Solvent-dependent selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid under neat conditions
2020-01-14KiJinLiuTngYuZengJiLeZengShoFengGongJunYiHeYingWuLinJiXiTnZhongCoWeiMinHe
Ki-Jin Liu,Tng-Yu Zeng,Ji-Le Zeng,Sho-Feng Gong,Jun-Yi He,Ying-Wu Lin,Ji-Xi Tn,Zhong Co,Wei-Min He,,*
a Department of Chemistry, Hunan University of Science and Engineering, Yongzhou 425100, China
b Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China
c School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China
d School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China
Keywords:
5-Hydroxymethylfurfural
2,5-Furandicarboxylic acid
Base-free
External initiator-free
Dioxygen
ABSTRACT
An eco-friendly and economical route for the oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid(FDCA)with atmospheric dioxygen as the sole oxidant under acid-,base-,metal-,and external initiator-free conditions in minimal solvent was reported.In the present reaction,the 1,2-diethoxyethylane has a dual role:reaction medium and free-radical initiator.The FDCA easily crystallizes during the reaction and was simple purified via recrystallization to provide the pure FDCA.
Nowadays, the conversion of renewable biomass into valuable chemicals and bio-fuels represents one of the most promising strategies to reduce the reliance on non-renewable fossil resources.Thus, extensive efforts have been dedicated for the transformation of readily available biomass into platform compounds and value-added chemicals [1].Among these bio-based chemicals,2,5-furandicarboxylic acid(FDCA),which was identified as one of the twelve pivotal value-added chemicals, has received particular attention in recent decades.FDCA is not only used extensively for production of bio-based polymers (polyethylane 2,5-furandicarboxylate) but also serves as versatile synthetic intermediates in organic synthesis, pharmaceuticals and metal organic framework materials [2].
FDCA could be produced by oxidation of abundant and readily accessible 5-hydroxymethylfurfural (HMF) with harmful (super)stoichiometric quantities of organic or inorganic oxidants(Scheme 1a)[3],which cause environmental issues and high cost.For the afore-mentioned drawbacks, dioxygen becomes a better choice because of its natural abundance,low cost and eco-friendly virtues [4].A number of homogeneous (Scheme 1b) [5]and heterogeneous(Scheme 1c)[6]metal-catalyzed oxidations of HMF with high pressure dioxygen as the terminal oxidant have been established.However, in the homogeneous metal catalysis, the metal salts are difficult to isolate and often results in the production of inorganic wastes as side-products.For the heterogeneous metal-catalyzed aerobic oxidations,the high manufacturing cost of noble-based or bimetallic catalysts, the potential leaching of metal ions and the employment of excessive inorganic base severely hamper their industrial applications.Thus, the development of practical and sustainable synthetic protocols for the construction of FDCA through oxidation with atmospheric dioxygen as the sole oxidant is strongly desirable.In light of the widespread utilities of FDCA and also continuing with our research interest in green organic chemistry [7], we herein report for the first time an eco-friendly and economical method for the oxidation of HMF into FDCA with atmospheric dioxygen as the terminal oxidant in 6 equiv.of 1,2-diethoxyethylane under acid-, base-,metal- and external initiator-free conditions (Scheme 1d).

