DESs: Green solvents for transition metal catalyzed organic reactions
2020-01-14LifenPengZhifngHuQichoLuZilongTngYinchunJioXinhuXu
Lifen Peng,Zhifng Hu,Qicho Lu,Zilong Tng,Yinchun Jio,Xinhu Xu
a Key Laboratory of Theoretical Organic Chemistry and Functional Molecule of Ministry of Education, Hunan Provincial Key Laboratory of Controllable Preparation and Functional Application of Fine Polymers, School of Chemistry and Chemical Engineering, Hunan University of Science and Technology,Xiangtan 411201, China
b State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China
Keywords:
DESs
Green solvents
Green chemistry
Transition metal
Catalysis
ABSTRACT
In this review,the recent development about using DESs as green solvents in transition metal catalyzed organic reactions was highlighted.Firstly, the development of DESs was simply introduced.After presenting the advantages of DESs, transition metals catalyzed organic reactions using DESs as green solvents were classified and introduced in detail.Different transition metals such as Au, metal impregnated on magnetite,Pd and Ru catalyzed organic reactions proceeded smoothly in DESs and gave corresponding products in good yields.And in some cases,the catalytic systems could be recycled up to several times without any decrease in activity.
1.Introduction
The twelve principles of green chemistry, including preventing waste, innocuous auxiliary substances, catalytic, atom-economical,safe for environment,mild reaction conditions,absence of cocatalysts,without protecting atmosphere and so on,was a widely accepted criteria for the quick assessment of the“greenness” of a given organic reaction[1].According to these important points in achieving a Catalytic Green Chemical Process, the choice of a safe,non-toxic, biorenewable and cheap solvent was crucial in organic reactions[2].Green solvents,meeting the following requirements such as easy availability,non-toxicity,biodegradability,inflammability, recyclability, renewability, low price, have got more and more attention during the last few decades in the ongoing effort to obtain green organic reactions [3].Instead of conventional hazardous volatile,high toxic,non-biodegradable, and flammable organic solvents (VOCs), ionic liquids were often applied as environmentally benign solvents for various organic reactions because of their thermal stability, negligible vapor pressure,reusability and nonflammability.But ionic liquids usually possessed some drawbacks such as toxicity,non-renewable resources,high cost and tedious preparation [4].
Most recently, one kind of appropriate alternative solvents known as deep eutectic ionic liquids,low melting mixtures or deep eutectic solvents(DESs)have attracted more and more significant interest as green solvents for a wide range of applications[5].DESs,defined as molecular complexes typically formed between a simple halide salt like choline chloride (ChCl) or phosphonium halides, etc.and a hydrogen bond donor such as urea, carboxylic acids or polyols,were obtained facilely by mixing a phosphonium or ammonium salt component with a hydrogen bond donor component and heating to form a new liquid phase with a melting point lower than that of the single component.The properties of DESs were kind of similar to ionic liquids.Importantly, DESs had some remarkable advantages over ionic liquids including straightforward synthesis, low cost, high biodegradability, low toxicity,comparatively renewable sources and high tunable solvent properties by simply changing the nature and the molar ratio of the components.Due to these above mentioned interesting features, DESs were widely applied in various fields of modern chemistry including organic synthesis,dissolution of metal oxides,nanotechnology, separation processes, stabilization of DNA,biocatalytic reactions, electrodeposition of metals, material chemistry and so on [5b,5g,6].
Transition metal-catalyzed organic reaction was one of the most frequently used powerful protocols in organic synthesis to achieve elevated levels of chemo-, regio-, and stereoselectivity.Many transition metal complexes such as Pd,Au,Zn,Co,Rh,Ru,Mo,Ni, Cu and Fe have been developed as catalysts to accelerate organic reactions [7].Although these metal catalysts were very efficient to promote organic reactions, the recovery and reuse of catalysts were kind of difficult [8].Thus, the discarded metal catalysts caused serious problems such as waste of resources,pollution of environment.In order to recycle transition metal catalysts and design green reactions efficiently,recently more and more efforts have been focused on proceeding transition metal catalyzed organic reactions in DESs green solvents [9].In this review,transition metal catalyzed organic reactions proceeding in DESs were presented.In most of cases, DESs enabled facile recovery and reuse of metal catalysts without any decrease in activity.
