Reductive immobilization and long-term remobilization of radioactive pertechnetate using bio-macromolecules stabilized zero valent iron nanoparticles
2020-01-14HaodongJiYangmoZhuJunDuanWenLiuDongyeZhao
Haodong Ji,Yangmo Zhu,Jun Duan,Wen Liu,Dongye Zhao,d,
a The Key Laboratory of Water and Sediment Science, Ministry of Education, College of Environment Science and Engineering, Peking University, Beijing 100871, China
b Environmental Engineering Program, Department of Civil Engineering, Auburn University, Auburn, AL 36849, United States
c The Beijing Innovation Center for Engineering Science and Advanced Technology (BIC-ESAT), Peking University, Beijing 100871, China
d Institute of Environmental Science, Taiyuan University of Science and Technology, Taiyuan 030024, China
Keywords:
Reductive immobilization
Radionuclide
Stabilized nanoparticles
Zero valent iron
Groundwater
Technetium
ABSTRACT
Reductive immobilization of radioactive pertechnetate(99TcO4-)in simulated groundwater was studied by prepared carboxymethyl cellulose(CMC)and starch stabilized zero valent iron nanoparticles(nZVI),and long-term remobilization of reduced Tc was also evaluated under anoxic and oxic conditions.The stabilized nZVI can effectively reduce soluble 99Tc(VII) to insoluble 99Tc(IV), and they can be easily delivered into a contaminated groundwater zone and facilitate in situ remediation.In this study, CMCstabilized nZVI showed higher reactivity than that using starch as the stabilizer.Batch experiments indicated that more than 99%of 99Tc(VII)(C0=12 mg/mL)was reduced and removed from groundwater by CMC-stabilized nZVI with a CMC content of 0.2%(w/w)at a broad pH of 5-8.X-ray diffraction(XRD)and X-ray photoelectron spectroscopy (XPS) analyses further confirmed that 99Tc(VII)O4- transformed into99Tc(IV)O2(s).The presence of bicarbonate exhibited insignificant effect on Tc immobilization,while humic acid (HA) inhibited reaction mainly due to retardation on electron transfer and formation of Tc(IV)-HA complexes.More interesting, the immobilized Tc(IV) remained insoluble even after 120 d under anoxic condition, while only~21% was remobilized when exposed to air.Therefore, biomacromolecules stabilized nZVI nanoparticles could be a viable alternative for in situ remediation of radioactive contamination in groundwater.
As a radioactive fission product from235U (primary) or239Pu,technetium (99Tc) is released to the environment due to atmospheric nuclear testing and reprocessing of spent fuel, and the increasing inventory of99Tc is waiting for final disposition[1].For example, in the accident on the TEPCO's Fukushima Nuclear Power Station in Japan, the discharge amount of99Tc into the environment is determined as >0.25 T Bq [2].Tc concentration in the groundwater can vary from~50 pCi/L to >14,000 pCi/L at the source.If the concentration of99Tc arises suddenly due to accidental release,widespread contamination of seawaters,rivers,subsurface pore waters, ground waters, and sediments will pose long-time (half-life is 2.14×105years) radiological threat to both plants and animals (lung cancer and related maladies) through uptake and food chain [1].For public health, the drinking water standard for99Tc(β-emitter)is 900 pCi/L(or 5.3×10-10mol/L;33.3 Bq/dm3),suggesting that the amount of99Tc allowed to be present in subsurface groundwater is considerably smaller.The main form of99Tc,99TcO4-(99Tc(VII)),is quite mobile,so the immobilization of99Tc represents a key strategy to keep it from spreading in liquid or aquifer phase.
Compared with99TcO4-,99Tc(IV)oxide is insoluble,which is the predominant product of99Tc(VII) reduction (Fig.S1 in Supporting information) [3].Previous works developed many technologies transformation from Tc(VII) to Tc(IV) so as to realize the immobilization, such as chemical reduction/precipitation [4-6],biological treatment[7]and photoreduction[8].Fe-based materials are widely used as reducing agents for99Tc(VII), such as zero valent iron(ZVI)[3],FeS[9]and pyrite[10].However,the ability of Fe(0) and/or Fe(II) to reduce or absorb99Tc(VII) depends on their physiochemical properties, including surface modification.
