Elucidating dominant factors of PO43-, Cd2+ and nitrobenzene removal by biochar: A comparative investigation based on distinguishable biochars
2020-01-14ZhnglinLiuDongTinFeiShenLuluLongYnzongZhngGngYngYongmeiZengJingZhngJinsongHeYingZhuShihuiDeng
Zhnglin Liu,Dong Tin,Fei Shen,*,Lulu Long,Ynzong Zhng,Gng Yng,Yongmei Zeng,Jing Zhng,Jinsong He,Ying Zhu,Shihui Deng
a Institute of Ecological and Environmental Sciences, Sichuan Agricultural University, Chengdu 611130, China
b Rural Environment Protection Engineering & Technology Center of Sichuan Province, Sichuan Agricultural University, Chengdu 611130, China
Keywords:
Biochars
Adsorption
Typical pollutants
Dominant factors
Path analysis
ABSTRACT
Biochars produced from crab shell(CSB),oak sawdust(OB),Jerusalem artichoke tuber(JAB)and sorghum grain (SB) displayed distinguishable adsorption-related characteristics, such as specific surface area(SSA), ash content and acidic oxygen-containing functional groups(AFGs), which linked to the biochar adsorption mechanisms of most pollutants.Herein,PO43-,Cd2+,and nitrobenzene(NB)were employed for adsorption by these biochars to elucidate the dominant factors for the adsorption.Adsorption performance of the three pollutants onto these four biochars varied considerably,as exemplified by the excellent adsorption of PO43-and Cd2+onto CSB(225.3 and 116.0 mg/g,respectively)as compared with onto the other three biochars(4.2-37.1 mg/g for PO43-and 9.7-41.0 mg/g for Cd2+).OB displayed the best adsorption of NB (72.0 mg/g), followed by SB (39.5 mg/g), JAB (31.1 mg/g), and CSB (23.6 mg/g).The kinetics and isotherm adsorption assessments couple with material characterization suggested that the sorption of selected pollutants on biochars was attributed to the multiple mechanisms involved,including coprecipitation, chemical bonds, cation exchange, physical absorption, and complexation.Further path analysis suggested that AFGs and ash content in biochars were more important than SSA with regards to pollutant removal,especially,with ash playing a crucial role in the removal of Cd2+and PO43-,and AFGs being mainly responsible for NB adsorption.These findings might offer guidance on the preparation or modification of biochar with a targeted function for pollutant removal through an understanding the dominant factors.
Biochar is a carbon-rich solid product generated from pyrolyzing various types of biomass at oxygen-limited conditions [1].Given their potential for soil amendment, waste management,pollution control, climate change mitigation and energy production [2,3], biochars have gained much interest in recent years.Particularly, biochars have been assessed as good adsorbents for the removal of various pollutants from aqueous solutions,including heavy metals, aromatics, pesticides, pharmaceuticals and over-enriched nutrients (N and P).Nevertheless, the varying nature of the different types of biochars leads to a varying adsorption performance [4,5].
The characteristics of biochars is greatly affected by feedstocks,preparation conditions,and modification methods[6].The feedstocks for biochar preparation extensively cover lignocellulosic biomass(e.g.,crop straws,and woody residues),the digested biomass (e.g., aerobic digestion residues, mushroom cultivation residues, animal manures, and sludges), and the double digested substrates (e.g., anaerobic digestion residues of animal manures,and earthworm manure).Biochar characteristics also are potentially correlated with the preparation conditions, including heating rate, atmosphere, and holding temperature [7].In addition, biochar can be target modified by acids, bases, steam,carbonaceous materials, metal oxides, clay minerals [8].Correspondingly, the resultant biochars generally displays different performances on removing various inorganic pollutants[9,10]and organic pollutants [11,12], moreover, the adsorption mechanisms are greatly affected by the properties of the employed biochar as well as of the target pollutants.It therefore follows that, through the careful matching of biochar and target pollutant characteristics, a designed adsorption performance can potentially achieved,thus promoting the applicability of biochars as pollutant adsorbents [13].
Given the varied physicochemical properties of biochars,assessment of the mechanisms of pollutant adsorption onto biochars is complicated.Moreover, the dominant factors of removing a pollutant are very hard to be clarified, leading to the limited application of biochars in pollutant control.Therefore, a good understanding the dominant mechanisms for the most common pollutants would be beneficial in order to standardize the process of biochar production, and to match suitable feedstocks,preparation conditions or modifications.
