2D Bi2WO6/MoS2 as a new photo-activated carrier for boosting electrocatalytic methanol oxidation with visible light illumination
2020-01-14HongminZhngJieHeChunyngZhiMingshnZhu
Hongmin Zhng,Jie He,Chunyng Zhi,Mingshn Zhu,*
a School of Environment, Jinan University, Guangzhou 510632, China
b School of Materials Science and Chemical Engineering, Ningbo University, Ningbo 315211, China
Keywords:
Methanol oxidation
Bi2WO6
MoS2
Visible light
Electrocatalysis
ABSTRACT
In this paper, a new two-dimensional (2D)/2D composite of Bi2WO6/MoS2 was facile synthesized,and then was used as supporting material for depositing Pt nanoparticles.The as-synthesized Pt-Bi2WO6/MoS2 was extended into photo-assisted electrocatalytic oxidation of methanol, which is a model anode reaction for direct methanol fuel cell.Compare with traditional electrocatalytic process,Pt-Bi2WO6/MoS2 displays 1.5 times enhanced electrocatalytic performance on methanol oxidation with assistance of visible light irradiation and 2.2 times for commercial Pt/C.Besides, from the results of chronoamperometric and chronopotentiometry experiments,the stability of Pt-Bi2WO6/MoS2 electrode is clearly improved under visible light irradiation.The synergistic effects of photo-and electro-catalytic in the heterojunction of Pt-Bi2WO6/MoS2 in favor of the above enhancement.This research gives more insights in the fields of photo-assisted traditional electrocatalytic application by constructing of semiconductor heterojunction carrier.
Owing to its high efficiency and low even zero emission,direct methanol fuel cell (DMFC), an energy converting devices which could take the chemical energy from fuel transform into electrical energy,has drawn increasing attention from the public[1-3].The key reaction of DMFC is methanol oxidation and noble metal Pt is through to be the most effective electrocatalyst.However,owing to its high-expense and easy to be poisoned of bare Pt,it is strongly to develop new anode catalysts to reach a success of DMFCs development [4-6].Lately, using semiconductors as carriers for noble electrocatalysts have been drawn a great deal of attention[7-14].Thanks to their optical properties, semiconductors would have strong oxidized ability when they are motivated by photoenergy upon light illumination.With the aid of photocatalytic process, the electrocatalytic performance and anti-poisoning ability towards alcohol oxidation on the surface of electrode will be enhanced.
As one of members of Auivillius oxides, bismuth tungstate(Bi2WO6)is the simplest material among these with unique layered structure and stabilized chemistry properties [15-18].Due to its narrow band gap (ca.2.8 eV) and excellent photocatalytic performance in the field of photocatalysis, it has drawn a mass of attention from researcher.However,there are still some defects which restrict its further applications.Firstly, pure Bi2WO6is merely excited under the wavelength shorter than 450 nm wavelength [19-27].Secondly, the photocatalytic activity would be reduced because of its high recombination ratio of e-/h+.Lastly,most of present applications of Bi2WO6focus on the water splitting and photodegradation organic pollutants, it's crucial to extend its application in new areas.
To address the above limitations, 2D-layered transition metal disulfide (TMD) such as molybdenum disulfide (MoS2) was introduced to hybridize with Bi2WO6.Firstly, both two-dimensional (2D) structure of Bi2WO6and MoS2endow the heterojunction of Bi2WO6/MoS2with abundant 2D coupling interfaces and strong interfacial interaction, which will facilitate charge separation during photocatalytic process[28].Secondly,due to its narrow band gap (1.2-1.9 eV) of MoS2[29-31], light harvesting ability of heterojunction of Bi2WO6/MoS2will be improved.These two advantages give big potential to use such 2D/2D heterojunction of Bi2WO6/MoS2in the application of photo-assisted electrocatalytic methanol oxidation reaction (MOR).Herein, a series of different ratio of MoS2in Bi2WO6/MoS2composites were fabricated facilely and then used as supporting material to decorate Pt nanoparticles.The as-prepared Pt-Bi2WO6/MoS2was further used as working electrode in the application of electrocatalytic MOR.Compared with traditional electrocatalytic process,the composite of Pt-Bi2WO6/MoS2electrode displays higher electrocatalytic activity and stability under visible light irradiation.To the best of our knowledge, this is first example of the new application of the heterojunction of Bi2WO6/MoS2for boosting electrocatalytic performance with the aid of visible light illumination.
