALF-GPI-AP-CrRLK1L复合物在植物生长发育中的功能有了初步认识,但对其精细的分子调控机制还缺乏深入研究。LLG如何精确感知胞外信号(如ROS水平、不同盐离子浓度、病原信号分子等),LRE如何根据外界条件调整自身构象,如何调节其与不同配体(如RALFs)结合,如何招募不同的CrRLKs并激活其下游级联信号通路[59],LRE家族成员含有的保守Cys位点如何精细调节其蛋白质构象[60-61],这些科学问题尚待研究。进一步利用分子遗传学策略并结合多组学技术整合分析LRE的分子调控机理具有重要意义。
参考文献:
[1] FUJITA M,KINOSHITA T.Structural remodeling of GPI anchors during biosynthesis and after attachment to proteins [J].Biochimica et Biophysica Acta-Molecular,2010,584(9):1670-1677.
[2] MU?IZ M,RIEZMAN H.Trafficking of glycosylphosphatidylinositol anchored proteins from the endoplasmic reticulum to the cell surface [J].Journal of Lipid Research,2016,57(3):352-360.
[3] ZURZOLO C,SIMONS K.Glycosylphosphatidylinositol-anchored proteins:membrane organization and transport [J].Biochim et Biophys Acta,2016,1858:632-639.
[4] LALANNE E,HONYS D,JOHNSON A,et al.SETH1 and SETH2,two components of the glycosylphosphatidylinositol anchor biosynthetic pathway,are required for pollen germination and tube growth in Arabidopsis [J].Plant Cell,2004,16(1):229-240.
[5] GILLMOR C S,LUKOWITZ W,BRININSTOOL G,et al.Glycosylphosphatidylinositol-anchored proteins are required for cell wall synthesis and morphogenesis in Arabidopsis [J].Plant Cell,2005,17(4):1128-1140.
[6] BUNDY M G,KOSENTKA P Z,WILLET A H,et al.A mutation in the catalytic subunit of the glycosylphosphatidylinositol transamidase disrupts growth,fertility,and stomata formation [J].Plant Physiology,2016,171(2):974-985.
[7] LIU X L,CASTRO C,WANG Y B,et al.The role of LORELEI in pollen tube reception at the interface of the synergid cell and pollen tube requires the modified eight-cysteine motif and the receptor-like kinase FERONIA [J].Plant Cell,2016,28:1035-1052.
[8] BORNER G H,LILLEY K S,STEVENS T J,et al.Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis:a proteomic and genomic analysis [J].Plant Physiology,2003,132(2):568-577.
[9] LIU L F,SHANGGUAN K K,ZHANG B C,et al.Brittle Culm1,a COBRA-like protein,functions in cellulose assembly through binding cellulose microfibrils [J].PLoS Genetics,2013,9:e1003704.
[10] HOU Y N,GUO X Y,CYPRYS P,et al.Maternal ENODLs are required for pollen tube reception in Arabidopsis [J].Current Biology,2016,26:2343-2350.
[11] FENG H Q,LIU C,FU R,et al.LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 regulate pollen tube growth as chaperones and coreceptors for ANXUR/BUPS receptor kinases in Arabidopsis [J].Molecular Plant,2019,12:1612-1623.
[12] YU S C,GUO Z W,JOHNSON C,et al.Recent progress in synthetic and biological studies of GPI anchors and GPI-anchored proteins [J].Current Opinion in Chemical Biology,2013,17:1006-1013.
[13] JOS?-ESTANYOL M,GOMIS-RUTH F X,PUIGDOMENECH P.The eight-cysteine motif,a versatile structure in plant proteins [J].Plant Physiology and Biochemistry,2004,42:355-365.
[14] SHEN Q J,BOURDAIS G,PAN H R.Arabidopsis Glycosylphosphatidylinositol-anchored protein LLG1 associates with and modulates FLS2 to regulate innate immunity [J].Proceedings of the National Academy of Sciences of the United States of America,2017,114:5749-5754.
[15] CAPRON A,GOURGUES M,NEIVA L S,et al.Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene [J].Plant Cell,2008,20(11):3038-3049.
[16] CHEUNG A Y,LI C,ZOU Y J,et al.Glycosylphosphatidylinositol anchoring:control through modification [J].Plant Physiology,2014,166:748-750.
