Synergistic co-removal of zinc(II)and cefazolin by a Fe/amine-modified chitosan composite
2020-01-14ChenLingYixunZhoZixiRenJingngHnChngqingZhuFuQingLiu
Chen Ling,Yixun Zho,Zixi Ren,Jingng Hn,Chngqing Zhu,Fu-Qing Liu,*
a College of Biology and the Environment, Nanjing Forestry University, Nanjing 210037, China
b State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023, China
Keywords:
Coadsorption
Heavy metal ions
Antibiotics
Biomass
Bridging effect
Polyamine
Hydrous ferric oxide
ABSTRACT
A novel Fe/amine modified chitosan composite (Fe/N-CS) was facilely synthesized and showed higher affinity to both Zn(II)and cefazolin(CEF)than its precursors.Synergistic co-adsorption of them by Fe/NCS was observed in varied conditions.The adsorption amount maximally increased by 100.1%for Zn and 68.2% for CEF in bi-solute systems.The initial adsorption rate of Zn(II) also improved with CEF.The increasing temperature facilitated coadsorption.The results of the preloading tests, FTIR/XPS characterizations and DFT calculations suggested that (1) both polyamines and Fe sites participated in the adsorption of Zn(II)and CEF,(2)Zn(II)could serve as a new efficient site for CEF,forming[amine- Zn-CEF]/[FeOH-Zn-CEF]ternary complexes, and (3) the co-presence of CEF shielded the electrostatic repulsion between protonated amines and Zn(II),contributing to the enhancement of Zn(II)adsorption.Further, the ion strength exerted positive and negative influences on the adsorption of Zn(II) and CEF,respectively.Additionally, CEF and Zn(II) were successively recovered by 0.1 mol/L NaOH followed by 2 mmol/L HCl.Fe/N-CS could be stably reused five times.The findings imply that Fe/N-CS is promising for the highly efficient co-removal of concurrent heavy metals and antibiotics.
In past decades, various kinds of pharmaceuticals were produced and overused in medical treatment, personal care and stock farming[1-3].Most of the main pharmaceutical compounds were lowly bio-used/degraded and excreted with urine,ending up in the environment.Livestock plants, in particular,with excessive growth promoters containing Zn(II), Cu(II), antibiotics, etc.were added to feeds to ensure the profitability[4].The concentrations of heavy metal ions(HMIs)and antibiotics(ANTs)were up to several mg/L in the livestock wastewaters and hardly reduced by the traditional biological treatments [5].The discharge and accumulation of HMIs and ANTs in the environment much threatened the human health due to the toxicity and risk of drug resistance.Advanced treatment of the wastewaters containing HMIs and ANTs is important for ensuring the safety of water resources.
Advanced oxidation process, membrane separation, carbonbased adsorption and plant remediation were implemented to remove the residual toxics after the bio-treatment[6-8].However,the former two suffered from considerable energy/reagent consumption, secondary pollution, membrane fouling/blocking and so on.The latter two are much simpler and use much less energy but are generally less effective with respect to the target pollutants due to both limited functional groups and strong interference by the coexistent substances.The large dosage of adsorbents and its regeneration as well as the huge wetland area also increase the cost and lead to limited practical applications.
Recently, biomass-based adsorbents have been developed and have exhibited high capacity to HMIs and ANTs.The high amount of carboxyl, amine and hydroxyl groups in the biomass (such as saccharides, chitosan and cyclodextrin) contributed to the adsorption[9-11].Most of studies focused on the sole adsorption of HMIs or ANTs.However,the actual wastewater often contained various solutes such as both HMIs and ANTs.With multiple polar groups in their molecules, ANTs easily complexed with HMIs,influencing their adsorption [12].It was reported that HMIs inhibited the adsorption of ANTs onto the chitosan and ANTs could decrease the uptake of HMIs onto the carboxyl-containing adsorbents [13].Some others found that HMIs can bridge for ANTs but the enhanced capacity was still too low [14].Therefore,synergistic and highly efficient co-removal of HMIs and ANTs using one kind of adsorbent is most promising strategy for treating the wastewater containing them.