2.Au catalyzed cycloisomerisation in DESs
In 2014, Álvarez reported the first iminophosphorane-Au(I)catalyzed cycloisomerisation of γ-alkynoic acids in DES(Scheme 1)[10].Treatment of γ-alkynoic acids 1 with Au(I)catalyst in 1ChCl/2Urea at room temperature under air afforded desired cycloisomerisation products 2 in excellent yields.Other DESs like 1ChCl/2glycerol (Gly), 1ChCl/2ethyleneglycol (EG) and 1ChCl/2lactic acid(Lac) were also efficient for the formation of products, but the eutectic mixture 1ChCl/2Urea enabled much higher activity of iminophosphorane-Au(I) catalyst leading to quantitative conversion in only 15 min.This fact could be probably attributed to basic and more dipolar nature of 1ChCl/2Urea.Most importantly, this catalytic system remained active(90%-99%) after recycling up to four consecutive runs, without a notable decrease of the activity after each cycle.Thus, this reaction in DESs provided important contribution to green chemistry.
The first cycloisomerisation reaction of (Z)-enynols in DES catalyzed by bis(iminophosphorane)-Au(I) was also reported by Álvarez (Scheme 2) [11].Bis(iminophosphorane)-Au(I) catalyzed cycloisomerisation of(Z)-enynols 3 proceeded smoothly in 1ChCl/2Gly at rt under air without co-catalyst and gave furans 4 in good to excellent yields.Compared to other solvents like water,glycerol or ionic liquid ([BMIM][BF4]), DESs enabled the reaction proceeding in higher rate.The catalytic system could be reused for 10 consecutive times without any loss of activity or selectivity.
The above catalytic system could be applied to one-pot tandem cycloisomerisation/Diels-Alder reaction as well.In the presence of bis(iminophosphorane)-Au(I),(Z)-enynol 5 reacted with activated diethyl acetylenedicarboxylate 6 in 1ChCl/2Gly at 45°C under air leading to the clean and high-yield formation(85%-91%) of the desired oxabicyclic products 7 (Scheme 3) [11].
When water or DES (1ChCl/2Urea) was used as solvents, Au(I)catalyzed cycloisomerization of alkyny amides 8 occurred at room temperature without co-catalysts and protecting atmosphere and produced corresponding alkylidene lactams 9 in moderate to excellent yields (Scheme 4) [12].Catalyst was recycled up to four consecutives runs albeit with a decrease in activity after each recycling step.This is the first example about the recyclability of a catalyst in cycloisomerization of alkynyl amides.
3.Metal impregnated on magnetite catalyzed reactions
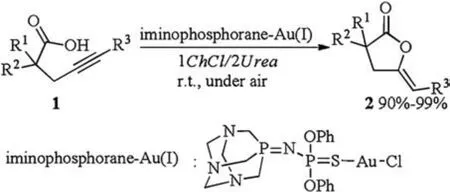
Scheme 1.Au(I)-catalyzed cycloisomerisation of γ-alkynoic acids.
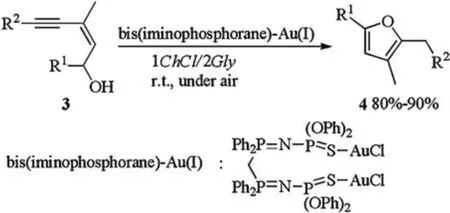
Scheme 2.Au(I)-catalyzed cycloisomerisation of (Z)-enynols.
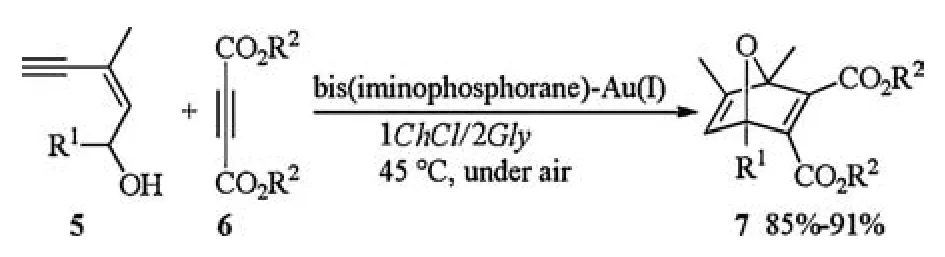
Scheme 3.Au(I)-catalyzed tandem cycloisomerisation/Diels-Alder reaction.