During the synthesis of nanoscale ZVI (nZVI) particles, both their spontaneous agglomeration and continued passivation by water molecules once particles formed can largely consume their reduction capacity onto target contaminants.A proper stabilizer is proposed to guarantee nZVI with both larger specific total surface area and much more targeted performance functionally.The food grade starch has been used as stabilizer for bimetallic nanoparticles (~55 m2/g) to degrade Trichloroethene (TCE) and polychlorinated biphenyls (PCBs) [11].In addition, carboxymethyl cellulose (CMC) was also found as an efficient stabilizer to manipulate the size and dispersibility of ZVI particles [12].The CMC-stabilized nZVI performed much smaller hydrodynamic size (18.6 nm) than bare ZVI (>3 μm)based on the dynamic light scattering(DLS)method[12],which showed high reactivity on most of heavy metals and organic contaminants compared with unstabilized ZVI due to the protection of formed nanoparticles from agglomeration[13,14].Therefore, in this study, soluble starch and CMC are applied as stabilizers to facilitate nZVI preparation, and the removal of99Tc(VII) from simulated groundwater by the two stabilized nZVI was compared.
The overall goal of this work was to test the effectiveness of CMC-and starch-stabilized nZVI for reductive sequestration of the highly mobile and soluble99Tc(VII)in simulated groundwater.The specific objectives were to: (1) test the removal efficiencies of99TcO4-by stabilized nZVI with different dosages, (2) study the effects of solution chemistry conditions such as pH, bicarbonate,and natural organic matter (NOM) on Tc immobilization, (3)elucidate the underlying99Tc(VII)reduction mechanism by means of material characterizations, and (4) evaluate the long-term stability of reduced products under aerobic and anaerobic circumstances.
All chemicals used in this work were of analytical grade or higher.Details related to the chemicals are provided in Text S1(Supporting information).CMC- or starch-stabilized ZVI were prepared through our previous reported methods [15-17].Specifically, 10 mL of 22.3 mmol/L FeCl2·4H2O was added to 25 mL 1 wt% stabilizer (CMC or starch) solution in a flask(250 mL), and the mixture was stirred at 600 rpm for 5 min.Then the flask was immediately sealed and fixed on the shaker and connected with vacuum.Then 10 mL of sodium borohydride solution based on the stoichiometric amounts was injected into the flask for 3 min to yield a dark and evenly distributed particle suspension.The suspension was then shaken for another 2 min followed by 2 min standing, in order to facilitate further growth and refinement of particles.Finally, the stabilized nZVI particle suspension is of 60 mg/L as Fe (also available from 10 mg/L to 100 mg/L)with CMC or starch concentration of 0.2 wt%eventually by mixing with contaminated DI water containing99TcO4-stock solution.
Batch kinetic experiments were carried out to test the effectiveness of stabilized nZVI nanoparticles for reductive removal of99Tc(VII).The initial99Tc(VII) concentration was fixed at 12 mg/L with nZVI dosage varying from 10 mg/L to 100 mg/L.Batch experiments were conducted in 30 mL glass vials with Teflon-lined caps, and the vial headspace was near zero to minimize dissolved oxygen(DO)effect.The initial pH was adjusted to~8.0 by 0.1 mmol/L HCl and NaOH.The mixtures were then placed on a shaker(200 rpm)and sampled at pre-determined time intervals and then filtered through a VSWP membrane (mixed cellulose esters,pore size=25 nm,Merck Millipore Ltd.,Germany).Control tests in the presence of CMC, starch, Fe(II) or sodium borohydride but without the nanoparticles were also carried out but under other identical conditions and same synthesized procedure.The99Tc(VII) concentration in the supernatant was measured by liquid scintillation counter (Beckman LS 6500 Multiple-purpose Scintillation Counter, USA), where each 5 mL Tc sample was mixed with 10 mL of the scintillation cocktail(Ultima-GoldTM,PerkinElmer,Inc.,MA,USA).The detection limit of99Tc was~5.98×10-10mol/L (15.33 CPM, or~5.92×10-5μg/L).
Experiments on effects of material dosage,pH,bicarbonate and HA were conducted similarly,and details are presented in the Text S2 (Supporting information).CMC-stabilized nZVI samples before and after99Tc(VII) reduction were characterized by X-ray diffraction(XRD)and X-ray photoelectron spectroscopy(XPS)(Text S3 in Supporting information).A long-term evaluation on reoxidation of reduced Tc was conducted over 120 days, and the method on the stability test on immobilized Tc is provided in the Text S4(Supporting information).