Chitosan,full of α- hydroxyl groups and primary amine groups,is one of the most studied biomass matrixes.It was usually crosslinked to improve the chemical stability [15].However, this decreased the available groups for HMI and ANTs.Therefore,further modification is a common strategy [16].As well known,polyamines can coordinate with many kinds of toxic metal ions instead of alkali (earth) cations, and also attract ANTs via electrostatic attraction or H-bonding [17,18].Other studies suggested that introducing hydrous ferric oxide (HFO) into the biomass also facilitated the selective adsorption of ANTs, forming an inner-sphere complex between Fe(III)and ANTs based on Lewis acid-base interactions [19].Therefore, an HFO/polyamines dualmodified chitosan composite(Fe/N- CS for short)was fabricated in this work and speculated to exhibit high capacity to both HMIs and ANTs.Zn(II) and cefazolin (CEF, Scheme S1 in Supporting information)were selected as models because both were typically present in medical and livestock sewage,however,no information was available about their coadsorption [20].
Fe/N-CS was synthesized using the same method as that described in our previous work [15].Briefly, chitosan powders were dissolved in acetic acid solution.Fe2(SO4)3and polyethyleneimine (PEI) were sequentially mixed with chitosan solution,and the mixture was stirred at 50°C until uniform.The obtained fluid was then dropped into a mixed alkaline solution by a springe.The formed hydrogel beads were further crosslinked using epichlorohydrin.Then the Fe/N-CS bead was obtained after washing and drying.The Fe(III)content in Fe/N-CS was 6.5%.The unmodified chitosan beads (CS) and the beads with sole PEI modification (N-CS) were prepared for control.More detailed information about the synthesis and reagents is shown in Text S1 (Supporting information).
The adsorption of Zn(II) and CEF in sole solute and bi-solute systems was conducted in a shaker at 25°C, expect for the temperature study.0.012 g beads were mixed with 20 mL solutions and sealed in the glass vials with PTFE-lined septum caps.The aqueous pH was initially adjusted to a pre-set value using diluted NaOH and HCl.The adsorption proceeded for 48 h via equilibrium tests,and the samples were withdrawn at various time intervals for kinetic studies.In the preloading experiments, the fresh beads were initially equilibrated with Zn(II)/CEF; subsequently, the Zn(II)/CEF preloaded beads were fed with CEF/Zn to attain second equilibrium.All the detail conditions are described below the corresponding figures.The analytical method for models and characterizations is provided in Text S2(Supporting information).
The surface charge of CS and its two derivatives was first determined.As shown in Fig.S1a (Supporting information), the zero-point-charge of CS was at pH 7.09,whereas N- CS and Fe/N-CS were apparently positively charged at all the tested pHs.Specifically,the zeta potential of CS exhibited two fast slide stages at 3.5-6.1 and 6.4-7.5, which probably corresponded to the deprotonation of -NH2and the dissociation of -OH, respectively.Grafting PEI macromolecule increased the zeta potential because the amines exerted different affinities toward H+depending on its location[21].Generally,the primary amine deprotonated first at a relatively lower pH.A considerable amount of secondary and tertiary amines was still positively charged at a higher pH(>7).The surface charge of Fe/N- CS changed similarly to that of N-CS because of the low HFO content.The pH buffering capacity of the three adsorbents could also indicate the charge information.As shown in Fig.S1b(Supporting information),the higher buffering abilities of N-CS and Fe/N-CS also proved that they owned more polar functional groups than the original CS.

Fig.1.Adsorbent comparison for the adsorption of Zn(II)(a)/CEF(b)in sole solute and bi-solute systems at different initial pH.The initial concentration of solute is 100 μmol/L; Dosage: 0.6 g/L.
The adsorption of Zn(II) and CEF by three adsorbents was compared at different environmentally-relevant pH (Fig.1).CS showed a very low capacity toward Zn(II).The capacity was almost the same at the initial pH 3 and pH 5 (~10 μmol/g) and then increased significantly (~40 μmol/g) at pH 7.0, related to the competition of H+for the amine groups.As expected, Zn(II)adsorption was enhanced by the modification of polyamine and further increased by the embedding of HFO.When compared with the natural amines in CS, PEI macromolecule contains a high density of multi-amine groups, leading to a high coordination affinity toward Zn(II) and forming stable chelating configuration.The coordination accompanied the deprotonation of amines which was proved by the decrease in solution pH during Zn adsorption by N- CS and Fe/N-CS(Table S1 in Supporting information).In case of Fe/N- CS,the Zn(II)loaded amount was increased by 60%than those by N- CS,suggesting that HFO can contribute to Zn(II)adsorption.It was reported that Fe-OH groups can capture Zn(II) via the inner complexation [22].Besides, Fe-OH and amine might synergistically coordinate with Zn(II) as bidentate ligands.
