Fabrication of niobium doped titanate nanoflakes with enhanced visible-light-driven photocatalytic activity for efficient ibuprofen degradation
2020-01-14WenLiuWeiZhngMushiLiuPenghuiDuChenyunDngJilingLingYunyiLi
Wen Liu,Wei Zhng,Mushi Liu,Penghui Du,Chenyun Dng,Jiling Ling,*,Yunyi Li,*
a Key Laboratory of the Three Gorges Reservoir Region's Eco-Environment of Ministry of Education, Chongqing University, Chongqing 400044, China
b The Key Laboratory of Water and Sediment Sciences, Ministry of Education, College of Environmental Sciences and Engineering, Peking University, Beijing 100871, China
c The Beijing Innovation Center for Engineering Science and Advanced Technology (BIC-ESAT), Peking University, Beijing 100871, China
Keywords:
Titanate
Niobium
Photocatalysis
Element doping
Ibuprofen
ABSTRACT
In this study,a novel class of niobium(Nb)doped titanate nanoflakes(TNFs)are fabricated through a onestep hydrothermal method.Nb doping affects the curving of titanate nanosheet,leading to the formation of nanoflake structure.In addition, Nb5+ filled in the interlayers of [TiO6]alters the light adsorption property of pristine titanate.The band gap of Nb-TNFs is narrowed to 2.85 eV, while neat titanate nanotubes(TNTs)is 3.4 eV.The enhanced visible light adsorption significantly enhances the visible-lightdriven activity of Nb-TNFs for ibuprofen (IBP) degradation.The pseudo-first order kinetics constant for Nb-TNFs is calculated to be 1.04 h-1, while no obvious removal is observed for TNTs.Photo-generated holes(h+)and hydroxyl radicals(· OH)are responsible for IBP degradation.The photocatalytic activity of Nb-TNFs depends on pH condition, and the optimal pH value is found to be 5.In addition, Nb-TNFs exhibited superior photo-stability during the reuse cycles.The results demonstrated Nb-TNFs are very promising in photocatalytic water purification.
Since reported by Daughton and Ternes in 1999 [1], the contamination of pharmaceutical personal care products (PPCPs)attract continuous concern in recent years.Various kinds of PPCPs havebeendetectedinsoil,rivers,lakesandgroundwaterinChina[2].The degradation efficiency of waste water treatment plants(WWTPs) towards PPCPs are relatively low, which leads to the frequently detection of PPCPs in the effluents.Ibuprofen (IBP) is knownasanantiinflammatoryandantipyreticdrug,andwidelyused all over the world.Through WWTPs effluents, IBP enters the environments and pose negative effects on species of flora and fauna in aquatic ecosystems.Since energy crisis and environmental pollution have been great challenges in the 21stcentury, photocatalysis is considered to be a promising technology for the elimination of organic pollutants in waste water[3-6].
TiO2is the most widely studied photocatalyst due to its low cost, high photocatalytic activity, and excellent stability [7].Whereas, pristine TiO2cannot efficiently utilize solar energy because of its large band gap(3.0-3.2 eV)[8].Thus,great efforts are devoted to improve the visible light irradiation of TiO2as well as TiO2based semiconductors.Among these strategies, morphology control and elemental doping are wide used and proven to be effective [7-11].Titanate nanotubes(TNTs), as a one-dimensional TiO2based photocatalyst gained tremendous attention recently,and exhibited good photocatalytic activity towards the degradation of organic pollutant[3]as well as heavy metal reduction[11].In addition, good ion-exchange property of TNTs makes it convenient to narrow the band gap by incorporating proper metal cations [7,9].Nb recently attracted much attention for TiO2modification due to its unique properties[12].Ji et al.found that Nb doping promoted the charge carrier transfer from the bulk phase to the surface of the catalyst[13].Similar ionic radius of Nb5+and Ti4+favors the doping process [7].In addition, the strong interactions between the 4d orbitals of Nb and 3d orbitals of Ti make Nb a good alternative for tuning the electronic structure of TiO2base semiconductors [7,9,14].