Scheme 4.Au(I)-catalyzed cycloisomerization of alkyny amides.
In 2014, Zhang investigated superparamagnetic CuFeO2nanoparticles catalyzed one-pot, three components reaction in DESs[13].Treatment of 2-aminopyridines 10,aldehydes 11,and alkynes 12 with CuFeO2in DES(2Citric acid/3dimethylurea (DMU))at 65°C yielded corresponding imidazo[1,2-a]pyridines 13.The catalytic system could be successfully recycled up to six times with keeping its catalytic performance (Scheme 5).
Magnetic Fe3O4nanoparticle catalyzed eco-friendly synthesis of cyanohydrins in DES was reported by Azizi in 2015.In the presence of Fe3O4catalyst, aldehydes 14 reacted with TMSCN 15 and afforded corresponding cyanohydrins 16 in moderate to excellent yields.This catalytic system was also used for synthesis of β-hydroxy nitriles.Treatment of epoxides 17,15 with Fe3O4catalyst afforded corresponding β-hydroxy nitriles 18 in good to excellent yields.The main products isolated were those arriving from the attack of the nucleophile to the less hindered position of the asymmetrical epoxide (Scheme 6) [14].
A plausible mechanism for the synthesis of cyanohydrins was shown in Scheme 7.The reaction probably took place via activation of the carbonyl group in 14 with hydrogen bonding ability of deep eutectic solvent.Magnetic Fe3O4nanoparticles catalyst could serve the dual role in this system,as a Lewis acidic surface and so could activate the carbonyl group by coordination of the Fe in the Fe3O4.Furthermore, Fe3O4assisted in improving reactivity of TMSCN through weak interaction between oxygen in Fe3O4with silicon in TMSCN,leading to the formation of intermediate.The nucleophilic attack of TMSCN on activated carbonyl formed corresponding products 16 [14].
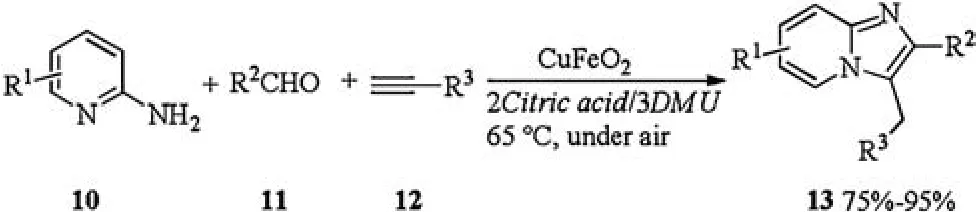
Scheme 5.CuFeO2 catalyzed one-pot, three component reaction.
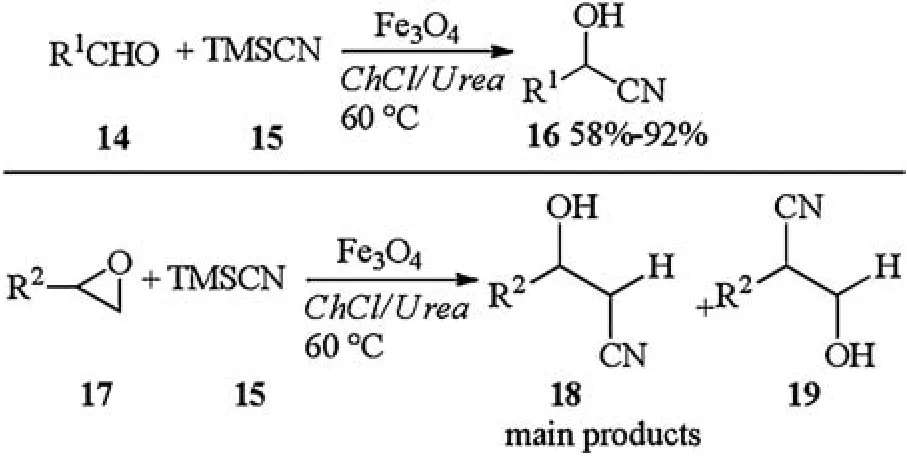
Scheme 6.Fe3O4 nanoparticle catalyzed synthesis of cyanohydrins in DESs.