The adsorption of CEF onto the three adsorbents decreased at higher pH.As the p Kaof carboxyl groups in CEF molecule was 3.0,most of CEF was in an anionic form at pH 6.0.The electrostatic force between CEF-and the protonated amines of CS mainly contributed in its adsorption.It significantly dropped at pH ≥5 because the amines deprotonated and the surface hydroxyls dissociated.Differently, N- CS and Fe/N-CS can retain high capacity to CEF at pH 5.0 and 7.0, much higher than those in CS group at the same conditions.The crosslinking effect of PEI and HFO not only consumed part of the hydroxyls in CS but also served as new active sites for CEF [23].Moreover, N- CS and Fe/N-CS were able to simultaneously adsorb Zn and CEF without any obvious competition between them.In case of the same bi-solute systems,the total adsorption amount of Zn(II) and CEF by Fe/N- CS was always highest at all the tested pH levels,5.65 times and 1.28 times of that obtained by CS and N- CS, respectively, in maximum.

Fig.2.Adsorption isotherms of Zn(II) (a) and CEF (b) onto Fe/N-CS in different systems.C0,Zn is 50-750 μmol/L,C0,CEF is 0,100 and 750 μmol/L for panel a; C0,CEF is 50-750 μmol/L,and C0,Zn is 0,100 and 750 μmol/L for panel b;Dosage 0.6 g/L.Solid and dash lines are the curves fitting by LM and FM, respectively.
The adsorption isotherms of Zn(II)and CEF onto Fe/N-CS in sole solute and bi-solute systems at a typical neutral pH(6.0)are shown in Fig.2.The adsorption of Zn(II)and CEF obviously enhanced each other, especially at a high initial concentration.The amount increased by 15.6%-100.1% for Zn and 68.2%-27.7% for CEF at the initial concentrations from 50 μmol/L to 750 μmol/L compared with the controls in the sole solute systems.The data were fitted by Langmuir model(LM)and Freundlich model(FM).According to the correlation coefficients ( R2), the sole adsorption of Zn(II) and CEF by Fe/N-CS both better conformed to LM with a higher R2,suggesting that their adsorption was approximately a one-layer uniform distribution onto the surface sites and less affected by the intermolecular force.However,the adsorption isotherm in the bisolute system more accorded with the FM than the LM,especially for Zn adsorption in the presence of 750 μmol/L CEF.It means that their adsorption became much heterogeneous which was probably attributed to the complexation between Zn and CEF in the aqueous and solid phases.The fitted values of Qmaxand Kfin the two models(Table S2 in Supporting information) can reflect the adsorption ability.In reliable fitting(R2>0.98),we found that the Qmaxof CEF improved by 1-2 times when Zn(II)coexisted while Kfof Zn(II)was also promoted by 0.6-2.5 times when CEF was present, implying that Fe/N-CS showed greater adsorption ability toward both Zn(II)and CEF when the two pollutants coexisted in the wastewaters.
The kinetic adsorption course is shown in Fig.S2 (Supporting information).The pseudo-second-order kinetic model better fitted all the adsorption (R2>0.98) than the pseudo-first-order model,suggesting a chemical reaction driven interaction between solutes and Fe/N-CS.The fitted parameters are present in Table S3(Supporting information).CEF obviously enhanced Zn adsorption after 200 min.The fitted equilibrium adsorption amounts were much close to the measured values,and increased with more CEF in the system.In general,the apparent adsorption rate( k2)reduced with the increased loading amount for the same adsorbent [24].The initial adsorption rate h (h=k2·Qe2) was then considered.h Values in different systems were in the order of 100 μmol/L CEF >0 CEF>750 μmol/L CEF, indicating that a moderate amount of CEF can accelerate the binding of Zn onto Fe/N-CS,however,excess CEF may retard it due to the site competition effect of free CEF.Much differently, the effect of Zn(II) onto CEF adsorption showed two different stages:The adsorption of CEF was apparently suppressed by Zn(II)in the initial 600 min but then revived and even exceeded the controls without the addition of Zn(II).The h values of CEF adsorption kept decreasing with the increase of Zn(II).We speculated that the complexation of Zn and CEF in the aqueous phase likely inhibited or delayed the interaction between CEF and N/Fe-CS.
