Design of a 1,8-naphthalimide-based OFF-ON type bioorthogonal reagent for fluorescent imaging in live cells
2020-01-14ZhuzhouShoChunZhngXiohuZhuYjunWngWenyunXuYuChenXiomingWngHilingZhuYongLing
Zhuzhou Sho,Chun Zhng,Xiohu Zhu,Yjun Wng,Wenyun Xu,Yu Chen,Xioming Wng,Hiling Zhu,*,Yong Ling,*
a State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, School of Chemistry and Chemical Engineering,Nanjing University, Nanjing 210023, China
b State Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Nanjing University, Nanjing 210023, China
Keywords:
Bioorthogonal cycloaddition
1,8-Naphthalimide
Photophysical property
Sydnone
Turn-on fluorescence
ABSTRACT
A novel 1,8-naphthalimide-based OFF-ON type fluorogenic sydnone (Naph-Syd) is designed as bioorthogonal probe for imaging.Sydnone moiety efficiently quenches the native fluorescence of 1,8-naphthalimide, which can be restored with the enhancement of about 300-fold, after reacting with strained cyclooctynes to form pyrazole products (Naph-Pyr).The second-order rate constant of this bioorthogonal cycloaddition can be up to 2.5 L mol-1 s-1,which benefits imaging of biomolecules at low concentrations in cellular environment.
1,8-Naphthalimide is a typical chromophore that possesses excellent fluorescent properties and high photostability [1-3].It has been extensively used in detecting germs [4], heavy metal ions [5-8]in aqueous and biological medium, as well as fluorescent sensors to track reactive oxygen species [9-12]and biomolecules in cells and animals [13-18].In 2016, the Zhang group reported a 1,8-naphthalimide-based fluorogenic probe with a 4-substituted terminal alkyne functional group (Naphyne), which undergoes copper(I)-catalyzed cycloaddition with azide efficiently [19].It was found that the alkynyl group could quench the fluorescence of 1,8-naphthlimide,and that the formed cycloadduct 1,2,3-triazole produced a 12-fold increase in fluorescence intensity at the maximum emission wavelength of around 460 nm.Compared to the conventional techniques using fluorescent dyes, this OFF-ON type method has a high signal-to-noise ratio[20],and avoids washing away redundant dyes,providing an important tool for real-time imaging in living systems.Owing to the unique photophysical properties of the Naph-yne and its adduct with azide, the Zhang group successfully realized the sialome imaging in zebrafish [19].
Sydnonesare1,3-dipolarazomethineiminesbridgedbyalactone,and react with alkynes to give pyrazoles afterthe extrusion ofcarbon dioxide [21].In recent years, sydnone and its derivatives have attracted increasing attentions as bioorthogonal reagents [22],which have been applied to protein labeling,drug release,and so on[23-28].Very recently,the Friscourt and Taran groups synthesized coumarin-sydnones,and found that they have the turn-on fluorescent properties upon cycloadditions with strained alkynes[29,30].Herein, with the aid of computations, we design and develop a novel bioorthogonal reagent, 1,8-naphthalimide substituted sydnone (Naph-Syd), which has superior OFF-ON photophysical property before and after cycloaddition reactions,and it has been successfully used for cell imaging under metal-free and no-wash conditions.
Before launching into experiments, we first predicted the photophysical properties of Naph-Syd 1a and its cycloaddition product 1,8-naphthalimide substituted pyrazole (Naph-Pyr 2a,Fig.1) using time-dependent density functional theory (TD-DFT)calculations.Based on the optimized geometries of 1a and 2a(Fig.S1 in Supporting information), the optical properties including the one-photon absorption and emission spectra for 1a and 2a were calculated in water at the B3LYP/6-31+G(d) level using the CPCM model.As listed in Table 1, the fluorescent emission of two molecules comes from the S1→S0transition.For Naph-Syd 1a, the computed maximum emission wavelength is 485 nm, but the oscillator strength is very small, only 0.0053,indicating that 1a may be non-emissive.However,for Naph-Pyr 2a,the oscillator strength at its maximum emission wavelength of 474 nm is 0.6108,115 times that of 1a.This shows that the strong fluorescence of 1,8-naphthalimide would be restored after the cycloaddition of sydnone with alkyne to form pyrazole.