As shown in Fig.1A, 2 D Bi2WO6and MoS2were both facile synthesized by hydrothermal methods and then 2D/2D heterojunction of Bi2WO6and MoS2were assembled with assistance of ultrasound process.Then, this 2D/2D heterojunction was acted as supporting material to deposit the Pt nanoparticles.SEM and TEM images of Bi2WO6and Bi2WO6/MoS2were shown in Figs.1B-D and Fig.S1(Supporting information).Firstly,2D rigidity sheet-like and soft sheet-like morphologies for Bi2WO6and MoS2were formed,respectively.Secondly, the sizes of the Bi2WO6nanosheets range from 500 nm to 2 μm, and for MoS2are around 100 nm.Fig.1E shows that small sizes of particles were deposited well on the surface of Bi2WO6/MoS2nanosheets, indicating Pt nanoparticles were formed in the composite of Pt-Bi2WO6/MoS2.The size distribution of Pt is shown in Fig.S2(Supporting information)with average diameter is around 5.90 nm.
The surface chemical compositions of Bi2WO6/MoS2and Pt-Bi2WO6/MoS2were measured by XPS and the results are shown in Fig.S3 (Supporting information).Figs.S3A and B show two characteristic peaks at 159.2 and 164.5 eV,36.1 and 38.3 eV,which correspond to Bi 4f7/2and Bi 4f5/2,W 4f7/2and W 4f5/2,respectively[32].Moreover, Fig.S3C exhibits two characteristic peaks at ca.229.0 and 235.3 eV, which were attributed to the doublet of Mo 3d5/2and Mo 3d3/2, respectively [33,34].For Pt-Bi2WO6/MoS2species, the binding energy of 71.9 and 75.3 eV are corresponding to Pt 4f7/2and Pt 4f5/2, respectively (Fig.S3D) [35], indicating the successful formation of metallic Pt.Besides,when Pt nanoparticles were deposited on the composite of Bi2WO6/MoS2,it is interesting to find a phenomenon that the binding energies of Bi 4f,W 4f and Mo 3d were all shifted to lower values about 0.5 eV.This phenomenon is explained that the introduction of Pt to the composite induced the change of chemical environment [36].

Fig.1.(A) Formation process of Pt-Bi2WO6/MoS2.SEM images of Bi2WO6 (B) and Bi2WO6/MoS2 (C).TEM images of Bi2WO6/MoS2 (D) and Pt-Bi2WO6/MoS2 (E).The insert in Fig.1D is the HRTEM image of Bi2WO6/MoS2.
To obtain the crystal structures and phase of as-prepared samples, XRD patterns of MoS2, Bi2WO6, Bi2WO6/MoS2, and Pt-Bi2WO6/MoS2were measured (Fig.S4 in Supporting information).For pure MoS2, three peaks at approximately 14.2°,33.4°, and 59.0° are exhibited, which could be put down to the(002),(101),and(110)planes of MoS2,respectively[37,38].Besides,Bi2WO6shows that the main diffraction peaks at ca.28.2°, 32.7°,47.1°, 55.8°, 58.5°, 68.6°, 76.1°, 78.3° could be ascribed to the diffraction of(113),(200),(220),(313),(226),(400),(333)and(420)crystal faces, respectively [39].The formed Bi2WO6/MoS2and Pt-Bi2WO6/MoS2composites show the similar diffraction profile as that of Bi2WO6,indicating that the introduced-MoS2does not exert a distinctly influence on the crystal structure of Bi2WO6.However,there are no significantly characteristic peaks belong to MoS2in the Bi2WO6/MoS2and Pt-Bi2WO6/MoS2.This could be ascribed to the low loading content and weak diffraction intensity of MoS2in the heterojunction.With the Pt nanoparticles decorating on the surface of Bi2WO6/MoS2afterwards, a weak peak located at ca.39.9° could be observed, which is the characteristic diffraction peak of Pt (111), further indicating the formation of Pt in the composite of Pt-Bi2WO6/MoS2.
The optical properties of as-prepared MoS2, Bi2WO6and Bi2WO6/MoS2were investigated by the UV-vis diffuse reflectance spectra.As shown in Fig.S5(Supporting information),on account of its narrow band-gap, pure MoS2shows nearly complete absorption in the UV and visible-light region.Then,the absorption edge of pure Bi2WO6was observed at about 459 nm,obtaining the band-gap of Bi2WO6is 2.70 eV.After hybridization with MoS2,the absorption edge of Bi2WO6/MoS2is located at ca.490 nm, which shifts to longer wavelengths as compared to pure Bi2WO6.This shift is due to the interaction of Bi2WO6and MoS2[32,40].