Scheme 1.Oxidation of HMF to FDCA.
Our preliminary investigation started with the oxidation of HMF with dioxygen as the sole oxidant in 6 equiv.of 1,2-diethoxyethylane at 115°C, and 87% yield of FDCA, 7% yield of HMFCA, 3% yield of FFCA and 2% yield of DFF based on 99%conversion of HMF were detected by HPLC after 24 h(Table 1,entry 1).As per our expectation, the nature of the reaction medium played a decisive role on the efficiency of the selective conversion.The other high-boiling-point etherate solvents gave relatively good conversions of HMF with lower selectivity for FDCA(entries 2-6),whereas the low-boiling-point etherate solvents (atmospheric boiling point ≤100°C) (entries 7 and 8) or other non-ether promoters resulted in lower or no conversions of HMF (entries 9 and 10).Reducing the amount of 1,2-diethoxyethylane to 5 equiv.led to relatively lower reaction efficiency(entry 11).Elevating the reaction temperature to 120°C (boiling point of 1,2-diethoxyethylane: 121°C) was not beneficial (entry 12).Decreasing the reaction temperature would lead to a distinct decrease in the conversion of HMF(entries 13-16).Importantly,no conversion was detected when the oxidation was conducted at 75°C (entry 16).Performing this transformation in the dark did not affect the reaction outcome clearly ruled out the possibility that this oxidation involves a photochemical process (entry 17).Taken together, these results suggested that this oxidation might be a thermodynamically controlled process.When dioxygen was replaced by ambient air, the HMF conversion was much lower(entry 18).When the oxidation reaction was performed in fresh distilled 1,2-diethoxyethylane,the conversion of HMF and the yield of FDCA showed no change(entry 19).No oxidation occurred in the absence of 1,2-diethoxyethylane, and the HMF was quantitatively recovered (entry 20).
Unlike the metal catalyzed oxidation of HMF in which the silicagel column chromatographic purification was necessary for the removal of metal catalysts and additives that might restrain subsequent conversions, this present oxidation was carried out under acid-,base-,metal-and external initiator-free conditions.To prove such an operational advantage, several one-pot transformations starting from HMF were carried out (Scheme 2).As anticipated, the crude substrate smoothly underwent the subsequent esterification (HMF →2a and 2b) [8], chloroformylation (HMF →2c) [9]and amidation (HMF →2d) with good conversions [10].
Most of the homogeneous catalyzed oxidation of HMF took place in copious amount of organic solvents or corrosive acids,which resulted in environmental contamination and equipment corrosion [5].In the aqueous conversion of HMF, the extraction workup would consume more organic extraction solvent than isolation of products from organic reaction medium, leading to high manufacture cost for the separation process.Moreover,organic pollutants and inorganic salts contained in waste would endanger the safety of the water resource.Therefore,oxidation of HMF under minimal solvent conditions became a better choice in the view of green chemistry.As stated above, charging HMF(1.2 mmol, yellow) and 6 equiv.of 1,2-diethoxyethylane (white)into a 10 mL glass tube with an O2balloon,a small amount of HMF dissolved in 1,2-diethoxyethylane.The insoluble portion of HMF and 1,2-diethoxyethylane formed a liquid/liquid mixture(Fig.1A).Upon stirring at 115°C for 30 min,a yellow emulsion was observed(Fig.1B).The FDCA crystallized during the oxidation progress and a white solid/liquid mixture was obtained at the end of the oxidation(Fig.1C), then the crude FDCA could be purified simply by recrystallization to give the pure FDCA in 76% isolated yield( Figs.1D and E).

Table 1 Optimization of reaction conditions.a

Scheme 2.One-pot transformations.Conditions: HMF (0.6 mmol), 1,2-diethoxyethylane (3.6 mmol), O2 balloon,115 °C.

Fig.1.The oxidation process of HMF.
It is well known that the etherate could be converted into peroxide in the presence of dioxygen at ambient temperature but the conversion rate is quite sluggish [11].We presumed that the concentration of dioxygen has an influence on the oxidation rate.By varying the N2/O2ratio under one atmosphere pressure, the factor of O2concentration was investigated as shown in Fig.2,and the results confirmed that decreasing the partial pressure of O2had a negative effect on the reaction rate,which is consistent with our speculation.

Fig.2.The HMF conversion of various O2 concentrations.

Fig.3.The time course experiment for the oxidation.
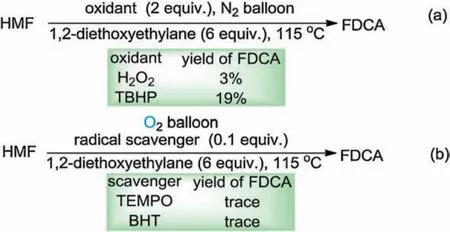
Scheme 3.Mechanism research.
Fig.3 shows the effect of time on the conversion of HMF and the selectivities on all oxidation products under the standard reaction conditions.In the initial reaction stage of 4 h, DFF (36% yield),HMFCA(19%yield)and FFCA(8%yield)were generated along with the consumption of the HMF (63% conversation), suggesting that the conversion of HMF to DFF is the dominant reaction[12].After reaction for 6 h, DFF (28% yield), HMFCA (30% yield), FFCA (6%yield) and FDCA (20% yield) were detected by HPLC.As the oxidation exceeded 12 h,HMF was almost completely converted to the oxidation products.These results suggest that the alcohol hydroxyl group in HMF is more liable to oxidation than the aldehyde group,indicating a different mechanism compared with previous reports[13]and the transformation of aldehyde group to carboxyl group is the rate-controlling step [14].
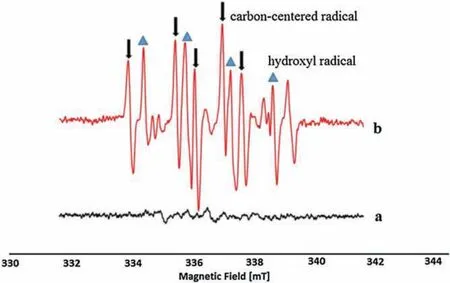
Fig.4.Electron paramagnetic resonance (EPR) experiment.

