Friedel-Crafts trifluoromethylthiolation of electron-rich(hetero)arenes with trifluoromethylthio-saccharin in 2,2,2-trifluoroethanol (TFE)
2020-01-14ShiyoLuWenboChenQilongShen
Shiyo Lu,Wenbo Chen,*,Qilong Shen
a Shanghai Key Laboratory of Materials Protection and Advanced Materials in Electric Power, Shanghai University of Electric Power, Shanghai 200090, China
b Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China
Keywords:
Fluorine
Trifluoromethylthio
Friedel-crafts
Arene
Trifluoroethanol
ABSTRACT
A promoter-free Friedel-Crafts trifluoromethylthiolation of electron-rich arenes and heteroarenes with N-trifluoromethylthiosaccharin 5 using 2,2,2-trifluoroethanol (TFE) as the solvent was described.The reactions were conducted at 40°C and a variety of common functional groups were compatible.
Owing to its high lipophilicity(Hansch's hydrophobicity parameter for SCF3=1.44)and strong electron-withdrawing property,the trifluoromethylthio group (CF3S-) is generally regarded by the medicinal chemists as one of the privileged druggable domains in improving the drug molecule's pharmacokinetic properties such as the cell membrane permeability[1-3].Consequently,the development of efficient methods for the incorporation of the trifluoromethylthio group into the target molecule has attracted great recent interests.Over the last ten years, numerous elegant trifluoromethylthiolating methodshave been reported that were able to directly introduce the trifluoromethylthio group into small molecules under mild conditions[4-10].
Among these methods, Friedel-Crafts type of direct trifluoromethylthiolation of an arene with an electrophilic trifluoromethylthiolating reagent represents a straightforward method for the preparation of trifluoromethylthiolated arenes.The first such reaction was reported by Sheppard in 1964 where trifluoromethanesulfenyl chloride(CF3SCl)was allowed to react with a variety of phenols to give the corresponding trifluoromethylthiolated phenol derivatives in high yields [11].Yet, the high toxicity of trifluoromethanesulfenyl chloride limited its practical applications(Scheme 1, Eq.1) [12].Fifty years later, Glorius and coworkers reported that, in the presence of 10.0 equiv.of NaCl, reactions of N- trifluoromethylthiophthalimide 4 or N-trifluoromethylthiosaccharin 5 with a variety of electron-rich arenes occurred smoothly to give the corresponding trifluoromethylthiolated products in good yields[13,14].It was proposed that trifluoromethanesulfenyl chloride, which is the reactive species that reacted with the substrates is generated in situ by mixing reagents 4 or 5 with NaCl at high temperature(Scheme 1,Eq.2).Alternatively,reactions of an electrophilic trifluoromethylthiolating reagent with an arene, in the presence of a strong Brøsted acid or Lewis acid-a common Friedel-Crafts promoter have been reported (Scheme 1, Eq.3)[15-21].For example, Billard, Langlois and their coworkers described that trifluoromethanesulfanylamide, readily synthesized from diethylaminosulfur trifluoride (DAST) reacted with indoles in the presence of p-TsOH [15].Likewise, Lu and Shen reported that the high yielding reactions of trifluoromethanesulfenate with indoles in the presence of TfOH[18].Very recently,several groups reported that in the presence of a phosphorus reductant,CF3SO2Na,CF3SO2Cl or their derivatives underwent an in situ reduction to generate an electrophilic trifluoromethylsulfinate species that reacted with indoles,followed by further reduction to give the corresponding trifluoromethylthiolated indole derivatives(Scheme 1,Eq.5)[22-30].Nevertheless,these approaches typically use moisture or air sensitive phosphorus reductant,which greatly hampers their broad applications.