The cyclic voltammograms (CVs) curves of as-prepared Pt-Bi2WO6and Pt-Bi2WO6/MoS2for photo-assisted electrocatalytic MOR in alkaline condition under dark and light irradiation were shown in Fig.2A.Firstly,as for all electrodes,two significant peaks were defined as forward(ca.-0.19 V) and backward(ca.-0.29 V)in the range of -0.7-0.3 V.Universally, the forward peak current density was used to estimate the electrocatalytic performance of electrode for MOR.The forward peak current density of Pt-Bi2WO6/MoS2was detected as 1781 mA/mgPt.Serving as a contrast, the corresponding forward peak current density of Pt-Bi2WO6was less than that of Pt-Bi2WO6/MoS2at the same condition,which is only about 522 mA/mgPt.Note that,when two electrodes were under visible light irradiation, the peak current densities were increased obviously.Under visible light irradiation,the peak current densities of Pt-Bi2WO6/MoS2is reached to 2743 mA/mgPt, which is improved 2.5 times compared to Pt-Bi2WO6at the same condition.Moreover, the peak current density of Pt-Bi2WO6/MoS2heterostructure under light irradiation condition is 1.5 times higher than that of under dark environment.This phenomenon indicates that under visible light irradiation,the Pt-Bi2WO6/MoS2electrode could promote the electrocatalytic activity in the MOR.The influence of GCE alone for KOH and CH3OH/KOH were investigated, which had no catalytic activities(Fig.S6 in Supporting information).Additionally,the influences of different weight ration of MoS2in the heterostructures of Pt-Bi2WO6/MoS2on MOR were researched and the results were shown in Fig.2B.The optimal ratio of MoS2in Bi2WO6/MoS2was observed to be 15 wt%.Besides that,the optimization of Pt amount was also investigated,and the best optimal ratio of Pt was 20 wt%(Fig.S7 in Supporting information).In addition, compared with commercial Pt/C electrode (1228 mA/mgPt), the Pt-Bi2WO6/MoS2electrode shows the much higher catalytic activity for MOR under visible light irradiation.The current of Pt/C under visible light irradiation was 1280.4 mA/mgPt, indicating that there was no photothermal effect on the enhancement of photo-assistance of MOR.
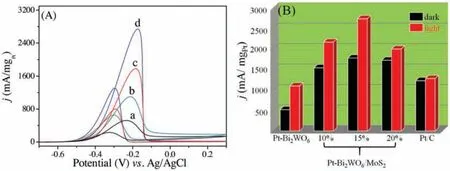
Fig.2.(A) 90th CVs of (a, b) Pt-Bi2WO6 and (c, d) Pt-Bi2WO6/MoS2 electrodes under dark (a, c) and visible-light irradiation (b, d) in 1.0 mol/L CH3OH/KOH solution.(B)Histogram of activities of different electrodes in photo-assisted electrocatalytic MOR under visible-light and dark conditions.
Apart from its catalytic performance,the long-term stability of the catalyst is another crucial point for fuel cells application.For the sake of researching the long-term of stability of samples,chronoamperometric (CA) and chronopotentiometry (CP) curves were carried out with and without visible light irradiation.As exhibited in Fig.3A, CA curves display the change of current density of Pt-Bi2WO6and Pt-Bi2WO6/MoS2with time in 1500 s at the potential of -0.2 V with light on or off.The voltage in the CA measurement was selected based on methanol oxidation potential,which is around -0.2 V.Owing to the adsorption of reactiongenerated intermediate on the surface of catalyst, the current densities were decreased rapidly at the initial stage, and then began to stabilize.It can be drawn a conclusion that the current densities of Pt-Bi2WO6/MoS2electrodes are higher than Pt-Bi2WO6electrodes.Moreover, the current densities of Pt-Bi2WO6and Pt-Bi2WO6/MoS2electrodes with light irradiation are consistently higher than those of without light irradiation,demonstrating that photo-assistance is beneficial for the catalyst's catalytic performance and stability.
The CP measurement is usually used to evaluate the antipoisoning capacity of catalysts for MOR.The CP curves of Pt-Bi2WO6and Pt-Bi2WO6/MoS2electrodes under visible light illumination and dark condition at 200 μA are shown in Fig.3B.Generally,the potential of electrode is gradually increased with the polarization time going on.When the potential increases rapidly,the catalyst has been poisoned.The longer polarization time of electrode means better anti-poisoning capacity.As exhibited in Fig.3B, the duration of Pt-Bi2WO6/MoS2electrode is 7229 s with visible light irradiation,which is 2.3,1.3,and 4.8 times higher than that of Pt-Bi2WO6with light irradiation (3135 s),Pt-Bi2WO6/MoS2(5527 s) and Pt-Bi2WO6(1501s) under dark condition.These data further ascertain the electrode has better anti-poisoning capacity under light irradiation.