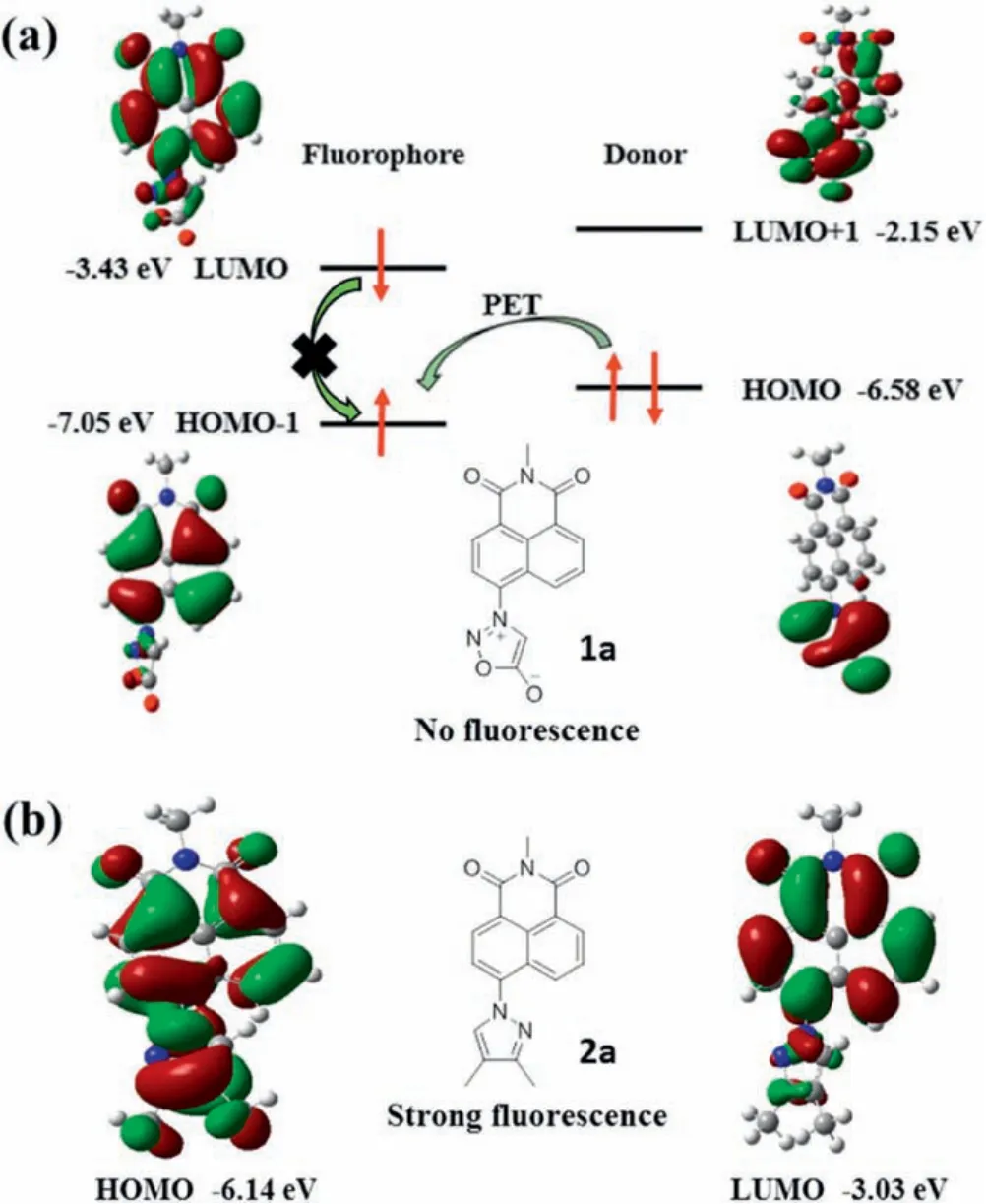
Fig.1.(a)Naph-Syd 1a and(b)Naph-Pyr 2a with their frontier molecular orbitals,and the illustration of the PET process for Naph-Syd 1a.