The effect of temperature(T)on the adsorption and coadsorption of Zn(II)and CEF is shown in Text S3(Supporting information).The distribution coefficient ( Kd=Qe/Ce) increased with T by 0.29-2.84 times for Zn(II)and 0.51-1.67 times for CEF(Fig.S3 in Supporting information).The enthalpy change(Δ H°) calculated by the Vant's Hoff equation was 6.06-32.9 kJ/mol for Zn(II)and 17.7-24.1 kJ/mol for CEF,suggesting that the adsorption of Zn and CEF is endothermic and mainly a chemical process in nature (Table S4 in Supporting information).The positive values of Δ S°implied an increased randomness at the solid-solution interface.The value of ΔG°was negative,indicating that the adsorption of Zn(II)and CEF by Fe/N-CS was both spontaneous.Then, comparing the sole and bi-solute systems,mutual promotion can be observed for the coadsorption of Zn and CEF at the same T,and the enhancement ratio(calculated as Kd,Zn/CEFinbi-solutesystem/Kd,Zn/CEFinsolesolutesystem) also increases with an increase in T at the same concentrations.Besides,it is wellknown that a more negative Δ G°indicates a greater driving force[25].As shown in Table S4,the ΔG°of Zn/CEF adsorption increased with both T and the concentration of CEF/Zn(the other solute).The aforementioned results prove that appropriately increasing T is favorable for interactions among Zn(II),CEF and Fe/N-CS.
To investigate the aqueous complexation between Zn(II) and CEF, the FTIR (Fig.S4 in Supporting information) of CEF aqueous solution with and without Zn(II) were measured to study the changes.Peaks at 1427 cm-1(possibly assigned to vC-N),1254 cm-1,and 1046-1104 cm-1(associated with β- lactam) exhibited strengthened intensities and had the shift of 2-24 cm-1when Zn(II) coexisted, whereas the v(C=O)amideat 1634 and 604 cm-1(azole related) remained unchanged [26].The results proved that Zn(II) and CEF partly complexed in the aqueous phase.Therefore,Zn(II),CEF and the[Zn-CEF]complex were coexisted in a typical bisolute system.On one side, Zn species could be captured by Fe/N-CS through the coordination with the neutral amines and the Fe-OH site(Eqs.(1) and(2))[27].On the other hand,CEF(mostly in anions form)could interact with the protonated amines and the Fe(III)/Fe-O site (Eqs.(3)-(5)) [23].Regardless of the interaction between CEF and Zn in the system, their adsorption should mutually restrain each other because they shared similar adsorption sites.However,the fact was that their adsorption on Fe/N-CS was both much promoted, implying that the bridging effects between Zn(II) and CEF occurred at the micro-interface.It can be possibly divided into two kinds:Complex adsorption(CA,Eq.(6))and site-bridging interaction (Eqs.(7) and (8)) [14,27-29].