[17] ZHANG W T,LIU J,ZHANG Y X,et al.A high-quality genome sequence of alkaligrass provides insights into halophyte stress tolerance [J].Science China:Life Sciences,2020,9:1269-1282.
[18] TSUKAMOTO T,QIN Y,HUANG Y,et al.A role for LORELEI,a putative glycosylphosphatidylinositol-anchored protein,in Arabidopsis thaliana double fertilization and early seed development [J].The Plant Journal,2010,62:571-588.
[19] EISENHABER B,WILDANER M,SCHULTZ C J,et al.Glycosylphosphatidylinositol lipid anchoring of plant proteins.Sensitive prediction from sequence-and genome-wide studies for Arabidopsis and rice [J].Plant Physiology,2003,133:1691-1701.
[20] MAO Y,ZHANG Z,WONG B.Use of green fluorescent protein fusions to analyse the N-and C-terminal signal peptides of GPI-anchored cell wall proteins in Candida albicans [J].Molecular Microbiology,2003,50:1617-1628.
[21] ROTMAN N,GOURGUES M,GUITTON A E,et al.A dialogue between the SIRENE pathway in synergids and the fertilization independent seed pathway in the central cell controls male gamete release during double fertilization in Arabidopsis [J].Molecular Plant,2008,1:659-666.
[22] KESSLER S A,SHIMOSATO-ASANO H,KEINATH N F,et al.Conserved molecular components for pollen tube reception and fungal invasion [J].Science,2010,330:968-971.
[23] LINDNER H,KESSLER S A,MULLER L M,et al.TURAN and EVAN mediate pollen tube reception in Arabidopsis synergids through protein glycosylation [J].PLoS Biology,2015,13:e1002139.
[24] DENNINGER P,BLECKMANN A,LAUSSER A,et al.Male-female communication triggers calcium signatures during fertilization in Arabidopsis [J].Nature Communication,2014,5:4645.
[25] DUAN Q H,KITA D,JOHNSON E A,et al.Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis [J].Nature Communication,2014,5:3129.
[26] NGO Q A,VOGLER H,LITUIEV D S,et al.A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery [J].Developmental Cell,2014,29:491-500.
[27] LI C,YEH F L,CHEUNG A Y,et al.Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis [J].Elife,2015,4:e06587.
[28] YADEGARI R,DREWS G N.Female gametophyte development [J].Plant Cell,2004,16:S133-S141.
[29] BERGER F,GRINI P E,SCHNITTGER A.Endosperm:an integrator of seed growth and development [J].Current Opinion in Plant Biology,2006,9:664-670.
[30] DUAN Q H,KITA D,LI C,et al.FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development [J].Proceedings of the National Academy of Sciences of the United States of America,2010,107:17821-17826.
[31] KEINATH N F,KIERSZNIOWSKA S,LOREK J,et al.PAMP(pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity [J].Journal of Biological Chemistry,2010,285:39140-39149.
[32] HUANG G Q,LI E,GE F R,et al.Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth [J].New Phytologist,2013,200:1089-1101.
[33] GUO H Q,LI L,YE H X,et al.Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana [J].Proceedings of the National Academy of Sciences of the United States of America,2009,106:7648-7653.
[34] DESLAURIERS S D,LARSEN P B.FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls [J].Molecular Plant,2010,3:626-640.
[35] SWANSON S,GILROY S.ROS in plant development [J].Physiologia Plantarum,2010,138:384-392.
[36] HARUTA M,SABAT G,STECKER K,et al.A peptide hormone and its receptor protein kinase regulate plant cell expansion [J].Science,2014,343:408-411.
[37] XIAO Y,STEGMANN M,HAN Z F,et al.Mechanisms of RALF peptide perception by a heterotypic receptor complex [J].Nature,2019,572:270-274.
[38] MONSHAUSEN G B,BIBIKOVA T N,MESSERI M A,et al.Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs [J].Proceedings of the National Academy of Sciences of the United States of America,2007,104:20996-21001.
[39] ZHU J K.Salt and drought stress signal transduction in plants [J].Annual Review of Plant Biology,2002,53:247-273.