Scheme 7.Plausible mechanism for the synthesis of cyanohydrins.
Cross-dehydrogenative coupling in DES catalyzed by copper(II)oxide impregnated on magnetite was introduced by Ram ón in 2016(Scheme 8) [15].Subjection of tetrahydroisoquinolines 20 and alkynes 21 to CuO-Fe3O4catalyzed dehydrogenative coupling in 1ChCl/2(CH2OH)2gave corresponding products 22 in moderate to excellent yields.The presence of 1ChCl/2(CH2OH)2was necessary for achieving reasonable yields since the reaction did not occur in some VOCs like DMSO,MeOH,toluene,H2O,DMF,etc.The catalytic system could be recycled up to ten times without detrimental effect on the yield of the reaction.
Palladium on magnetite accelerated cycloisomerization of 4-pentynoic acid in H2O as well as in DESs(1ChCl/2urea,1acetylcholine chloride (AcChCl)/2urea, 1ChCl/1resorcinol, 1ChCl/2EG, 1ChCl/2Gly) was also described in 2018 by Ram ón [16].Treatment of 4-pentynoic acid 23 with PdO-Fe3O4catalyst in DESs or water formed the desired enol-lactone 24 in 67%-100% yields (Scheme 9).
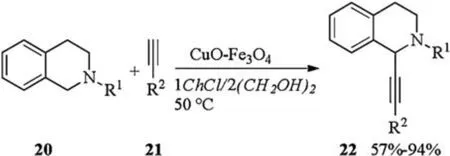
Scheme 8.CuO-Fe3O4 catalyzed dehydrogenative coupling.
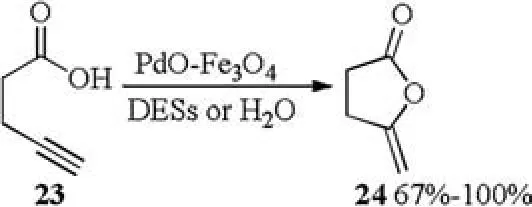
Scheme 9.PdO-Fe3O4 catalyzed cycloisomerization of 4-pentynoic acid.
Magnetically separable graphene oxide anchored sulfonic acid(Fe3O4/GO/SO3H) nanoparticles catalyzed one-pot, three components reaction of 1-phenyl-3-(pyridin-3-yl)-1H-pyrazol-5-amine with 3-oxo-3-(pyridin-3-yl)propanenitrile and aldehydes in ChCl/Gly was applied for synthesis of 3,6-di(pyridin-3-yl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles.As shown in Scheme 10, Subjection of amine 25,aldehydes 26 and nitrile 27 to CoFe2O4/GO/SO3H catalyzed one-pot reaction under microwave irradiation using ChCl/Gly as a green solvent gave corresponding products 28 in good to excellent yields [17].
4.Pd catalyzed coupling and other reactions
In 2006, K önig described Pd catalyzed Suzuki coupling of aryl halides with phenyl boronic acid in DESs [18].Aryl bromides 29 was coupled with phenyl boronic acid 30 in different sugar/urea/salt DESs at 90°C using Pd(OAc)2as a catalyst and Na2CO3as a base to afford corresponding coupling products 31 in good to excellent yields (Scheme 11).
In 2009, the same research group reported Pd catalyzed Heck cross coupling and Sonogashira cross coupling in DESs [19].Subjection of iodide 32 and n-butyl acrylate 33 to different Pd(Pd/C, Pd(OAc)2or PdCl2(PPh3)2catalyzed Heck cross-coupling in different DESs(3D-mannose/7DMU or 2L-carnitine/3urea)gave the desired coupling products 34 in 62%-91% yields.PdCl2(PPh3)2catalyzed Sonogashira coupling of bromide 35 and phenyl acetylene in 3D-mannose/7DMU without copper co-catalyst formed corresponding coupling products 36 in moderate yields(Scheme 12).