For Zn(II) adsorption,
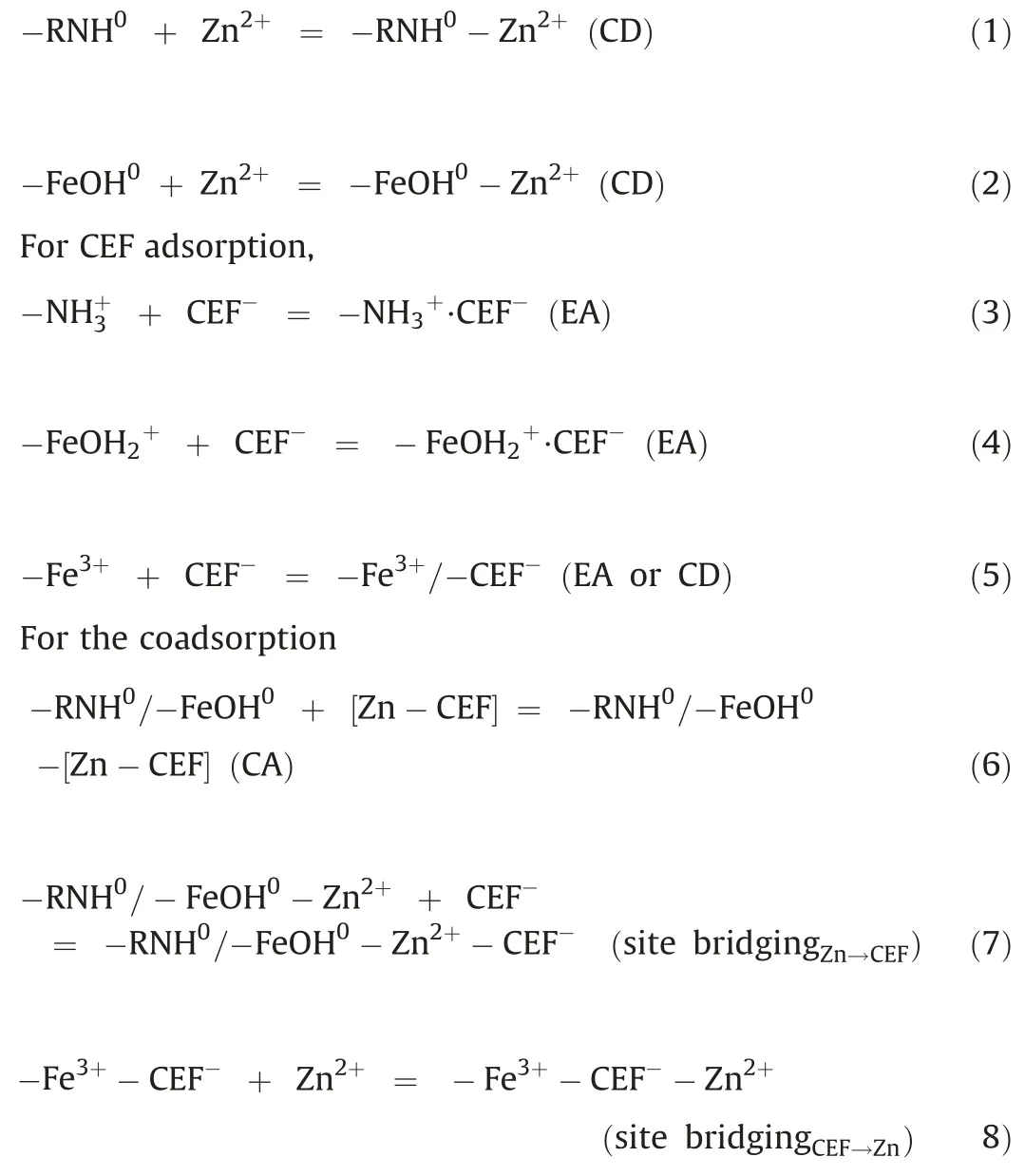
where CD=coordination; EA= electrostatic attraction; CA=complex adsorption
To clarify the dominant mechanisms behind the synergistic coadsorption of Zn and CEF by Fe/N-CS,the sequential adsorption(one solute was preloaded and followed by another solute) was conducted to compare with the regular coadsorption(two solutes pre-mixed together).As shown in Fig.3a, CEF uptake by Fe/N-CS was both increased with the amount of adsorbed Zn in the two adsorption modes.Moreover, we observed that more CEF can be adsorbed in preloading mode than that in the co-addition mode at the same QZn(II).Then,the release of Zn(II)after CEF adsorption was detected to be 5.8%-10.2% of its preloading amount.The trends implied that Zn(II) served as a new efficient site for CEF (Eq.(8)).Since a few part of CEF complexed with Zn(II) in the co-addition system, there was the competition between free Zn(II) and [Zn-CEF]complex for their common favorite sites (polyamine/HFO).The former may be preferable,and less free CEF could be attracted onto the surface Zn sites, leading to a lower bridging effect efficiency in the co-addition mode than that in the preloading mode [14].