[40] FENG W,KITA D,PEAYCELLE A,et al.The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling [J].Current Biology,2018,28:666-675.
[41] BORASSI C,SEDE A R,MECCHIA M A,et al.An update on cell surface proteins containing extensin-motifs [J].Journal of Experimental Botany,2016,67:477-487.
[42] ZHAO C Z,ZAYED O,YU Z P,et al.Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis [J].Proceedings of the National Academy of Sciences of the United States of America,2018,115:13123-13128.
[43] CANNON M C,TERNEUS K,HALL Q,et al.Self-assembly of the plant cell wall requires an extensin scaffold [J].Proceedings of the National Academy of Sciences of the United States of America,2008,105:2226-2231.
[44] CHEN J,YU F,LIU Y,et al.FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis [J].Proceedings of the National Academy of Sciences of the United States of America,2016,113:5519-5527.
[45] SUN Y D,LI L,MACHO A P,et al.Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex [J].Science,2013,342:624-628.
[46] FRYE C A,TANG D,INNES R W.et al.Negative regulation of defense responses in plants by a conserved MAPKK kinase [J].Proceedings of the National Academy of Sciences of the United States of America,2001,98:373-378.
[47] SHI H,SHEN Q J,QI Y P,et al.BR-SIGNALING KINASE1 physically associates with FLAGELLIN SENSING2 and regulates plant innate immunity in Arabidopsis [J].Plant Cell,2013,25:1143-1157.
[48] ZOU Y,AGGARWAL M,ZHENG W G,et al.Receptor-like kinases as surface regulators for RAC/ROP-mediated pollen tube growth and interaction with the pistil [J].AoB Plants,2011,201(8):plr017.
[49] GE Z X,CHEUNG A Y,QU L J.Pollen tube integrity regulation in flowering plants:insights from molecular assemblies on the pollen tube surface [J].New Phytologist,2019,222:687-693.
[50] GE Z X,BERGONCI T,ZHAO Y L,et al.Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling [J].Science,2017,358:1596-1600.
[51] FRANCK C M,WESTERMANN J,BOISSON-DERNIER A.Plant malectin-like receptor kinases:from cell wall integrity to immunity and beyond [J].Annual Review of Plant Biology,2018,69:301-328.
[52] LI H J,YANG W C.Ligands switch model for pollen-tube integrity and burst [J].Trends in Plant Science,2018,23:369-372.
[53] ZHU L,CHU L C,LIANG Y,et al.The Arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required for normal growth of pollen tubes in the pistil [J].The Plant Journal,2018,95(3):474-486.
[54] KAYA H,NAKAJIMA R,IWANO M,et al.Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth [J].Plant Cell,2014,26:1069-1080.
[55] MANGANO S,JUAREZ S P D,ESTEVEZ J M.Ros regulation of polar-growth in plant cells [J].Plant Physiology,2016,171:1593-1605.
[56] MECCHIA M A,SANTOS-FERNANDEZ G,DUSS N N,et al.RALF4/19 peptides interact with LRX protein to control pollen tube growth in Arabidopsis [J].Science,2017,358:1600-1603.
[57] MIYAZAKI S,MURATA T,SAKURAI-OZATO N,et al.ANXUR1 and ANXUR2,sister genes to FERONIA/SIRENE,are male factors for coordinated fertilization [J].Current Biology,2009,19(15):1327-1331.
[58] STEGMANN M,MONAGHAN J,SMAKOWSKA-LUZAN E,et al.The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling [J].Science,2017,355:287-289.
[59] XU G Y,CHEN W J,SONG L M,et al.FERONIA phosphorylates E3 ubiquitin ligase ATL6 to modulate the stability of 14-3-3 proteins in response to the carbon/nitrogen ratio [J].Journal of Experimental Botany,2019,70:6375-6388.
[60] DUAN Q H,LIU M C J,KITA D,et al.FERONIA controls pectin-and nitric oxide-mediated male-female interaction [J].Nature,2020,579(7800):561-566.
[61] YU J J,LI Y,QIN Z,et al.Plant chloroplast stress response:insights from thiol redox proteomics [J].Antioxidants and Redox Signaling,2020,33(1):35-57.
(責任编辑:顾浩然,郁慧)