Previous studies have reported that the adsorption mechanisms may greatly depend on biochar properties, including mineral components, surface functional groups (AFGs), specific surface area(SSA)[3,14].In this context,four feedstocks,namely crab shell(CS), oak sawdust (OS), Jerusalem artichoke tuber (JA) and sorghum grain (SG) were selected for preparing biochars, and the detailed information for biochar preparation and characterization can be checked in Supporting information.The resultant biochars exhibited distinguishable characteristics (Tables S1 and S2 in Supporting information), and thereby were employed to investigate the adsorption performances of three typical pollutants, namely phosphate (PO43-), cadmium (Cd2+) and nitrobenzene (NB), representing over-enriched nutrients, metal ions, and organic contaminants, respectively, as well as their possible adsorption mechanisms onto these four selected biochars.Path analysis model, a multi-statistical regression method, was employed to correlate the basic characteristics of biochars and their pollutant adsorption, by which the dominant factors for the adsorption of three typical pollutants by biochars were clarify.Overall, this work aimed to provide a new insight for the preparation of biochars with better adsorption performances for the corresponding pollutants.
Generally,kinetic parameters are very important to understand the adsorption mechanisms of biochars [15].The pseudo-secondorder model canwell describe the adsorptionprocess of PO43-,Cd2+and NB(Fig.S1 and Table S3 in Supporting information).The pseudo second-order equation is widely accepted to describe the chemisorption involving valency forces through the sharing or exchange of electrons between the sorbent and adsorbate.Therefore, the adsorption process of the selected contaminants by the four biochars was greatly controlled by the chemical adsorption mechanism[16].
Besides,in order to evaluate the maximum adsorption capacity to PO43-,Cd2+ad NB by the four biochars,the adsorption data were simulated using Langmuir and Freundlich models(Fig.1),and the isotherm parameters calculated from these models were given in Table S4(Supporting information).For PO43-,the Langmuir model exhibited a good agreement for CSB, JAB, SB and OB, which was better than that of the Freundlich model.These results potentially suggest that a monolayer adsorption occurred with the involvement of chemical and physical adsorption [17].The maximum capacity for PO43-adsorption of CSB was calculated to be 225.3 mg/g, which was 6.1-fold higher than that for JAB(37.1 mg/g), 13.5-fold higher than that of SB (16.7 mg/g), and 54.1-fold higher than that of OB(4.2 mg/g).For Cd2+,the Langmuir model was more suitable for the fitness of the adsorption onto CSB,whereas that onto JAB, SB, and OB was better simulated by the Freundlich model.These results suggest that Cd2+adsorption onto CSB mainly occurred in monolayers, yet that onto JAB, SB and OB were not only affected by the surface porosity, but also by the internal porosity [18].The adsorption capacities of Cd2+onto the tested biochars followed the order of CSB >>JAB >OB >>SB,with CSB exhibiting greater sorption capacity than that of plant-based feedstocks, likely due to the poorly-developed pore structure on JAB, SB, and OB.However, it should be noted that the sorption affinity of the biochars to Cd2+correlated poorly with their SSA,therefore suggesting that factors other than SSA might also be responsible for the sorption of Cd2+onto biochars [14].Finally,regarding organic pollutants,NB removal by the four biochars was better described by the Langmuir model.Moreover,JAB,SB and OB exhibited much greater adsorption capacities (31.1, 39.5 and 72.0 mg/g, respectively), compared with that of CSB (23.6 mg/g).According to these adsorption capacities, it was obvious that the selected biochars exhibited a distinguishable performance in the removal of PO43-,Cd2+and NB,which may be potentially attributed to the significant difference of the physical and chemical characteristics on the employed biochars.