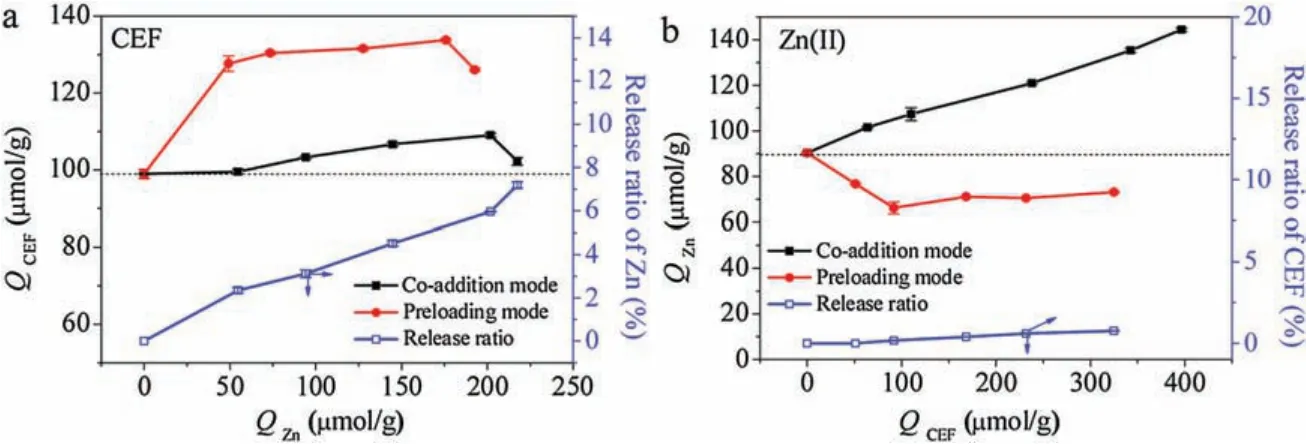
Fig.3.The adsorption amounts of CEF(a)and Zn(II)(b)as a function of the adsorption amount of CEF or Zn(II)in co-addition and preloading systems,respectively.The release of targets in preloading system was shown in the corresponding right Y-axis.C0, Zn is 100 μmol/L,and C0, CEF is 50-1000 μmol/L for panel a; C0, CEF is 100 μmol/L,and C0, Zn is 50-1000 μmol/L for panel b; Dosage 0.6 g/L.
The impact of CEF on Zn(II) was much different in the two modes (Fig.3b): QZnincreased with QCEFin the co-addition mode but decreased with it in the preloading mode.The inhibition effect in CEF preloading system directly ruled out the mechanism Eq.(8)in which Zn(II) was bridged by the newly produced CEF site and Site-CEF-Zn ternary structures formed.Alternatively,we speculated that the promotion of Zn(II) adsorption by CEF in bi-solute system was probably resulted from the electrostatic shielding of protonated amines(-+NH3,-+NH2-and-+NR-)by huge amount of CEF-anions.Further, more amines were available for Zn(II)adsorption.The mechanism was similar to those in the promoted interaction between HMIs and polyamine-contained materials by inorganic anions(NO3-,SO42-,etc.)[15,30].Some may debate that Fe/N-CS could capture the[Zn-CEF]complex for preference in case of a higher affinity (Eq.(6)).However, if so, the promotion efficiency of CEF adsorption in co-addition mode should not be lower than that in the Zn preloading mode.Then,the inhibition of Zn(II)adsorption by the preloading of CEF was easy to understand because part of the primary amine site was occupied by the CEF in advance and few anions contributed to the shielding effect.Besides,less than 1.0%of the preloaded CEF was detected during Zn adsorption probably because the replaced CEF was promptly bridged by Zn(II).
The FTIR spectra of Fe/N- CS treated in different systems is present in Fig.S5 (Supporting Information).The peaks at 1593.7,1072.6 and 597.5 cm-1corresponded to the vibrations of -NH-,C--O and Fe-O bonds in Fe/N--CS, respectively, which obviously shifted after the adsorption of Zn(II)/CEF[31].Moreover,the C--N vibration at 1458.9 cm-1almost disappeared in the samples loading Zn/CEF [32].The changes directly proved our speculation that both polyamines and HFO participated in the adsorption of the two target contaminants.