This desired OFF-ON character of fluorogenic probe can be rationalized by frontier molecular orbital (FMO) analysis.For Naph-Syd 1a, the whole molecular structure has a large torsion angle of 58.6°(Table 1).Therefore, the 1,8-naphthalimide unit serves as a free fluorophore,and the sydnone moiety refers to a free donor, as shown in Fig.1a.The HOMO energy of the fluorophore(i.e., the HOMO-1 of the whole molecule 1a) is -7.05 eV, and it is lower than that of the HOMO energy of the donor(-6.58 eV,i.e.the HOMO of the whole molecule 1a), which evokes the photoinduced electron transfer (PET) process.The fluorescence is quenched as the consequence of the electron transfer from the sydnone moiety to the 1,8-naphthalimide fluorophore.However,after the sydnone-alkyne cycloaddtion, Naph-Pyr 2a has a much smaller torsion angle of 31.5°(Table 1).As a result, there is much better conjugation between pyrazole and 1,8-naphthalimide,which blocks the PET process and produces strong fluorescence.
Encouraged by the theoretical prediction, we designed a synthetic route to Naph-Syd 1b as depicted in Scheme 1.From commercially available 4-bromo-1,8-naphthalic anhydride 3, the 1,8-naphthalimide scaffold was obtained in 65%yield by refluxing 3 with n-butylamine in ethanol [31].Then compound 4 reacted with glycine under Ullmann's conditions to afford crude 5 for further transformation.Finally, the sydnone ring was generated after nitrosation of the amino group with tert-butyl nitrite and subsequent dehydration by trifluoroacetic anhydride.The overall yield of target molecule 1b is 51% from compound 4.
We prepared pyrazole products 2b and 2c from cycloaddition reactions of Naph-Syd 1b with cyclooctynes DIBAC-COOH 6 and endo-BCN 7,respectively(Fig.2a).Then we obtained their UV-vis and fluorescence spectra at room temperature, and summarized them in Fig.2b and Figs.S2-S6 (Supporting information).As expected, Naph-Syd 1b does not show any fluorescence intensity(Φ=0.001,black line,λexc=375 nm,Fig.2b).By contrast,Naph-Pyr 2b(Φ=0.343,red line,λexc=375 nm)and Naph-Pyr 2c(Φ=0.335,blue line,λexc=368 nm)exhibit strong blue fluorescence(Fig.2b).The maximum emission wavelengths of 2b and 2c are both at around 480 nm,at which the fluorescence intensity is increased by about 300 times(315-fold for 2b,250-fold for 2c)as compared to that of Naph-Syd 1b.These experimental results shows that the OFF-ON photophysical property of the newly designed bioorthogonal probe Naph-Syd before and after cycloadditions is superior to those of Naph-yne (12-fold enhancement) [19]and coumarinsydnones (ca.100-fold enhancement) [29,30].
Furthermore,we studied the kinetics of the reactions of Naph-Syd 1b with cyclooctynes DIBAC-COOH 6 and endo-BCN 7.As shown in Fig.S7-S8,the measured second-order rate constants(k2)were 2.5±0.4 L mol-1s-1(with 6) and 0.26±0.04 L mol-1s-1(with 7) in 1:1 DMSO/H2O at 25°C, which are about two times larger than those of coumarin-sydnones with corresponding alkynes [29,30].The reaction rates of Naph-Syd with strained alkynes,especially with DIBAC,are suitable for metal-free labeling the low concentration of biomolecules in the cellular environment[32-34].

Table 1 Photophysical properties of Naph-Syd 1a and Naph-Pyr 2a calculated in water at 298 K.a
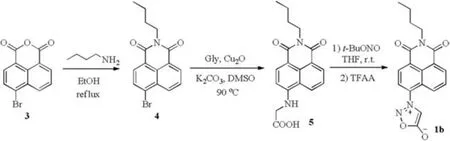
Scheme 1.Synthetic route to Naph-Syd 1b.