In this work,a novel class of Nb doped titanate nanoflakes(Nb-TNFs)were fabricated via a one-step hydrothermal process.Briefly,1.2 g TiO2(P25, Degussa Corporation) and 48 mg of Nb2O5were mixed and then dispersed into 8 mol/L NaOH solution (66.7 mL).After the mixture was magnetically stirred for 12 h, it was transferred into a Teflon reactor with stainless steel cover to undergo a hydrothermal treatment at 150°C for 48 h.Finally,Nb-TNFs were harvested after being washed to neutral with deionized water and dried at 105°C for 4 h.TNTs were prepared through the same procedure except for the addition of Nb2O5.Since the unique electronic band structure, the incorporation of Nb is expected to extend the light adsorption edge of TNTs and thus enhanced its visible-light-driven photocatalytic activity towards IBP degradation.
The morphology of Nb-TNFs was characterized by TEM images.As displayed in Fig.1a, Nb-TNFs exhibit a morphology of twodimensional nanoflakes.In addition, small amount of onedimensional nanotubes are also observed at the edge of the nanoflakes.The unique morphology of Nb-TNFs can be attributed to the synthetic method.Previous studies found that pure TNTs fabricated via the same procedure without Nb have a regular tube morphology, which originated from the curving of tianate nanosheet[15].Whereas,the addition of small fraction of Nb2O5affects the curve of titanate nanosheet, leading the formation of the mixture of nanoflake and nanotubes.The high resolution TEM images in Fig.1b show that there are some nanotube structure in the nanoflakes, and an interlayer spacing of 0.8 nm is observed,which corresponds to the (002) facet of sodium titanate [14,15].The unchanged crystal layer spacing indicates that the addition of Nb does not change the origin[TiO6]structure in TNTs.In addition,no lattice for[NbO6]is observed,indicating Nb may exist as doping atoms in Nb-TNFs.
The XRD patterns of Nb-TNFs, TNTs and the precursors are displayed in Fig.2a.The characteristic diffraction peaks for pristine TNTs can be indexed to sodium titanate,which could be described as the formula NaxH2-xTi3O7·nH2O (x relies on the content) [14].For Nb-TNFs,the characteristic peaks for TNTs remain unchanged,indicating that the presence of Nb does not affect the structure of[TiO6]layers.However, the strong peaks at around 10°, which is assigned to the interlayer of the[TiO6]octahedrons,shifts towards larger angel after the incorporation of Nb.Since Na+and H+within the interlayer are easy to be exchanged with cations, Nb5+is suggested to be incorporated between the[TiO6]layers during the hydrothermal process.In addition, no peaks corresponding to Nb2O5or other impurity is observed in Nb-TNFs.The observations above reveal that all Nb is in the form of doping atoms instead of metal oxides (NbO, NbO2or Nb2O5).
Survey XPS spectrum(Fig.2b)shows that Nb-TNFs were mainly composed of Ti(21.8%)and O(73.6%),which corresponding to the formation of TNFs.In addition,4.1%of Na and 0.5%of Nb was also detected, which are suggested to present between the [TiO6]layers.In the high resolution spectrum of O 1s,the peaks at binding energy of 531.3 and 529.0 eV are assigned to O atoms from surface bonded hydroxyl groups (O--H) and the lattice structure (metaloxygen,Me--OH,Me=Ti or Nb)[9,15](Fig.2c).XPS results show Nb is successfully doped into TNFs.
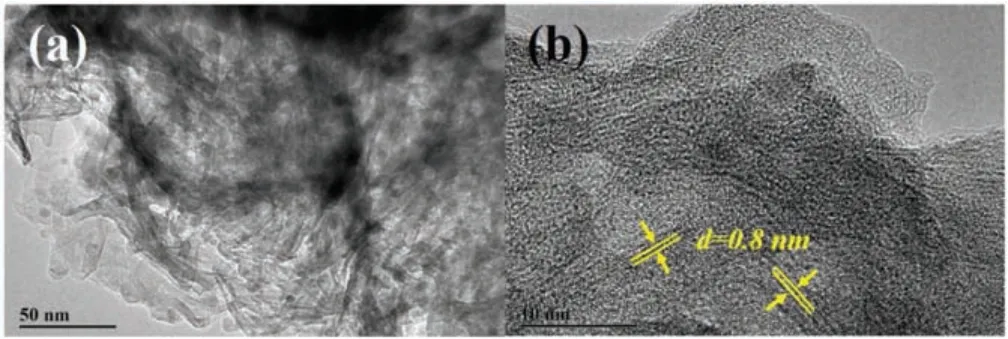
Fig.1.TEM (a) and HRTEM (b) images of Nb-TNFs.