Pd catalyzed Stille alkylation of arylbromides and tributylphenylstannane in DESs was also studied[20].When Pd2(dba)3/AsPh3was applied as a catalyst, Stille alkylation of bromides 37 and tributylphenylstannane occurred in different DESs (6Lactose/3DMU/1NH4Cl, 5Mannitol/4DMU/1NH4Cl, 5Maltose/4DMU/1NH4Cl or 7Sorbitol/2DMU/1NH4Cl etc.)and afforded biphenyl products 38 in 80%-100% yields (Scheme 13).
PdI2/KI-catalyzed heterocyclization in DESs was researched by Mancuso and Gabriele in 2016(Scheme 14)[21].In the presence of PdI2and KI, heterocyclization of 1-mercapto-3-yn-2-ols 39 proceeded smoothly in 1ChCl/2Gly and led to corresponding thiophenes 40 in moderate to good yields.After extraction of the thiophene products with hexane or Et2O,the DES/catalytic system could be easily recovered and reused for several times without appreciable loss of activity.

Scheme 10.CoFe2O4/GO/SO3H catalyzed one-pot reaction.

Scheme 11.Pd catalyzed Suzuki coupling.

Scheme 12.Pd catalyzed Heck coupling and Sonogashira coupling.
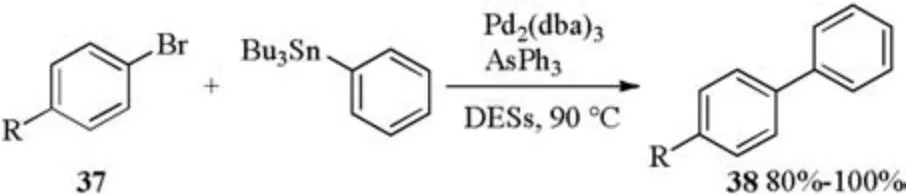
Scheme 13.Pd catalyzed Stille alkylation.

Scheme 14.Pd catalyzed heterocyclization.
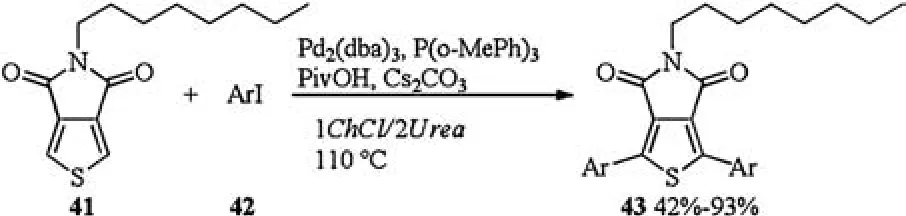
Scheme 15.Pd catalyzed thiophene-aryl coupling.
In 2017, Farinola studied the first Pd-catalyzed thiophene-aryl coupling reaction in DESs (Scheme 15).When Pd2(dba)3/P(o-MePh)3was applied as a catalyst, PivOH was used as an additive and Cs2CO3was applied as a base,direct thiophene-aryl coupling of 5-octylthieno[3,4-c]pyrrole-4,6-dione 41 with a series of functionalized aryl iodides 42 via C-H bond activation occurred in DES(1ChCl/2Urea) and gave products 43 in 42%-93% yields.Pd and ligand were found to be the key for successful implementation of this thiophene-aryl coupling.PdCl2(CH3CN)2, Pd(OAc)2, and Pd(PPh3)4were found to be much less effective than the Herrmann-Beller catalyst, leading to low yields of 43, while Pd2(dba)3and PdCl2(PPh3)2shew higher efficiencies,affording 43 in 82%and 55%yields,respectively.Ligand P( o-MeOPh)3afforded 43 in 82%yield,whereas Low yield (24%) were obtained in the presence of less electron-rich ligand P( o-MePh)3.When less hindered PPh3was used,the desired product was obtained in 55%yield.These results suggested that electron donating group (MeO) in P( o-MeOPh)3is beneficial for a smooth reaction course,probably due to its capacity to increase the electron density and the stability of the Pd complex in the reaction media [22].