Based on the above results, the as-prepared Bi2WO6/MoS2composites not only improve the electrocatalytic activities of MOR effectively with the aid of visible light irradiation, but also show high anti-poisoning capacities.The mechanism of photo-assisted electrocatalytic oxidation of methanol is exhibited in Scheme 1.Firstly, for electrocatalytic process (EC), methanol molecules are easily oxidized through direct or indirect ways by traditional electrocatalytic oxidation.Secondly, for photocatalytic (PC) process,the semiconductor properties of Bi2WO6and MoS2mean that the heterojunction of Bi2WO6/MoS2was excited upon light illumination, it will give rise to generate electrons and holes separately in the conduction band (CB) and valence band (VB) on Bi2WO6and MoS2,respectively.According to previous reports,the CB and VB of Bi2WO6and MoS2were 0.46 and 3.26 eV, -0.12 and 1.78 eV vs.normal hydrogen electrode(NHE),respectively[20,41-43].Base on these energy band positions, the holes from VB of Bi2WO6were easily transferred to the VB of MoS2, thereby achieving high efficiency of charge separation.After that,the holes will be reacted with the OH-/H2O which was adsorbed on the surface to form hydroxyl radicals (·OH).And the adsorbed methanol would be oxidized because of these·OH have strong oxidizing capacity.On the other hand, the photo-generated electrons were easily transferred from the CB of MoS2to the CB of Bi2WO6,and these electrons were lastly transferred to the circuit by external electric field.Thanks to the Bi2WO6/MoS2heterojunction, the high-efficiency separation of the photo-generated charge supporter, thus distinctly decreased the recombination of electron-hole pairs in Bi2WO6and MoS2, respectively, enhancing the photo-assisted electrochemical performance.
To confirm the charge separation in the heterojunction of Bi2WO6/MoS2, the photocurrent response was investigated.As shown in Fig.S8(Supporting information),the current densities of Pt-Bi2WO6and Pt-Bi2WO6/MoS2electrodes display higher current densities when the light turned on.On the contrast, the current densities fall back instantly when the light turned off.The average photocurrent response of Pt-Bi2WO6produced about 2.4 mA/mgPtduring the operation of light “off” and “on”.Nevertheless,Pt-Bi2WO6/MoS2electrode as working electrode with the same condition, the photocurrent response reaches to 42.6 mA/mgPt,which is 17.8 times higher than that of Pt-Bi2WO6electrode.The result indicates that the Pt-Bi2WO6/MoS2nanocomposite has the better photoelectric properties than Pt-Bi2WO6.The slight declining trend of curve is caused by the poisoning of intermediate species on the surface of the catalyst during catalytic process[44].

Fig.3.Chronoamperometric curves(A)at-0.2 V and chronopotentiometry curves(B)at 200 μA of Pt-Bi2WO6(a,b)and Pt-Bi2WO6/MoS2(c,d)in 1.0 mol/L CH3OH+1.0 mol/L KOH solution under dark condition (a, c) and visible light irradiation (b, d).