The FT-IR spectra of JAB,SB,and OB before PO43-,Cd2+and NB sorption (Fig.S2 in Supporting information) showed bands at approximately 3435 cm-1, attributed to hydroxyl (alcohols, phenols, and organic acids) stretching [19].The absorption band at 2920 cm-1was mainly attributed to the vibration of methylene-CH2in the aliphatic chain[20].COO-asymmetric stretching was detected at approximately 1590 cm-1.The band at 1384 cm-1was attributed to symmetric bonding vibration of methylene or the phenolic-OH stretching vibration [4].The band at 1100 cm-1was attributed to P-O or Si-O stretching vibration, and the band at 874 cm-1was possibly due to stretching vibration for C-O groups[21].CSB exhibited relatively weak bands from -OH stretching(3430 cm-1), -CH2vibration (2920 cm-1) and COO- asymmetric stretching (1590 cm-1), indicating that CSB contained relatively fewer functional groups.Additionally,the bands at 1420 cm-1and 874 cm-1were due to the presence of the CO32-group due to the abundant calcium carbonate content in CSB [9].Following PO43-sorption, the bands at 3435 cm-1(-OH) (OB), 1384 cm-1( phenolic-OH)(CSB,JAB, SB,and OB),and 874 cm-1(CO32-group)(CSB)tended to be weak,and the bands at 1590 cm-1(COO-)(CSB),1100 cm-1(P-O)(CSB,JAB,SB,and OB),and 570 cm-1(PO43-group)(CSB, JAB and SB) became stronger [9].These changes indicated that the adsorption of PO43-by the different biochars was attributed to various factors such as AFGs and mineral element content.Following Cd2+sorption, the band at 3435 cm-1(-OH)(CSB, JAB, and OB) was weakened, implying that adsorption may be attributed to the existing AFGs [22]; conversely, the bands at 1420 cm-1and 874 cm-1(CSB, JAB, SB, and OB) were stronger,indicating that mineral precipitation(CdCO3,etc.)in biochars was formed during Cd2+adsorption.Finally,following NB sorption,the stretching vibration peak for C-N located at 874 cm-1and the stretching vibration peaks at 1420 cm-1can be attributed to the benzene skeleton (OB), indicating that the NB adsorption onto biochars occurred substantially[23].The band at 3435 cm-1(-OH)(CSB, JAB, SB and OB) tended to be weaker, and shifted to 3425 cm-1, 3414 cm-1, 3424 cm-1, and 3430 cm-1.Further, the bands at 1590 cm-1(COO-) shifted to 1575 cm-1,1574 cm-1and 1598 cm-1(JAB, SB and OB, respectively).These results indicated that AFGs may be involved in NB adsorption.
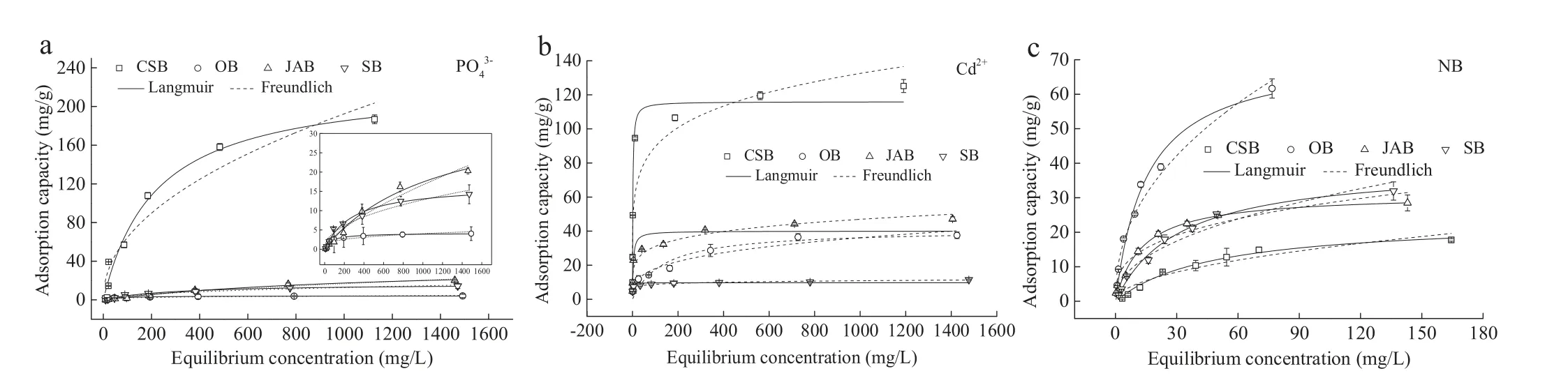
Fig.1.Adsorption isotherms for PO43- (a), Cd2+ (b) and NB (c).
The crystal structure of biochars was assessed by powder XRD(Fig.S3 in Supporting information).Calcite was detected as the major component in CSB and OB,whereas the major component in JAB was sylvite.SB did not form any obvious crystal structure.Following PO43-or Cd2+adsorption,new mineral peaks appeared in CSB,OB,and JAB,with phosphorus or cadmium immobilization being observed.Thus, surface precipitation may be an important mechanism in the removal of PO43-or Cd2+by biochars.