In 2017, Ram ön described DES as a green solvent for the Pd catalyzed C-S Bond Formation[23].When PdCl2/phosphine L1 was applied as a catalyst and sodium metabisulfite was used as SO2source, the sulfonylation reaction of boronic acids 44 and subsequent alkylation of the sulfinate salt with pentyl bromide proceeded smoothly in 1ChCl/2acetamide in one pot manner and afforded corresponding products 45 in moderate to excellent yields(Scheme 16).The catalytic system could be reused for three times without decrease in the yield of the reaction.
PdCl2in combination with cationic pyridiniophosphine ligands in DESs, was successfully applied to different cross coupling reactions like Suzuki-Miyaura,Sonogashira or Heck couplings[24].As shown in Scheme 17,subjection of halides 46 and phenylboronic acid to PdCl2/L2 catalyzed Suzuki-Miyaura coupling in 1ChCl/2Gly gave the desired biaryls 47 in good to excellent yields.PdCl2/L2 catalyzed Sonogashira coupling between halides 48 and phenyl acetylene occurred in Ph3PMeBr/2Gly and led to the desired coupling products 49 in 67%-99% yields.When 1ChCl/2Gly was applied as a solvent, PdCl2/L2 catalyzed Heck coupling between iodide 50 and methyl acrylate proceeded smoothly to form corresponding products 51 in moderate to excellent yields.In Suzuki and Sonogashira couplings, the catalytic system could be recycled up to 5 times without a significant drop in the catalytic activity.
When aryltrifluoroborates were applied as coupling partners,ligand-free Pd(OAc)2catalyzed Suzuki-Miyaura couplings could be carried out in DES[25].With Na2CO3as a base and 1ChCl/2Gly as a sustainable and environmentally responsible solvent, Pd(OAc)2catalyzed Suzuki-Miyaura couplings between aryl halides 52 and versatile, moisture-stable potassium aryltrifluoroborates 53 proceeded efficiently and chemoselectively in air,furnishing a series of valuable biaryls and terphenyl derivatives 54 in 53%-98% yields(Scheme 18).Catalyst, base and DES have been facile and successfully recycled up to six times with an E-factor as low as 8.74.
The first ligand free Pd catalyzed aminocarbonylation of(hetero)aryl iodides in DESs was reported by Capriati and Salomone in 2018 (Scheme 19) [26].The reaction of iodides 55 with amines 56 under a pressure of CO using Pd(OAc)2as a catalyst and K2CO3as a base straightforwardly led to the desired aminocarbonylated product 57 in moderate to excellent yields in 1ChCl/2Urea or 1ChCl/2Gly.The catalytic system could be recycled easily and remained active for over 5 cycles.
5.Ru catalyzed isomerisation of allylic alcohols
In 2017, Álvarez and Capriati reported Ru catalyzed one-pot sustainable synthesis of tertiary alcohols through redox isomerization of allylic alcohols assembled with the chemoselective addition of organolithium or organomagnesium reagents to the in situ formed ketones employing DESs as environmentally friendly reaction solvents.When allylic alcohols 58 and catalyst[Ru(η3:η3-C10H16)Cl(κ2-O,O-CH3CO2)]in 1ChCl/2Urea or 1ChCl/1Sorbitol(Sorb) solvent was heated at 50 or 75°C followed by the addition of n-BuLi/n-Bu2Mg as the organometallic reagent to the reaction mixture, 58 could be converted into the corresponding tertiary alcohols 60 in moderate to excellent yields without any additional step of purification or isolation of transiently formed ketones 59(Scheme 20) [27].

Scheme 16.Pd catalyzed C-S bond formation.
