A chlorinated non-fullerene acceptor for efficient polymer solar cells
2020-01-14MeiLuoCnZhuJunYunLiuyngZhouKeshtovYuGodovskyYingpingZou
Mei Luo,Cn Zhu,Jun Yun,Liuyng Zhou,M.L.Keshtov,D.Yu Godovsky,Yingping Zou,*
a College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
b Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow 119991, Russia
Keywords:
Electron acceptor Y15
Chlorinated
Non-fullerene electron acceptors
Efficient polymer solar cells
Narrow bandgap
ABSTRACT
Improving the performance and reducing the manufacturing costs are the main directions for the development of organic solar cells in the future.Here, the strategy that uses chemical structure modification to optimize the photoelectric properties is reported.A new narrow bandgap (1.30 eV)chlorinated non-fullerene electron acceptor(Y15),based on benzo[d][1,2,3]triazole with two 3-undecylthieno[2′,3′:4,5]thieno[3,2-b]pyrrole fused-7-heterocyclic ring,with absorption edge extending to the near-infrared (NIR) region, namely A-DA'D-A type structure, is designed and synthesized.Its electrochemical and optoelectronic properties are systematically investigated.Benefitting from its NIR light harvesting,the fabricated photovoltaic devices based on Y15 deliver a high power conversion efficiency (PCE) of 14.13%, when blending with a wide bandgap polymer donor PM6.Our results show that the A-DA'D-A type molecular design and application of near-infrared electron acceptors have the potential to further improve the PCE of polymer solar cells (PSCs).
Polymer solar cells(PSCs)have attracted much attention due to low cost,light weight,high flexibility and molecular design ability[1-4].Recently, the PSC devices with bulk heterojunction (BHJ)structure based on non-fullerene small molecular acceptors have achieved some exciting results with the power conversion efficiencies (PCEs) over 15% for single junction cells [5], and 17%for tandem solar cells [6], which are higher than those of devices based on fullerene and its derivatives.Although PSCs have made great breakthrough in PCEs [7-9], it is believed that further improvement is needed in the performance of the PSC devices towards commercialization.
To date, large number of small molecules and polymers with absorption edge extending to the near-infrared(NIR)region have been synthesized and applied to semitransparent PSCs [10-12],single junction-PSCs [13,14]and tandem solar cells [15]due to strong light absorption from the NIR region [16-18].Many exciting results have been achieved in organic electronics.For example, an ultra-narrow bandgap (ultra-NBG) non-fullerene acceptor (NFA), namely IEICO-4F, has been applied in PSCs with the absorption onset of ≈1000 nm,corresponding to low Egoptof 1.24 eV[19],and an impressive short-circuit current density(Jsc)of 25.3 mA/cm2is obtained,but the open circuit voltage(Voc)is only 0.73 V, indicating a problematic trade-off between the Vocand Jscin the photovoltaic devices.Therefore,it is still a big challenge for efficient improvement of the PCE of the PSC devices based on lowbandgap NFAs by designing and synthesizing new photovoltaic materials.
To further broaden the absorption spectra of NFAs, many efficient molecular design strategies have been successfully applied,such as side chain engineering[20-22]and the extended conjugation of core unit [23].By proper manipulation of the intramolecular charge transfer (ICT) effects through attaching electron-deficient unit (F or Cl atom)[18,24]or electron-rich unit(CH3)onto the end-capping unit,[25,26]the absorption spectra of NFAs can be extended to NIR region.Very recently,some new NIR acceptor-(donor-acceptor-donor)-acceptor (A-DA'D-A) type electron acceptors (such as Y1 and Y5) [27,28]with absorption onset at ≈900 nm,have been firstly reported as outstanding NFAs by our group.Moreover, Y1-based device achieved a good PCE of 13.4%with very low voltage loss of 0.57 V and high Jscof 22.0 mA/cm2.Hence, incorporating electron-withdrawing unit benzotriazole into fused ring core can possess red-shifted absorption, which is beneficial for improving PCE.