Given that the removal of99Tc(VII)in this study was carried out by stabilized nZVI, control tests of99Tc(VII) reduction in the presence of stabilizer (CMC or starch), or sodium borohydride (a strong reducing agent) used in the preparation of the nZVI nanoparticles were also carried out.Fig.S2 (Supporting information)shows that after 24 h reaction >99%of99Tc(VII)removal was achieved by 60 mg/L CMC-nZVI and starch-nZVI.However, single CMC,starch or sodium borohydride did not show any reduction of99Tc(VII) and single Fe(II) presented the limited reduction efficiency (18.6%) of99Tc(VII), indicating that the observed99Tc(VII)reduction was solely attributed to the nZVI nanoparticles.The reason for almost no reduction of99Tc(VII) by control NaBH4solution was due to the experimental procedure,and NaBH4could quickly react with H2O before Tc(VII) addition due to its high activity.Detailed discussion was presented in Text S5(Supporting information).
Fig.1 shows the reductive removal of99Tc(VII) by CMCstabilized (Fig.1a) and starch-stabilized nZVI (Fig.1b) at various dosages (10-100 mg/L as Fe).Rapid and high (>92%) removal of99Tc(VII) was observed within 0.5 h for both CMC- and starchstabilized nZVI at material dosage >60 mg/L.The pseudo-first order kinetic model is used to describe the kinetic data (Eq.1) [18,19]:

where C0and Ctare the Tc concentrations(mg/L)at time 0 and t(h)in aqueous phase,respectively;and kobsis the observed first-order rate constant (h-1).
Table S1 (Supporting information) summarizes the best-fitted parameters,and the pseudo-first order model can well describe the kinetic data when material dosage ≥40 mg/L (R2>0.9).The rate constant(kobs)increased with increasing material dosage for both CMC-and starch-stabilized nZVI.Especially,for CMC-nZVI(Fig.1a),the kobsincreased from 0.105 h-1to 9.179 h-1(by 87 times) as the material concentration increased from 10 mg/L to 100 mg/L as Fe.The dosage effect was more profound when it increased from 10 mg/L to 20 mg/L, as the kobssharply raised from 0.105 h-1to 2.431 h-1(by 23 times).The mass transfer limited the reactions at higher material dosage.Similarly,for starch-nZVI(Fig.1b),the kobswas increased from 0.080 h-1to 8.348 h-1(by 104 times) as the material concentration increased from 10 mg/L to 100 mg/L as Fe,and quick kinetics were only found when starch-nZVI dosage≥60 mg/L.Generally,though both materials can efficiently remove99Tc(VII)from the aqueous phase,the reduction rate was higher by CMC-nZVI than by starch-nZVI (Table S1).Therefore, in the following experiments and characterizations, CMC-nZVI was focused.The enhancement of the99Tc(VII) reaction rate at higher stabilized nZVI dosage can be attributed to more available reactive sites.

Fig.1.Kinetics of 99Tc(VII) reduction by CMC-stabilized (a) and starch-stabilized nZVI (b).(Initial 99Tc(VII)=12 mg/L; nZVI concentration=10-100 mg/L as Fe; CMC or starch=0.2 wt%; initial pH 8.0; CTL: control text without material addition).
Nano-size ZVI particles are a strong reducing agent and can be much stronger after stabilization [14].In this way, nZVI nanoparticles have relatively large surface area,numerous reaction sites and high chemical reactivity [12].That explains the consistent quick kinetic and high removal efficiency of99Tc(VII) using either CMC-or starch-stabilized nZVI.However,as consequences of high reactivity, a good fraction of reductive capacity of nZVI was consumed by water molecules especially when the particles were freshly prepared [20].For example, in Fig.1a, although 10 mg/L CMC-nZVI was adequate to convert initial 12 mg/L99Tc(VII) by stoichiometry, more than 99% of99Tc(VII) cannot be removed within 24 h.The same phenomenon was observed from Fig.1b,except that the barrier of rapid kinetics initiated by starch-nZVI was more significant than that by CMC-nZVI,which suggested that the negative charges carried by CMC facilitated not only the repulsion forces between nZVI particles when generating CMCnZVI but also the attraction from nZVI to99TcO4-.In addition,CMCnZVI usually has smaller particle size and higher surface area than starch-nZVI [12].Meanwhile, starch prevented starch-Fe(II) from agglomeration without charging particles[15,21],ferrous ions also acted as a reducing agent to convert99Tc(VII) to99Tc(IV), and greater rate and extent of99Tc(VII) reduction were promoted by higher amounts of Fe(II) at a given pH.