Fig.2.(a)XRD patterns of different photocatalysts;(b)Survey XPS spectrum of Nb-TNFs;(c)High resolution XPS spectrum of O 1s;(d)UV-DRS spectra of TNTs and Nb-TNFs.
The optical adsorption property of Nb-TNFs and neat TNTs are characterized by UV-DRS spectra.As shown in Fig.2d, the light adsorption of TNFs mainly distributes in the UV region.The light adsorption edge for TNTs is estimated to be 365 nm, which corresponds to a band gap of 3.40 eV.In contrast, Nb doping significantly enhanced the light adsorption of TNFs especially in visible light region.The light adsorption edge exhibits an obvious red shift to 436 nm,and the band gap was accordingly narrowed to 2.85 eV.The incorporation of Nb introduced new orbitals into original TNTs, which may act as midgap states and subsequently enhanced the visible light utilization [16].
The photocatalytic activities of as-prepared photocatalysts towards IBP degradation are tested using a simulated solar irradiation with a visible light intensity of 0.58 mW/cm2.Details about the photocatalytic experimental devices are provided in Supporting information.Under the simulated solar irradiation,the concentration of IBF did not decrease within 4 h, indicating a negligible contribution of photolysis (Fig.3a).In addition, neither the presence of P25 nor TNTs exhibit obvious photocatalytic degradation of IBP within the experimental duration.The weak photocatalytic activity of P25 and TNTs can be attributed to their large band gaps,which limit visible light utilization.In contrast,Nb doping significantly enhanced the IBP degradation efficiency.Specifically, over 95% of IBP was degraded by Nb-TNFs under the same condition, and the pseudo-first order kinetics constant (k)was calculated to be 1.04 h-1.
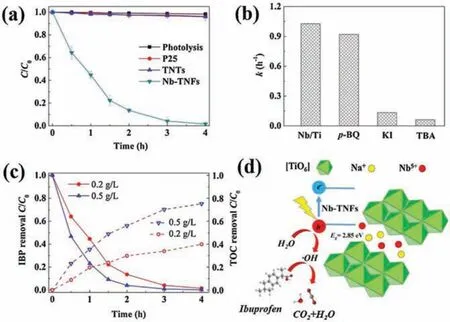
Fig.3.(a) Degradation of IBP by different photocatalysts (photocatalyst dosage:0.2 g/L;IBP concentration:4 μmol/L;initial pH:5;(b)The effect of quenchers on the pseudo-first order kinetics constant (k) of IBP degradation by Nb-TNFs; (c) The removal of IBP and total organic carbon(TOC)with different Nb-TNFs dosages;(d)Schematic illustration of photocatalytic mechanisms for IBP degradation.
In order to clarify the individual contribution of reactive species,1 mmol/L of p-benzoquinone(p-BQ)[17],KI and tert-butyl alcohol (TBA) [18]were chosen as the quenchers for superoxide radical (·O2-), photo-generated holes (h+) and hydroxyl radical(·OH), respectively.Blank control trials (Fig.S1 in Supporting information)reveal that ROS quenchers alone have no effect on the IBP concentration under visible light irradiation.Results show that p-BQ does not significantly affect the photocatalytic activity of Nb-TNFs (Fig.3b).The k value slightly drops by 13.2% to 0.903 h-1.However, when KI and TBA were added into the reaction system,the degradation of IBP was greatly inhibited.Specifically, k value decreases by 86.3% and 93.5% for the addition of KI and TBA,respectively.Thus, h+and·OH are found to be the dominated reactive species responsible for IBP degradation.On the other hand, organic pollutants usually undergo a stepwise degradation in photocatalytic system.Degradation intermediates before complete mineralization (be completely degraded into inorganic substances) might pose higher toxic effect than their mother molecules[19].Thus,mineralization is also very important for IBP removal.To clarify the mineralization of IBP, total organic carbon(TOC)of the reaction suspension within the experimental duration was detected using a Shimadzu TOC-L analyzer.The variation of TOC reflects the mineralization of carbon atoms in IBP and indirectly characterizes the mineralization of IBP.Results show that 200 mg/L of Nb-TNFs almost completely remove IBP within 4 h(Fig.3c).However,the mineralization ratio is only around 40%.It is easy to be accepted that the degradation of IBP molecule is only the initial step of IBP mineralization, which requires much more reactive species.With the dosage of Nb-TNFs increasing to 500 mg/L, mineralization of IBP is elevated to 78%.Based on the results,high mineralization is expected to be achieved by Nb-TNFs in case of enough dosage and reaction time.