As discussed above, it appears that multiple mechanisms,including coprecipitation, chemical bonds, cation exchange,physical adsorption, and complexation, are involved in pollutant removal by biochars.However, it is hard to differentiate the dominant factors, potentially complicating the modification or preparation of biochars targeted towards the removal of a certain pollutant.
In order to elucidate the dominant mechanism of biochar adsorption of PO43-, Cd2+and NB, the widely accepted physicochemical properties for adsorption, such as SSA, AFG and ash content, were correlated with adsorption capacities using path analysis model.The direct and indirect effects of the investigated factors on PO43-, Cd2+, and NB adsorption were assessed (Fig.2).The direct influence rate of ash content on PO43-adsorption reached 75.5%, which was much higher than that of AFGs (0.28%)and SSA (0.67%).The indirect effects, including the interaction of ash content×AFGs, ash content×SSA, and AFGs×SSA, were negligible, suggesting that the ash content in biochars played a leading role in the adsorption of PO43-,whereas the AFGs and SSA had little effect on PO43-adsorption.The mineral elements in ash(such as Mg2+,Ca2+,and Al3+)have been reported to exhibit some positive correlations with PO43-adsorption,and precipitation with metal oxides or metal ions was correspondingly considered as a major mechanisms for the adsorption PO43-[24,25].Based on these results, the preparation of biochars with a high phosphate removal capacity can be achieved by selecting feedstocks with high ash content such as anaerobically digested wastes, sludge and external skeleton of shellfish [24].Additionally, the targeted biochars can be designed at a relatively higher pyrolysis temperature using the feedstocks with low or medium ash content or modified by adding minerals or forming metal oxides [26,27].
With regards to the adsorption of Cd2+by the four biochars(Fig.2b),the direct influence rate of ash content,AFGs and SSA was 34.6%,6.2%and 3.0%,respectively,suggesting that the ash content in biochar played an important role in Cd2+adsorption.Ion exchange(Ca2+,Mg2+,K+and Na+)and surface precipitation(such as Cd-phosphate precipitate) were the possible mechanisms involved in Cd2+sorption [2].The role of AFGs on the surface was also considerable in Cd2+sorption by biochars,with Cd2+easily forming strong surface complexes with these functional groups[14].However, the indirect effects of AFGs on Cd2+sorption were negatively related to ash content in biochars,moreover,the degree of the indirect effects of AFGs were stronger than the direct effects AFGs.These results implied that the direct effects of AFGs on Cd2+adsorption were potentially due to the decrease in pH of the solution by the greater number of AFGs on biochar, which may weaken the occurrence of adsorption behaviors related to ash content(such as coprecipitation).Based on these facts,in order to achieve biochars with a high capacity for metal ion removal,feedstock selection should consider the ash content and volatile matter in compromise so that the function of ash and the AFGs of biochars can be developed in balance.The desirable feedstocks would therefore consist of agricultural residues with high ash content or their digested residues at medium pyrolysis temperatures [2,14].Further, integrating biochars from different feedstocks into a biochar compound with abundant AFGs and ash fraction may be another possible way to improve their metal adsorption performances.Finally, for NB adsorption (Fig.2c), the direct influence rate of the AFGs content reached 31.2%, followed by ash content (17.2%), and considerably higher than that of SSA(0.11%).Generally, the interactions between NB and biochars may be attributed to π-π interactions; although most AFGs would reduce the surface electron density of the biochar, and thus weaken the π-π interactions, AFGs increase the oxygen content and thus increase the adsorption of polar contaminants [28].Further,AFGs may also facilitate the formation of hydrogen bonds,which partially contribute to NB sorption on polar surfaces[29,30].In addition, previous studies have indicated that biochars rich in minerals might selectively adsorb NB onto the minerals, thus forming organo-mineral complexes and facilitating NB removal[31].Additionally,the values of A,A-IndB and A-IndC were almost zero,implying that the direct effect of SSA and the indirect effects of SSA on NB sorption by ash and AFGs were quite weak.These results indicated that the AFGs content was the most important factor affecting the adsorption of NB by the four tested biochars,followed by ash content,rather than SSA,despite previous studies indicating that pore-filling is greatly involved in organic contaminant removal [11].Furthermore, ash content had a direct contribution on NB adsorption, however, the indirect effects of AFGs on NB adsorption by ash was negative, which can partially weaken the adsorption,because higher amount of ash fraction can hinder the contact of AFGs on the surface of biochar with NB.Thus,for the preparation of biochars with special adsorption performance for polar organic pollutants,feedstocks with a high volatile matter and low ash content should be considered at the low/medium temperature pyrolysis or by hydrothermal carbonization in order to achieve abundant AFGs [29,30].Additionally, other targeted modification pathways to achieve AFGs by acids or oxidation (e.g., HNO3, HCl, H2O2) deserve further investigation[8,10].