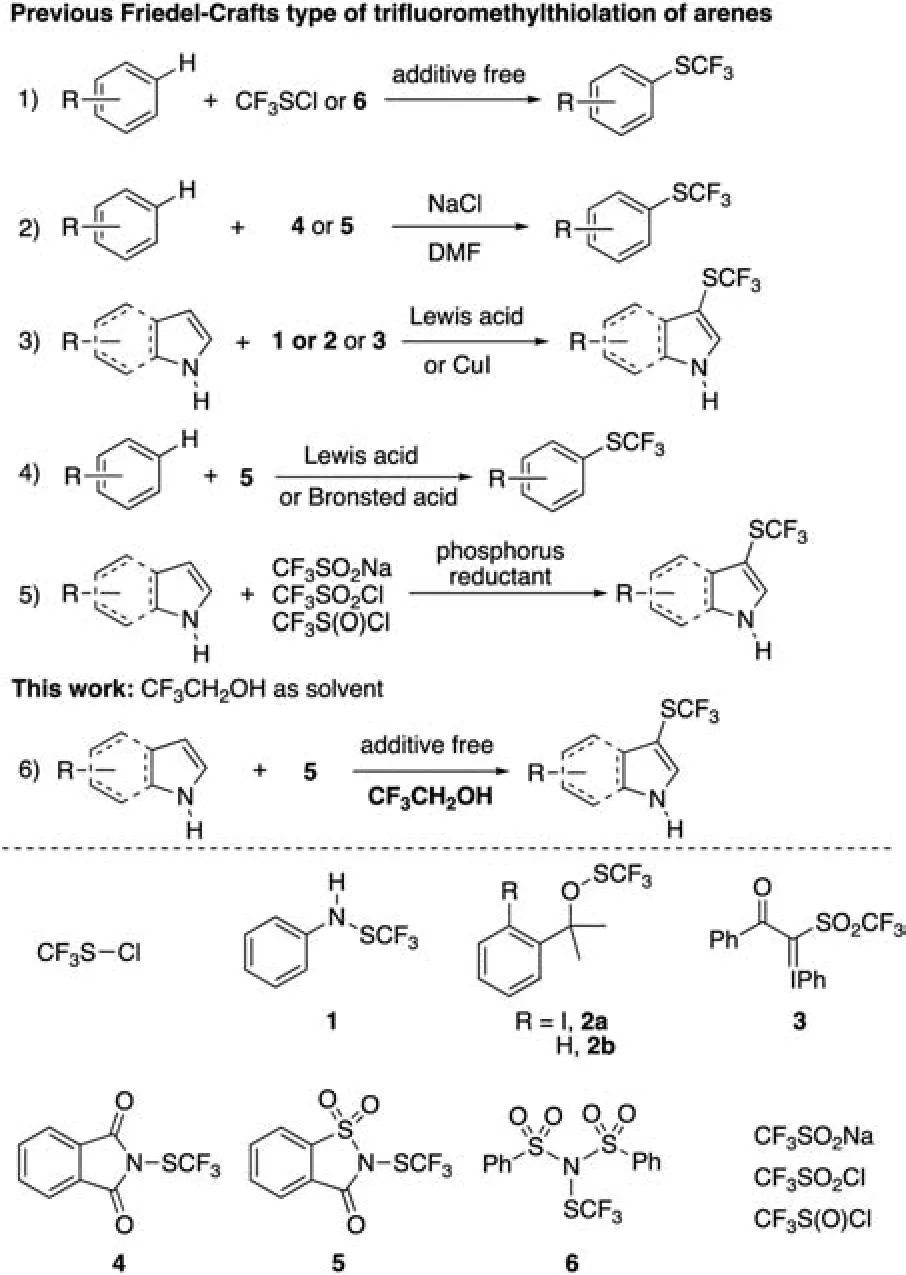
Scheme 1.Friedel-Crafts-type trifluoromethylthiolation of arenes.
In 2014, we discovered that N-trifluoromethylthiosaccharin 5 was a highly reactive electrophilic trifluoromethylthiolating reagent that reacted with various electron-rich arenes when Me3SiCl or CF3SO3H was used a promoter (Scheme 1, Eq.4) [20].Later on, we reported that Friedel-Crafts-type of trifluoromethylthiolation of electron-rich arenes in DMF could take place even in the absence of Brøsted acid or Lewis acid promoter when a highly reactive electrophilic trifluoromethylthiolating reagent Ntrifluoromethylthiodibenzene sulfonimide 6 was used(Scheme 1)[21].Because N-trifluoromethylthio dibenzenesulfonimide 6 is moisture-sensitive and difficult in handling while N-trifluoromethylthiosaccharin 5 is not sensitive to moisture, we wonder whether analogous promoter-free Friedel-Crafts-type of trifluoromethylthiolation with N-trifluoromethylthiosaccharin 5 could be developed.
It has been previously observed that promoter-free Friedel-Crafts alkylation and acylation often occurred smoothly when hexafluoro-2-propanol (HFIP) was used as a solvent, because of HFIP's unique properties including high ionizing power, low nucleophilicity, mild acidity and more importantly, strong hydrogen bond donating ability [31-35].We thus envisaged whether hydrogen bond between the oxygen of N-trifluoromethylthiosaccharin and hydrogen of HFIP could be established to activate N-trifluoromethylthiosaccharin for Friedel-Crafts trifluoromethylthiolation.Herein,we report the realization of such a reaction using HFIP's cheaper homologue 2,2,2-trifluoroethanol (TFE).