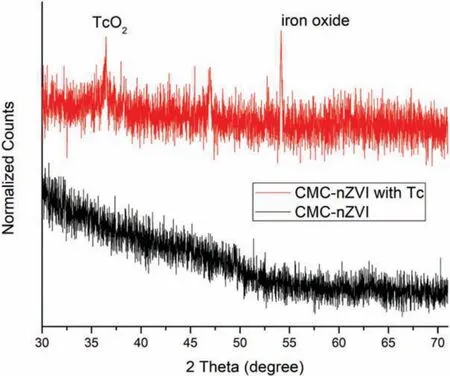
Fig.2.XRD patterns of CMC-nZVI before and after 99Tc(VII) reduction.
Fig.2 displays XRD patterns of CMC-nZVI before and after reacting with99Tc(VII).There are no significant peaks of assigned to Fe in the CMC-nZVI spectra, which is consistent with previous findings due to the large amount of coated stabilizers (CMC) and less ordered Fe0structure [14].After reaction, two distinct crystalline phases appeared, as indicated by the two new characteristic peaks of TcO2plane (JCPDS No.00-022-0910) at 36.2°and Fe2O3(116) plane at 54.2°(JCPDS No.33-0664),respectively [22-24], suggesting successful reduction of99Tc(VII).The weak peak of TcO2can be due to the low concentration of Tc.The XRD agrees with the Fe0-facilitated redox reaction mechanism illustrated below.
Fig.3 presents the XPS spectra of CMC-nZVI before and after99Tc(VII)reduction,and Table S2(Supporting information)lists the atomic percentage of the main elements.The main elements of neat CMC-nZVI were Fe (2.4%), O (32%), C (59.1%) and Na (6.5%),where the coated CMC will contribute to the C, O and Na.Furthermore,due to the high reactivity and water erosion,partial oxidation of Fe0can happen and introduce O to the material[25].For reduction of99Tc(VII)with CMC-nZVI at 12 h,it is clearly show that a Tc 3d peak appeared (Fig.3a), indicating deposition of Tc reduced products onto nZVI particles.The high resolution of Tc 3d further revealed reduction of99Tc(VII) to99Tc(IV) (Fig.3b).Specifically, the peaks at ca.257.6 eV and 255.7 eV assigned to Tc(VII)and Tc(IV),respectively[9,26],which consists of 80.1%of Tc as Tc(IV)and 19.9%as remaining Tc(VII).Therefore,efficient reductive immobilization of99Tc(VII)was achieved,and the formed99Tc(IV)is widely confirmed as TcO2(s),which can precipitate onto nZVI due to low solubility [3].99Tc(VII) reduction kinetics results, XRD and XPS analysis all indicated that the reduced product, TcO2(s),precipitated onto the Fe nanoparticles,so the Tc removal process is a combination of reduction and precipitation/adsorption.