Based on the aforementioned statements, the mechanisms involved in the enhanced photocatalytic degradation of IBP are summarized and schematically illustrated in Fig.3d.In presence of Nb5+between [TiO6]layers narrows the band gap of Nb-TNFs,which significantly enhanced the visible light utilization.Water molecules on the surface of Nb-TNFs are then oxidized by h+and transformed into·OH.As the dominated reactive species, h+and·OH degrade IBP into H2O and CO2.
pH condition is an important factor for photocatalytic reactions.To investigate its effect on the degradation of IBP by Nb-TNFs, experiments were also conducted under various pH conditions.Prior to each experiment, the initial pH condition of IBP solution is individually adjusted to the desired value using 0.1 mol/L NaOH or HClO4.As displayed in Fig.S2 (Supporting information), the photocatalytic activity of Nb-TNFs is highly depended on pH condition.The photocatalytic activity of Nb-TNFs under different pH conditions follow the order:pH 5 >pH 3 >pH 7 >pH 9 >pH 11.The observation can be explained by the reaction mechanisms as well as the unique nature of Nb-TNFs.Acid condition favors the oxidative capacity of h+, which is the most active species for IBP degradation [20,21].Thus, the photocatalytic activity of Nb-TNFs continuously decreased with the increased pH value at pH >5.On the other hand, due to the excellent ion-exchange property of TNFs [15], H+in bulk solution will substitute Na+or Nb5+in TNFs,which poses negative effect on the electronic structure.Therefore, pH 5 is found to be optimal reaction condition.
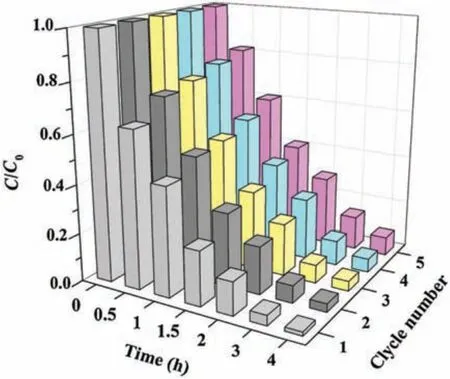
Fig.4.The degradation of IBP in the consecutive recycling experiments(photocatalyst dosage: 0.2 g/L; IBP concentration: 4 μmol/L; initial pH: 5).
In addition to the photocatalytic activity,reusability is also very important for a photocatalyst.After the photocatalytic experiment,Nb-TNFs can be easily collected by filtration through a 0.22 μm nylon membrane.The collected solids were washed and dried overnight to undergo the subsequent recycle tests.The reaction conditions for the recycle tests are the same with the first trial.Results show that Nb-TNFs exhibit excellent photocatalytic activity in the consecutive recycle experiments.Over 93% of IBP is still removed in the fifth reuse cycle(Fig.4).The observation indicates good photo-stability as well as reusability for Nb-TNFs.
In summary, Nb is successfully doped into TNTs and finally forms a novel class of Nb-TNFs via a one-step hydrothermal process.Owing to the unique layered structure of TNTs,Nb5+can be incorporated into the interlayer of [TiO6], which results in the formation of nanoflakes structure.Nb doping not only affects the morphology, but also significantly enhances the visible light adsorption of Nb-TNFs.Via the enhanced generation of h+and·OH,the visible-light-driven photocatalytic degradation of IBP by Nb-TNFs is superior to neat TNTs.Under optimal condition(pH 5),the degradation kinetic constant of IBP removal for Nb-TNFs is found to be 1.04 h-1,while pure TNTs exhibit almost no activity.In addition,excellent reusability is observed for Nb-TNFs within at least 5 reuse cycles.Both high photocatalytic activity and good photo stability make Nb-TNFs a promising alternative for visible-light-driven water decontamination.
Acknowledgments
This work was supported by the Natural Science Foundation Project of Chongqing Science and Technology Commission (CQ CSTC) (No.cstc2018jcyjAX0320) and the Fundamental Research Funds for the Central Universities (No.2018CDXYCH0013).Financial supports from the National Nature Science Foundation of China (NSFC) ( Nos.91647211 and No.51539001) and the Innovative Research Group of NSFC(No.51721006)are also greatly acknowledged.
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.07.050.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