We began our investigation by choosing the reaction of fluoroindole with reagent 5 as a model reaction to optimize the reaction conditions.As shown in Table 1,reaction of 6-fluoroindole with 1.5 equiv.of reagent 5 occurred after 12 h at 40°C in 49%-81%yield when the reaction was conducted in solvents such as CH2Cl2,THF, DMF or CH3CN, while reaction in HFIP or TFE gave the corresponding product in 93%and 94%yields,respectively(Table 1,entries 1-6).Next, the effect of the amount of reagent 5 was studied.Decreasing the amount of the reagent 5 led to significantlydecreasing the yield of the product (Table 1, entries 7 and 8).Finally,we studied the effect of reaction temperature.It was found that the yield decreased when the reaction was conducted at ambient temperature, while no significant change in yields when the reaction was conducted at 50-60°C.

Table 1 Optimization of conditions for Friedel-Crafts trifluoromethylthiolation of indole with reagent 5.a
Under the optimized conditions (Table 1, entry 6), we next investigated the generality of the substrates.In general,reactions of electron-rich heteroarenes such as indoles, pyrroles or 1Hindazole with reagent 5 occurred smoothly after 12 h at 40°C occurred smoothly to give the corresponding trifluoromethylthiolated products in 68%-99%yields.Specifically,indoles with both electron-donating group and electron-withdrawing group reacted with reagent 5 under the optimized conditions to give the trifluoromethylthiolated products in high yields.For example,reaction of 6-nitroindole with reagent 5 gave compound 7e in 93%yield,while reaction of 6-methoxyindole with reagent 5 generated the corresponding product 7h in 99%yields(Scheme 2,7e and 7h).Reactions of pyrroles with electron-withdrawing group such as enolizable ketone or ester group occurred in 91% and 73% yields,respective(Scheme 2, 7k and 7l).In addition, N-arylated pyrroles also reacted successfully with reagent 5 to give the desired trifluoromethylthiolated products in 68%-81% yields (Scheme 2,7m-p).Furthermore, 1H-indazole also reacted with reagent 5 to give 3-((trifluoromethyl)thio)-1H-indazole in 70%yield(Scheme 2,7i).In addition, reaction of thiophenes with electron-rich substituents reacted with N-trifluoromethylthiosaccharin to give the corresponding products in 40% and 85% yields, respectively(Scheme 2, 7q and 7r).However, reactions of thiophenes with electron-withdrawing groups, benzothiophene or benzofuran occurred in less than 5% yield even if the reactions were heated at 60°C.Next, we further studied the reactions of electron-rich arenes with reagent 5 under the optimized conditions.Reactions of arenes with two or three methoxy groups reacted successfully to give the corresponding products 7s and 7t in 89% and 96% yields,while reaction of anisole did not react at all when it is heated at 60°C for 12 h(Scheme 2,7s and 7t).Thus,the scope of the reaction is limited to strongly activated heteroarenes and arenes.Nevertheless, because of the mild conditions, various common functional groups such as halides including fluoride(7a),chloride(7c and 7n),bromide(7d and 7p)and iodide(7m),nitro(7e),cyano(7f),enolizable ketone(7k)and ester(7o and 7l)were compatible.Overall,the current method represents a complementary method for Brøsted acid or Lewis acid-promoted trifluoromethylthiolation of arenes with reagent 5.

Scheme 2.Scope for Friedel-Crafts trifluoromethylthiolation of indole with reagent 5 in TFE.Reaction conditions:arene(0.5 mmol),reagent 5(0.75 mmol)in 1.5 mL of CF3CH2OH at 40°C for 12 h; Isolated yields.
Mechanistically, 2,2,2-trifluoroethanol (TFE) might play two roles in Friedel-Crafts trifluoromethylthiolation of indole with reagent 5.As we expected previously, TFE may act as a hydrogen bond donor to activate reagent 5 which weakens the N-SCF3bond of reagent 5 and consequently,facilitates the reaction.Alternative,TFE may react with reagent 5 to give intermediate CF3CH2O-SCF38 which then is activated by TFE to react with arene.To probe whether intermediate 8 could be generated, we studied the reaction of reagent 5 in TFE.Interestingly, no new fluorine signal was observed as monitored by19F NMR spectroscopy.Therefore,we suggest that it is the hydrogen bond between TFE and reagent 5 that facilitates the Friedel-Crafts trifluoromethylthiolation of electron-rich arenes.
In summary, we developed a promoter-free Friedel-Crafts trifluoromethylthiolation of electron-rich arenes and heteroarenes with N-trifluoromethylthiosaccharin 5 using 2,2,2-trifluoroethanol(TFE) as the solvent.The reactions were conducted at room temperature and a variety of common functional groups were compatible.Reactions of reagent 5 with other nucleophiles in TFE are undergoing currently in our laboratory.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China(Nos.21625206,21632009, 21572258, 21421002), the Natural Science Foundation of Shanghai (No.17ZR1447100) and the Science and Technology Commission of Shanghai Municipality (No.14DZ 2261000).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.07.060.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