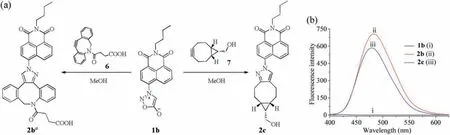
Fig.2.(a)Strain-promoted cycloadditions of Naph-Syd 1b.aOnly one regioisomer is depicted.(b)Fluorescence emission spectra of Naph-Syd 1b(curve i),Naph-Pyr 2b(curve ii), and Naph-Pyr 2c (curve iii) solutions of 1.1×10-5 mol/L in MeOH.
In addition, Naph-Syd 1b is inert to norbornene (stable coexistence in CDCl3for 24 h, Fig.S9 in Supporting information).It is well-established that tetrazines react smoothly with norbornene [35-37]but not with DIBAC [38,39].Notably,fluorogenic tetrazines can be efficiently turned on with green fluorescence enhancement upon Diels-Alder cycloadditions with strained alkenes [40-42].Therefore, the fluorogenic sydnone-DIBAC and fluorogenic tetrazine-norbornene cycloadditions, as mutually orthogonal reaction pairs [38,43-49], can be used for dual color (blue and green) turn-on fluorescent imaging of two biomolecules simultaneously.
Previous studies indicated that the skeletons of 1,8-naphthlimide [39,50], cyclooctynes [51,52], and sydnone [28]have excellent biocompatibility and low cytotoxicity.Our results of MTT assay in living Hela cells showed that the cell viability was more than 80% upon addition of Naph-Syd 1b (50 μmol/L) or Naph-Pyr 2b(50 μmol/L)at 37°C for 12 h(Fig.S10 in Supporting information), confirming that 1,8-naphthalimide substituted sydnone and pyrazole exhibit poor cytotoxicity.Then we evaluated the fluorogenic turn-on efficiency of Naph-Syd 1b as a bioorthogonal probe for imaging DIBAC-tagged mannosyl glycoproteins.Hela cells were metabolically labeled with 50 μmol/L pretreated unnatural sugar (ManDIBAC, Fig.3a) for 12 h as previously reported [52].The control groups were treated with D-mannosamine instead.After the cells were incubated with Naph-Syd 1b at 37°C for 1 h, they were analyzed by confocal microscopy using 405 nm as the excitation wavelength.As shown in Fig.3b, bright blue fluorescence was observed in the ManDIBAC pretreated cells,while there was no fluorescent signal detected in the control cells(D-mannosamine pretreated and others, Fig.S11 in Supporting information).
In conclusion, we have reported a novel OFF-ON type fluorogenic probe Naph-Syd with 1,8-naphthlimide fluorophore and sydnone functionality.Sydnone is the critical quenching group of 1,8-naphthlimide in no-fluorescence state due to PET mechanism, and it can undergo fast bioorthogonal cycloadditions with strained alkynes.After formation of pyrazole products, the strong fluorescence of 1,8-naphthlimide can be restored with the enhancement of about 300-fold.Due to excellent photophysical and kinetic properties, the current method is suitable for fluorescent imaging in live cells under metal-free and no-wash conditions, and it could be employed for simultaneous turn-on labeling of multiple targets through the combination of the fluorogenic tetrazine cycloadditions with strained alkenes.

Fig.3.(a) Cycloaddition of Naph-Syd 1b with ManDIBAC.(b) Fluorescent imaging of the ManDIBAC (top row) and D-mannosamine (bottom row) treated cells with bioorthogonal probe Naph-Syd.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.21803030), the National Thousand Young Talents Program,the Jiangsu Specially-Appointed Professor Plan, and the NSF of Jiangsu Province (No.BK20170631)in China.We are grateful to the High Performance Computing Center (HPCC) of Nanjing University for doing the numerical calculations in this paper on its blade cluster system.
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.06.023.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