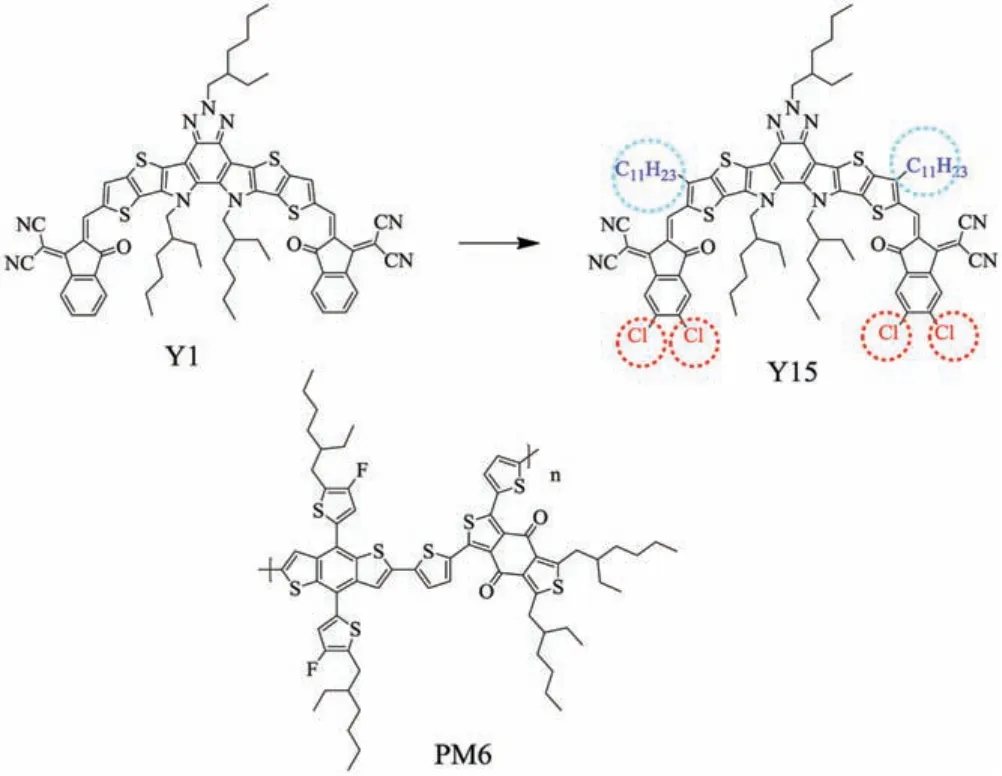
Fig.1.The structures of Y1, Y15 and PM6.
In this work, electron acceptor Y1 was structurally modified to extend absorption by using stronger substituents to enhance the ICT effects.[29]Therefore, a new low bangap A-DA'D-A type small molecule electron acceptor, named 8-(4-(diphenylamino) phenyl)-5-(2-ethylhexyl)-10,11-(2-ethylhexane) -bis(undecylthieno(2′,3′:4,5)thieno)-pyrrole(3,2-b)benzo(3,2b) (1,2,3)triazolo(4,5-e)Indole-2-bis(5,6-dichloro-3-oxo-2,3-dihydro-1H-inden-1-ylidene))dimalononitrile (Y15), was designed and synthesized (Fig.1).The weak electron-rich unit(undecyl)and strong electron-deficient chlorine atom (Cl) were attached to its DA'D core and end-group unit,respectively.Due to large dipole moment of C-Cl,the ICT effects are strengthened and thus lower energy levels and wide absorption spectrum can be obtained[24,30].In addition,the introduction of electron-deficient chlorine atom (Cl) can form more obvious molecular stacking to broaden absorption spectrum [14,31-35].Compared with electron acceptor Y1 [27], the absorption edge of Y15 is red-shifted about 70 nm.Moreover,in the DA'D-type fusedring core,the introduction of the weak electron-rich unit(undecyl)in the thieno[3,2-b]thiophene can increase the steric hindrance to form favored configuration at a zero torsion angle,according to the reported literature [13,36,37].According to the onset absorption[38], the Egoptof Y15 was estimated to 1.30 eV.Blending with the wide bandgap polymer PM6 [39], the device obtained a PCE of 11.54%.To further improve the efficiency of PSCs,the morphology of the active layer was optimized through 1,8-diiodooctane (DIO)additive[40]and thermal annealing(TA)treatment[41].A PCE over 14% was obtained.These results show that introduction of Clatomin the NFAs end-group unit is an effective strategy to extend the absorption,and thus improve PCEs of PSC devices.
The NFAs(Y1,Y15)and polymer donor PM6 are shown in Fig.1.The synthetic route of Y15 is shown in Fig.S1 (Supporting information).The target molecule was analyzed via1H NMR and13C NMR.1H NMR and13C NMR are shown in Figs.S2-S6(Supporting information).Y15 shows high solubility in common organic solvents, such as dichloromethane (DCM), chloroform(CHCl3) and chlorobenzene at room temperature.