Fan et al.[27]and Huo et al.[10]observed that the precipitation of the resultant TcO2under iron reducing conditions.Based on these observations and the above experimental results, the reductive immobilization of99Tc(VII) by stabilized nZVI is contributed by:(1)direct sorption of99TcO4-on the nanoparticle surface, (2) Fe0-mediated heterogeneous reduction, and (3)chemical co-precipitation of the resultant TcO2.Fig.4 illustrates the schematic diagram of99Tc(VII) reduction on the CMCstabilized nZVI.The following Eq.2 depicts the redox reaction:

Meanwhile, Ponder et al.claimed that the nZVI nanoparticles(Fe0) can also react with water to form Fe(II) ions as (Eqs.3-5)[20,28]:
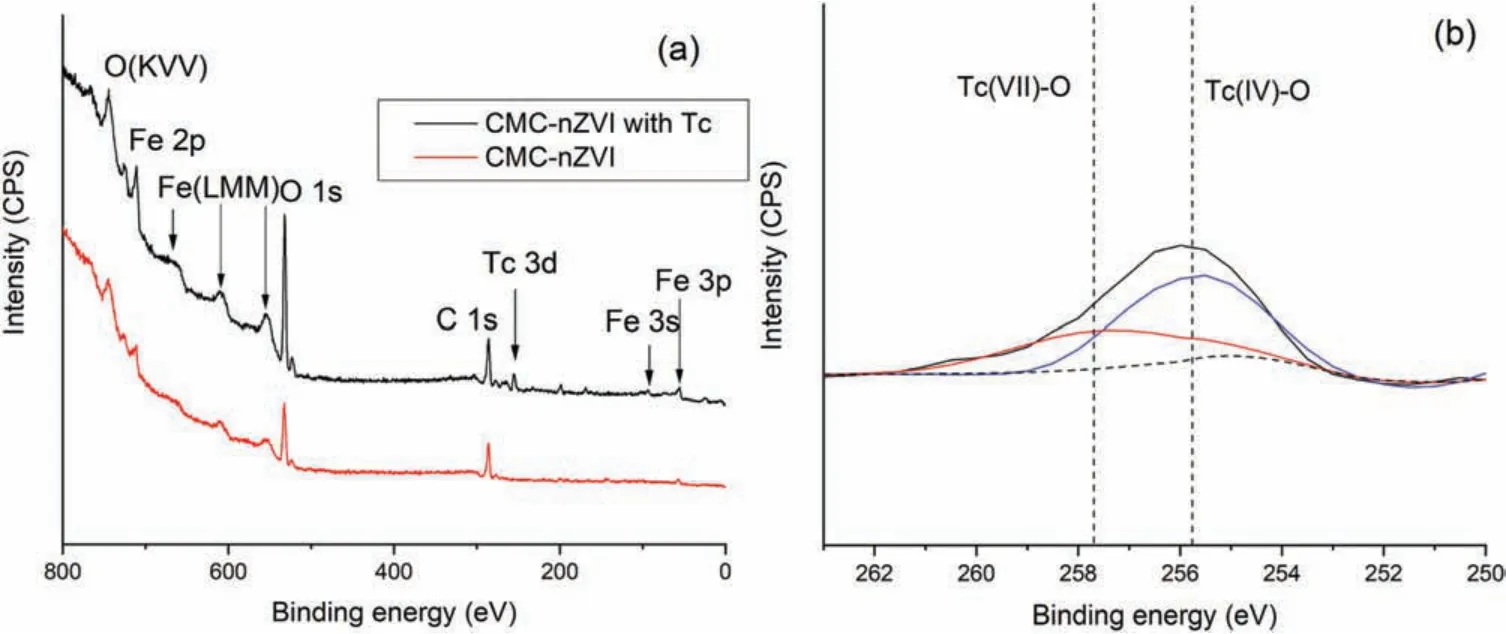
Fig.3.XPS spectra of CMC-nZVI before and after reduction of 99Tc(VII): (a) survey XPS, (b) high resolution of Tc 3d.
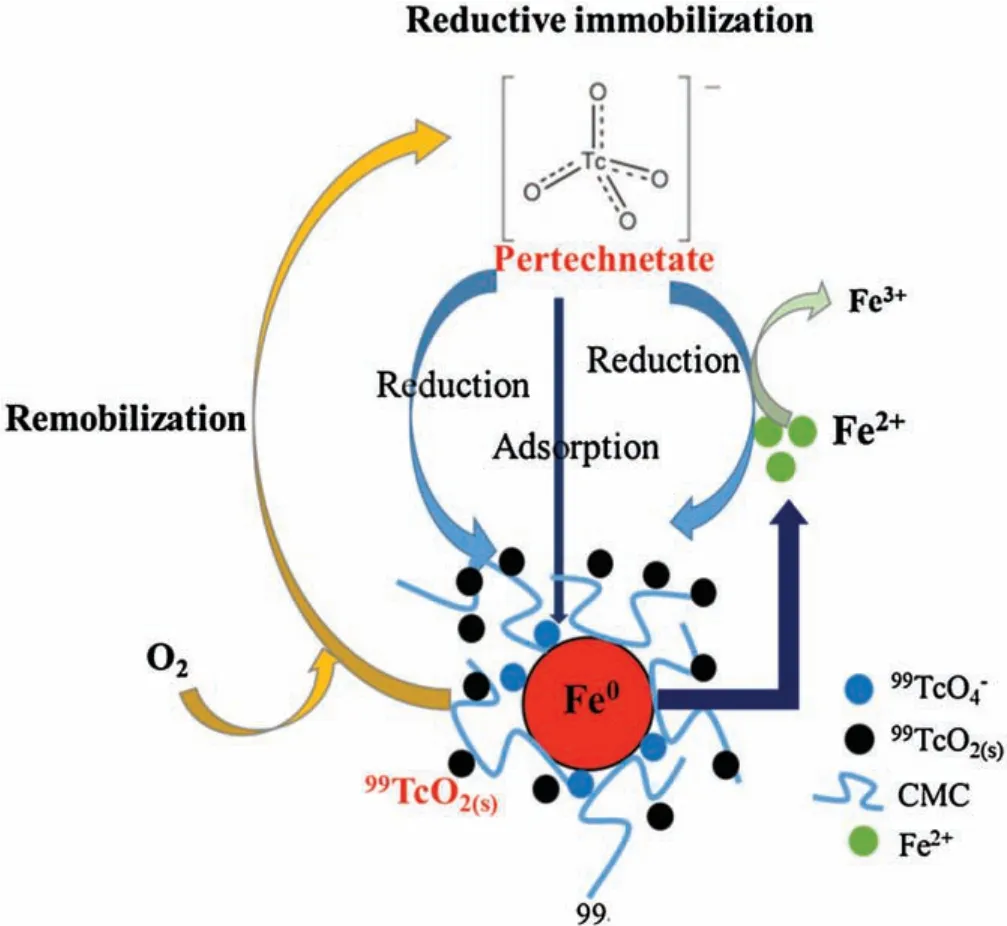
Fig.4.Schematic diagram on 99Tc(VII) reductive immobilization and remobilization on the CMC-stabilized nZVI.

The following homogeneous redox reaction between Fe(II)and99Tc(VII)can also occur(Eq.6)[21].However,in the heterogeneous Fe(II)reaction system,i.e.,adsorbed ferrous ions on the surface of nZVI,99Tc(VII) will be reduced to99TcO2as Eq.7 [29].
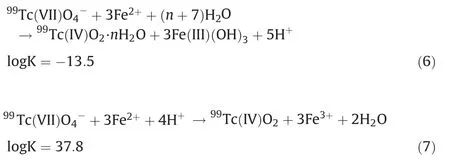
where K is the equilibrium constant under standard conditions.
Besides direct reduction of99Tc(VII) by Fe0, Eqs.5 and 7 also reveal that the indirect formation of99Tc(IV)O2(s),and the reaction is strongly pH-dependent and favors at low pH.