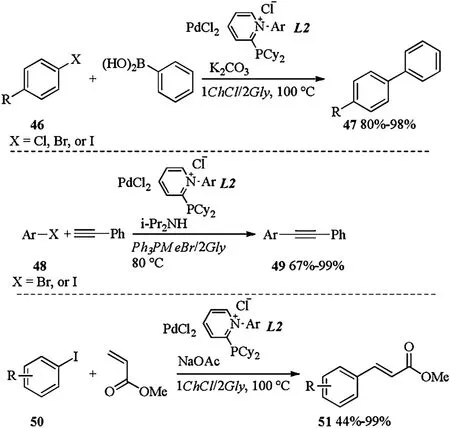
Scheme 17.Pd catalyzed Suzuki-Miyaura, Sonogashira or Heck couplings.

Scheme 18.Pd catalyzed Suzuki-Miyaura couplings of aryltrifluoroborates.
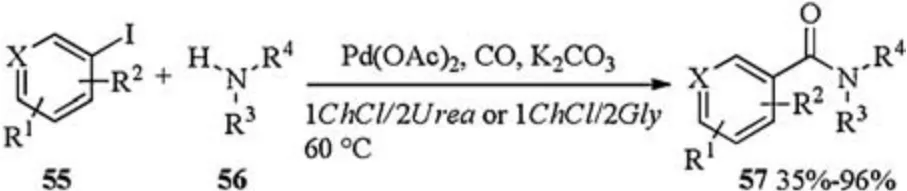
Scheme 19.Pd catalyzed aminocarbonylation.

Scheme 20.Ru catalyzed one-pot synthesis of tertiary alcohols.
One-pot ruthenium-catalyzed allylic alcohol isomerization followed by asymmetric bioreduction in DES-buffer mixtures could yield chiral alcohol[28].Subjection of allylic alcohol 61 to Ru (η3:η3-C10H16)Cl(κ2-O,O-CH3CO2)catalyzed isomerization and the following purified ketoreductases (KRED) catalyzed reduction provided chiral alcohols 63 in excellent yields.This reaction proceeded in one-pot manner and intermediates 62 was not required to be isolated (Scheme 21).
Other transition metal catalyzed reactions proceeding in DESs such as Cu catalyzed azide-alkyne 1,3-dipolar cycloaddition [19],Zn catalyzed rearrangement of aldoximes [29], Rh catalyzed hydrogenation of methyl α-cinnamide and hydroformylation[19,30,31], Al catalyzed furfural production from Xylose or Xylan[32],Fe catalyzed toluene oxidation[33],Sc catalyzed Diels-Alder reactions [34]and Fe, Zn or Cr catalyzed dehydration of fructose[35]were also reported in recent years.

Scheme 21.Ru catalyzed one-pot allylic alcohol isomerization followed by asymmetric bioreduction.
In summary,transition metal catalyzed reactions in DESs have attracted more and more attention because DESs have some remarkable advantages including thermal stability, negligible vapor pressure, reusability, nonflammability, straightforward synthesis, low cost, high biodegradability, low toxicity, comparatively renewable sources and high tunable solvent properties.Numerous reactions such as Au catalyzed cycloisomerisation,Metal impregnated on magnetite catalyzed organic reactions, Pd catalyzed coupling reactions, Ru catalyzed isomerization etc.proceeded smoothly in DESs and gave the desired products in reasonable yields.In some cases, the catalytic systems were very easy to recover and reuse for several times.
6.Limitations and perspectives
Although these above transition metal catalyzed organic reactions proceeding in DESs were useful and convenient for synthesis of organic compounds,some limitations existed such as expensive Ru or Pd complexes catalysts were often used to realize reasonable yields,poor functional group tolerance and so on.Thus,establishment of cheap and general catalytic system should be focused in future research.And application of DESs green solvents in other transition metal catalyzed organic reactions was still in high need.
Acknowledgments
We gratefully acknowledge the National Natural Science Foundation of China (Nos.21802040, 21877034), the Natural Science Fund Youth Project of Hunan Province (No.2018JJ3145),the General project of Hunan Education Department (No.17C0629), and the Open Foundation of Key Laboratory of Theoretical Organic Chemistry and Functional Molecule of Ministry of Education,Hunan University of Science and Technology(No.E21843) for financial support.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