The absorption spectra of Y15 are depicted in Fig.2a, in dilute chloroform(CF)solution and in the film.It is observed that the Y15 shows broad and strong absorption band in the 600-1000 nm.In the CF solution, Y15 exhibits a maximum absorption peak at 777 nm.In the solid thin films, the maximum absorption peak of Y15 has redshifted approximately 98 nm with Egopt=1.30 eV,corresponding to absorption onset of ≈949 nm, according to Egopt=1240/λonset.Compared with Y1 (Egopt=1.44 eV) [27], the Egoptof Y15 was lowered by 0.14 eV.Since Y15 has weak absorption in the 300-600 nm, a wide bandgap polymer donor PM6 with complementary absorption as shown in Fig.S7 (Supporting information) is chosen to boost efficiency in the photovoltaic devices.
The electrochemical curve of Y15 was measured in CH3CN/0.1 mol/L Bu4NPF6,as shown in Fig.S8a(Supporting information).The LUMO energy level can be estimated by the onset potentials of reduction part.The HOMO energy level can be calculated by the onset oxidation potentials[42].The HOMO energy level(-5.56 eV)and LUMO energy level (-3.93 eV) of Y15 were observed,respectively.The HOMO energy level(-5.43 eV)and LUMO energy level (-3.42 eV) of PM6 are shown in Fig.S8b (Supporting information).The energy levels of Y15 are obviously lower than those of Y1(HOMO=-5.42 eV,LUMO=-3.64 eV)[27],which may be related to the chlorine atomic effects in the end-group of NFAs.The low-lying energy levels of Y15 may have some advantages in polarization and photochemical stability, and the related photophysical data are shown in Table S1 (Supporting information).Molecular energy level diagrams of PM6 and Y15 are shown in Fig.2b.
To investigate the photovoltaic properties of the electron acceptor(Y15),we fabricated normal PSC device with the structure of ITO/PEDOT:PSS/PM6:Y15/PDINO/Al, where PM6 is used as polymer donor and Y15 as acceptor.When using CF as processing solvent,the optimal PM6/Y15 wt ratio(w/w)is 1:1.8.Subsequently,the morphology of the PM6:Y15-based devices are optimized by additives and thermal annealing (TA) treatment.It is found that PM6:Y15-based device with 0.3 vol%DIO and TA at 80°C for 10 min showed the best device performance.The current density-voltage(J-V)curves of the PM6:Y15-based devices are shown in Fig.2c and related data are listed in Table S2(Supporting information).PM6:Y15-based as cast device delivered a moderate PCE of 11.54%with Jscof 21.88 mA/cm2,Vocof 0.884 V and FF of 59.62%.Compared with as cast device, the optimal device yields an outstanding PCE of 14.13%with relatively higher Jscof 23.79 mA/cm2and FF of 68.49%,respectively,showing that the addition of DIO and TA can improve Jscand FF.
The external quantum efficiency (EQE) curves [43]of PM6:Y15-based devices are shown in Fig.2d.The EQE curves of devices both possess a broad photoresponse in 300-1000 nm, which are consistent with the absorption of the active layer.The highest EQE value of PM6:Y15-based device reached 74% in 500-660 nm.For these devices,the Jsccalculated from EQE spectra(21.525 mA/cm2for as cast film and 23.401 mA/cm2for optimized film)are similar with the values from the J-V curves.

Fig.2.(a)The absorption spectra of Y15 in the solution and in the film;(b)Molecular energy level diagrams of PM6 and Y15;(c)J-V curves of PM6:Y15 as cast and optimized devices; (d) EQE curves of PM6:Y15 as cast and optimized devices.
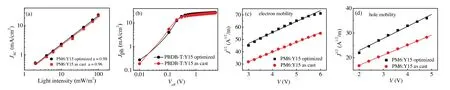
Fig.3.(a)Light intensity dependence of the Jsc of the PM6:Y15 based optimized and as cast devices;(b)Photocurrent density(Jph)versus effective voltage(Veff)characteristics of the PM6:Y15 based-optimized and as cast devices;(c)The electron mobilities of the PM6:Y15 based optimized and as cast devices;(d)The hole mobilities of the PM6:Y15 based optimized and as cast PSCs.
To study the bimolecular recombination behavior in the PM6:Y15-based device, the dependence of Jscon light intensity (Plight)was measured.Correlation between Jscand Plightis ascribed to a formula: Jsc∝Pα[44].When α value is close to 1, bimolecular recombination can be negligible in the PSC devices.As seen in Fig.3a,α value of the PM6:Y15-based at cast device is 0.96,while α value of optimized device is increased to 0.98.After device optimization, the α value is close to 1, which is beneficial to enhance charge transfer and decrease bimolecular recombination and thus improve Jscand FF.