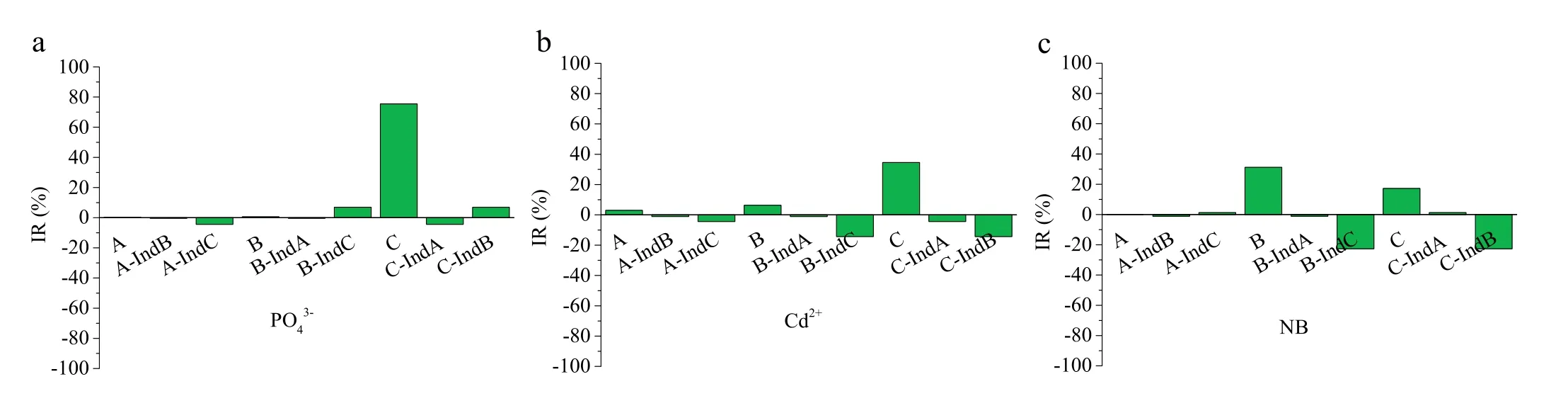
Fig.2.Direct and indirect influences of SSA,ash content and AFGs on the adsorption of PO43-(a),Cd2+(b)and NB(c)by biochars.A:the direct effect of SSA;B:the direct effect of AFGs; C: the direct effect of ash content; u-Indv represents the indirect effect of independent variable u through independent variable v (except for u).
Besides, another popularly investigated pollutants, such as methylene blue (MB), rhodamine B (RB), congo red (CR), methyl orange(MO),Cu2+,and p-nitroaniline(PNA),were also investigated on the adsorption performances by the four biochars using the path analysis(Figs.S4a-f in Supporting information).Basically,the uniform results can be achieved, in which ash content and the derived AFGs were mainly responsible for the adsorption of ionic or polar organic pollutants (MB, RB, CR and PNA), and metal iron(Cu2+).SSA of biochars was not the dominant factor to affect the adsorption.
In summary,the four biochars produced from CS,OS,JA,and SG feedstocks displayed different physicochemical properties and sorption performances for PO43-, Cd2+and NB.Based on the investigations on adsorption models and biochar characterization,coprecipitation, chemical bonds, cation exchange, complexation,and physical adsorption were involved in the removal of these pollutants.Path analysis indicated that the derived AFGs and ash fraction were mainly responsible for pollutant removal rather than SSA, and further that ash content was the dominant factor in promoting PO43-and Cd2+adsorption.The amount of AFGs on the surface of biochars may improve their NB adsorption capacity.The obtained results are useful for estimating biochar adsorption performance for different pollutants, offering a prepartion guidance to achieve a more accurate matching of biochar with pollutant removal.
Acknowledgment
This work was supported by the Department of Science and Technology of Sichuan Province(Nos.2017SZ0028,2017HH0047).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.04.016.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