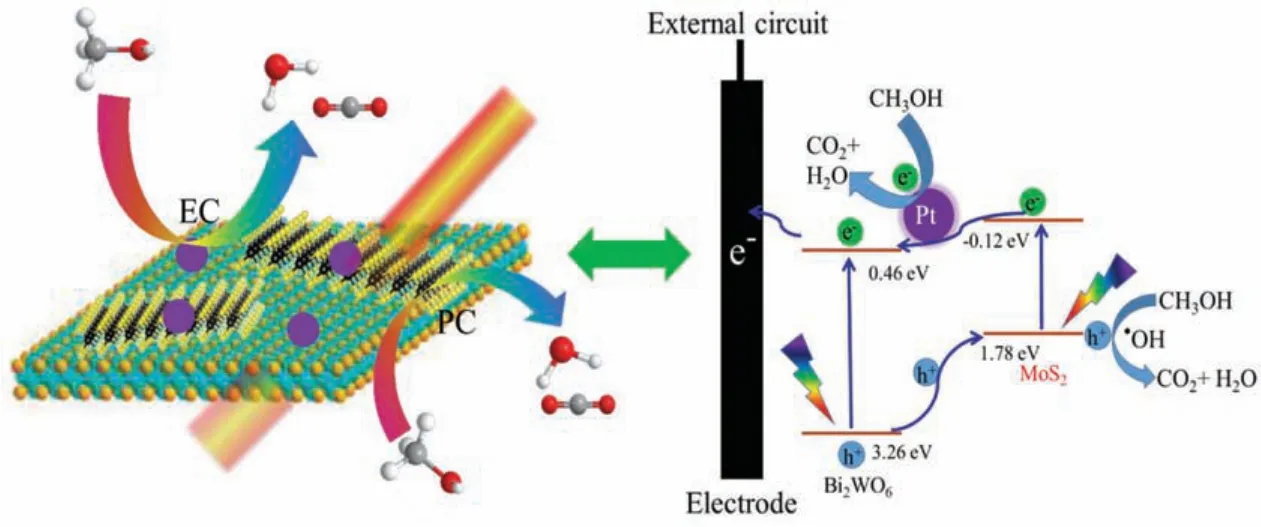
Scheme 1.Scheme of electro- and photo- catalytic MOR on the Pt-Bi2WO6/MoS2 electrode under light illumination.
In order to further evaluate the interfacial charge transport between electrode and electrolyte, the EIS data of as-prepared electrodes were measured.In the analysis of EIS, the diameter of arc (DIA) is usually used as an evaluation of interfacial charge transport, which signifies that the smaller arc the sample, the faster interfacial charge transfer it has.As shown in Fig.S9(Supporting information),under the dark condition,the DIA of Pt-Bi2WO6/MoS2electrode shows much smaller than corresponding of Pt-Bi2WO6electrode.In addition, when both electrodes are under light irradiation, the DIA further decreases and it is clearly seen that the Pt-Bi2WO6/MoS2electrode shows the smallest DIA.The corresponding equivalent circuits are used to fit the EIS data(inset of Fig.S9), where Rctis relative to the charge-transfer resistance at electrode/solution surface, and the data of Rctare summarized in Table S1(Supporting information).It can be easily found that under light illumination condition, Pt-Bi2WO6/MoS2electrode shows the smallest resistance, which further demonstrates that Pt-Bi2WO6/MoS2electrode is beneficial to interfacial charger transport under photo-irradiation [10].
To give the evidence of photocatalytic oxidation abilities of Bi2WO6and Bi2WO6/MoS2,the samples were further employed as photocatalysts to degrade the ciprofloxacin (CIP) (Fig.S10 in Supporting information).There was almost no degradation performance for CIP (6.3%) for pure MoS2under 2 h visible light irradiation.For Bi2WO6towards degradation of CIP,ca.42.3%of CIP were degraded.However, after the hybridization with MoS2, the degradation ability was enhanced to 53.9% under the same condition.Additionally, the stability of sample was studied by XRD patterns, from Fig.S11 (Supporting information), it can be seen that all the characteristic diffraction peaks of sample after reaction were consistent with the samples before reaction,indicating that the catalyst kept stable during the reaction.
The formation of·OH was detected by 5,5-dimethyl-1-pyrroline N-oxide(DMPO)which was usually used as a spin trapping adduct.Fig.S12(Supporting information)displayed four-line spectra with relative intensities of 1:2:2:1 in the system of Bi2WO6after visible light irradiation, suggesting the existence of Bi2WO6could generate·OH.Notably, after the hybridization with MoS2, the intensities of signals were significantly enhanced.This phenomenon indicated that efficient charge separation in the heterojunction of composite, leading to enhanced photo-assisted electrocatalytic performance.
In summary,a new 2D/2D heterojunction of Bi2WO6/MoS2was constructed and then was used to be a carrier for the deposition of Pt.After deposition of Pt,the catalytic activity of Pt-Bi2WO6/MoS2modified electrode for electrocatalytic of MOR was investigated both in dark and visible light irradiation condition.Compared with traditional electrocatalytic process, the new Pt-Bi2WO6/MoS2modified electrode shows 1.5 times enhanced electrocatalytic MOR activities with the aid of visible light irradiation.In addition,the stability of corresponding electrode is also improved obviously under visible light irradiation.Efficient charge separation between Bi2WO6and /MoS2is beneficial to enhance photo-assisted electrocatalytic performance.This research contributes to a new diagram to construct 2D heterojunction as a promising photoassistant carrier in the application of traditional electrocatalytic applications.
Acknowledgment
We appreciate the support of the National Natural Science Foundation of China (No.21603111).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.07.021.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