In order to explore the efficiencies of the exciton dissociation and charge collection in the PM6:Y15-based PSC devices, the photocurrent density(Jph)versus the effective applied voltage(Veff)analysis [45]was measured (Fig.3b).Under short-circuit conditions, the value of Jph/Jsat(Jsatstands for saturation photocurrent density)of 91.2%is obtained for the PM6:Y15-based as cast PSC device, while that of the optimized PM6:Y15-based device is 92.2%,indicating the optimized device has better exciton dissociation and charge collection properties.
To investigate charge transport of the PM6:Y15blend film,the space charge limited current (SCLC) method was used to measure charge mobilities[46].The structure of electron-only or hole-only device is ITO/ZnO/PM6:Y15/PDINO/Al or ITO/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)(PEDOT:PSS)/PM6:Y15/gold (Au), respectively.As seen in Figs.3c and d, the hole-only (μh) or electron-only (μe)mobility is calculated to be 1.52×10-4or 2.77×10-4cm2V-1s-1for PM6:Y15-based as cast device, respectively, corresponding to μe/μhratio of 1.82.After the optimization,the μhor μemobility of PM6:Y15-based device is increased to 3.25×10-4and 5.22×10-4cm2V-1s-1,respectively.The μe/μhratio is decreased to 1.61.The optimized device shows higher charge mobilities,which is attributed to reducing charge recombination and enhancing Jsc, and thus improving overall photovoltaic performance.
The microstructures of neat PM6 film,neat Y15 film and PM6:Y15 blend films were characterized using the grazing-incidence wideangle X-ray scattering(GIWAXS).The GIWAXS patterns of neat Y15 film,neat PM6 film and PM6:Y15 blend film are shown in Figs.4 a-c.The corresponding intensity profiles in the in-plane(IP)and out-of plane (OOP) directions are shown in Fig.4d.The neat Y15 film(Fig.4a)possesses a strong π-π stacking peak in the OOP direction at q =1.77 Å-1(d~3.548 Å), indicating a face-on orientation of Y15.Interestingly, there exists a diffraction peak in the IP direction at q =0.42 Å-1(d~14.95 Å), showing the coexistence of two distinct structure orders.The neat PM6 film(Fig.4b)presents a π-π peak in the OOP direction at q=1.695 Å-1(d~3.705 Å)and bimodal lamellar peaks in both IP and OOP directions at q=0.28 Å-1(d~22.428 Å)and q =0.36 Å-1(d~17.44 Å), respectively.The PM6:Y15 blend films(Fig.4c)displays as a strong diffraction peak in the OOP direction at q=1.735 Å-1(d~3.62 Å),associated with the π-π stacking of Y15.In the IP direction,the scattering peak at q=0.385 Å-1(d~16.31 Å)can be assigned to the lamellar stacking of either PM6 or Y15.

Fig.4.(a-c)GIWAXS image of PM6 film,Y15 film and PM6:Y15 blend film;(d)GIWAXS intensity profiles along the in-plane(dotted line)and out-of-plane(solid line)directions.Tapping-mode AFM height images of PM6:Y15 blend films as cast(e)and with TA+0.3%DIO(f);TEM images of PM6:Y15 blend films as cast(g)with TA+0.3%DIO(h).
The morphologies were further investigated by atomic force microscopy (AFM) and transmission electron microscopy (TEM)[47].As shown in Figs.4e and f, the root-mean-square (RMS)roughness (Rq) values of the PM6:Y15-based blend films with uniform and smooth morphology are approximately 1.56 nm and 1.53 nm, respectively.From the TEM images ( Figs.4g and h, it is known that the bright and dark zones represent the donor-rich and the acceptor-rich domain due to their different electron densities,respectively[40].Proper phase separation of PM6 and Y15 with a bicontinuous interpenetrating network can be observed in the blend film.This benefits exciton dissociation and charge transport,which results in high Jscand FF.
In summary, by the introduction of Cl atom into end groups of NFAs, a new small molecule acceptor (Y15) is designed and developed.Due to the electron-withdrawing ability of Cl-atom,chlorinated electron acceptor Y15 shows lower molecular energy levels compared to Y1 counterpart with no Cl attachment.After the addition of 0.3% DIO and TA treatment of 80°C, optimized device shows relatively higher charge mobilities,better exciton dissociation and charge collection.Therefore,a high PCE of 14.13%for the PM6:Y15 based optimized devices was obtained for binary single junction solar cells.Our work shows that rational optimization of molecular structures is extremely important to improve the PCE of OSCs.
Acknowledgments
This work has been financially supported by the National Natural Science Foundation of China (Nos.51811530096,21875286), the National Key Research & Development Projects of China (No.2017YFA0206600), Science Fund for Distinguished Young Scholars of Hunan Province (No.2017JJ1029).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.07.023.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