The changes in XPS fine spectra of main elements were analyzed to verify the coadsorption mechanisms (Fig.S6 in Supporting information).Their spectra were divided into several characteristic peaks summarized in Table S5(Supporting information).As expected,uncharged amines and Fe-OH were the active sites for Zn(II)according to the generation of N species at 401.4 eV,the disappearance of O1 and the shift of Fe2.Further, both the charged and uncharged amines as well as the Fe(III) sites(Fe1) in the adsorbent participated in the binding of CEF as N1,N2 and all the Fe species showed significant variations.Unfortunately, the N and O species are of several types and may have overlapped;thus,it was difficult to distinguish whether the changes were produced by the interaction or blending.To confirm the bridging effect between Zn(II) and CEF, the spectra of Zn 2p and S 2p in the sole solute and bi-solute systems were compared in Figs.S6c and S6d.The two kinds of Zn peaks were both shifted to a lower binding energy in bi-solute Zn system when compared with those in sole system.Similarly,S species which can only come from CEF showed significant changes when Zn and CEF were co-adsorbed.The results well confirmed that Zn(II) served as a new site for CEF(Eq.(7))and that S in CEF participated in the complexation with Zn(II).In addition, the higher intensity of both Zn and S in bi-solute system again proves the greater capture of Zn and CEF by Fe/N-CS.
The complex configuration of Zn(II) and CEF was then speculated and optimized using GaussView 5.0.8.Six possible structures were presented in Table S6 (Supporting information).The one with lower binding energy should be more stable [27].Notably, the Δ E value of complex (bN-S2-O1) was the lowest,indicating that β- lactam N, sulfydryl (S2) and carboxyl in CEF are inclined to bond simultaneously with Zn(II) and Zn(II) sites.This result is consistent with the results obtained from FTIR analysis.Furthermore, the interface interactions behind the synergistic coadsorption of Zn(II) and CEF are illustrated in Fig.4.
The anti-interference ability and regeneration stability of Fe/NCS were tested.Increasing the ion strength, QZn(II)in Zn sole and Zn+CEF bi-solute systems increased by 18.2%-42.3% and 5.8%-17.1%, respectively.This enhancement also resulted from the“electrostatic shielding effect” of anions at protonated amines sites.However, CEF was less adsorbed owing to the competition from dense anions.The inhibition was 12.8%-40.1%in the sole CEF system and 9.8%-24.3% in the bi-solute system.At the same salt condition, the adsorption of CEF in the presence of Zn(II) was always higher than that without Zn(II).Additionally, humic acid showed a slight influence (<6.5%) on the adsorption of them.
The regeneration of Fe/N-CS in this study contained a sequential recovery of CEF followed by Zn(II).0.1 mol/L of NaOH solution was the best choice to separate CEF and Zn.More than 97.2% of CEF desorbed from the beads without releasing Zn(II).Subsequently,more than 98% of Zn(II) was recovered by 2 mmol/L of HCl.The selection of the eluting reagents has been explained in Text S5(Supporting information).Further,the regenerated Fe/N-CS can be used after washing until neutrality.The adsorption capacities of Fe/N-CS for the coremoval of Zn(II) and CEF were less than 7.8% and 12.1%, respectively, in the fifth time.In addition, preliminary economic analysis was conducted for the new process using Fe/N-CS when compared with the processes using activated carbon and polymer resins(Text S6 in Supporting information).The price of Fe/N-CS was approximately 5 RMB/kg.The total cost was the lowest while using Fe/N-CS than that using the other tested adsorbents under similar conditions.
In summary,Fe/N- CS showed an excellent coadsorption ability toward Zn(II) and cefazolin which are commonly used.Their adsorption on Fe/N- CS could be promoted under various conditions.HFO and amines were both effective sites for the adsorption of Zn(II) and CEF.On one hand, the preloaded Zn(II)could bridge CEF through coordination with its β- lactam N, S and carboxyl groups to form a ternary complex on the HFO or amine site of Fe/N- CS.On the other hand,the electrostatic shielding effect of CEF anions onto the protonated amines mainly contributed to the enhanced adsorption of Zn(II).Fe/N- CS exhibited good antiinterference ability and regeneration stability.The findings of our study imply the excellent potential of Fe/N- CS in co-removal of heavy metals and antibiotics from wastewater, and may advance the mutual interaction between combined pollutants at the solidwater interface.

Fig.4.Proposed mechanisms behind the synergistic coadsorption of Zn(II) and CEF by Fe/N-CS.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.51708281),Natural Science Foundation of Jiangsu Province,China(No.BK20170647)and State Key Laboratory of Pollution Control and Resource Reuse Open Funding Project(No.PCRRF18022).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.09.035.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