Scheme 4.Proposed reaction mechanism.
Preliminary mechanistic studies were carried out to disclose some mechanistic information in the present aerobic oxidation (Scheme 3).In consideration of the fact that the noble metal catalyzed conversion of HMF has been established,to rule out the possibility that traces of metal catalysts may promote this oxidation, the 1,2-diethoxyethylane was checked by ICP-MS.The results showed no metal contaminants in the 1,2-diethoxyethylane.Treatment of HMF with H2O2or TBHP under nitrogenous atmosphere resulted in low yield of FDCA(Scheme 3a).When HMF was treated under standard reaction conditions in the presence of 0.1 equiv.of radical scavenger(TEMPO or BHT) (Scheme 3b), the oxidation was seriously inhibited, suggesting that the oxidation might proceed through a free radical process.To further clarify that a free-radical intermediate is involved in the developed reaction, the electron paramagnetic resonance (EPR) experiments were conducted.The treatment of 1,2-diethoxyethylane under dioxygen at room temperature for five minutes, no signal was observed (Fig.4,curve a).When the mixture was heating at 115°C under dioxygen atmosphere for five minutes, the signal of hydroxyl radical(g=2.002, AN=1.46 mT, AH=1.46 mT,) and the signal of carboncentered radical (g=2.002, AN=1.54 mT, AH=2.206 mT) were both detected (Fig.4, curve b).
On the basis of the above above-mentioned results and relevant literatures [5b,15], two tentative mechanistic pathways were proposed(Scheme 4).The present oxidation reaction is started by the generation of peroxide, in situ from the reaction of 1,2-diethoxyethylane and dioxygen at 115°C.In pathway A, the peroxide reacted with HMF to form the carbon-centered radical intermediate 3a with the release of a hydroxyl radical.Subsequently, the combination of the radical 3a and hydroxyl radical generated intermediate 4a, which facilely dehydrated to produce the DFF.The DFF was easily oxidized by hot dioxygen to the FDCA via radical autoxidation.According to the time course experiment for the oxidation, the pathway A might be the main reaction pathway for the present reaction.In pathway B,the HMF was first oxidized by hot dioxygen to generate the HMFCA through radical autoxidation.Then, HMFCA reacted with peroxides to afford 5a,which easily converted into FFAC with concomitant release of a molecular of H2O.Finally,the FFCA was further transformed to the expected FDCA through radical autoxidation again.
In conclusion,we have reported for the first time an acid-,base-,metal-and external initiator-free production of FDCA through 1,2-diethoxyethylane-promoted oxidation of HMF with atmospheric dioxygen as the sole oxidant under neat conditions.The desired FDCA crystallized directly from 1,2-diethoxyethylane during the oxidation process and was purified via recrystallization to provide the pure FDCA without further chromatographic purification.Onepot construction of 2,5-furandicarboxylate,2,5-furandicarbonyldichloride and 2,5-furandicarboxamide from readily available HMF were achieved with unprecedented simplicity.The alcoholic hydroxyl group in HMF could be oxidized preferentially to form DFF as the primary reaction intermediate instead of HMFCA,which is different from the previous HMF conversion strategies.Given the low cost and abundance of 1,2-diethoxyethylane and the simplicity of the operation,this eco-friendly oxidation protocol is expected to be practical for the conversion of HMF to FDCA.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We are grateful for financial support from the Hunan Provincial Natural Science Foundation of China (Nos.2019JJ40090 and 2019JJ20008).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.10.031.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