The rate and extent of99Tc(IV)dissolution was highly related to the organic ligands of chemical and structural properties [30],hence also causing significant implications for the99Tc(VII)reduction.Furthermore, as a common buffer and a popular composition in natural subsurface water, natural organic matter(e.g., humic acid) and high concentration of bicarbonate was usually found in natural water matrix [31].Based on the pseudofirst order model fitting,Fig.S3(Supporting information)displays the reduction rate constants(kobs)in the absence and presence of bicarbonate and HA at various pH.It was evident that bicarbonate showed minor effect while HA inhibited the reduction reaction of99Tc(VII).50 mg/L HA retarded the reduction as the kobsvalue decreased from~0.2 h-1to~0.1 h-1(by 2 times) in all the pH range from 5.0 to 8.0.However,additional 10 mmol/L HCO3-only slightly decreased the reaction rate at pH 5.0 and 6.0.
As shown in Eqs.5 and 7, H+plays an important role in the reduction of99Tc(VII), and the redox reaction is highly pHdependent.In addition, Tc(IV)O2(s) is more favorable to form at lower pH(Fig.S1).However,reductive removal of99Tc(VII)will be affected by pH in some contrasting ways.pH can strongly affect the surface charge of CMC-nZVI and then involve in the reaction between nanoparticles and99Tc(VII).He et al.[32]reported that CMC-nZVI was negatively charged at pH 5.0-8.0, which is consistent with pKavalue of 4.3 of CMC, suggesting the CMCstabilized nZVI showed electrostatic repulsion effect on99TcO4-.The kobsvalue slightly decreased from 0.193 h-1to 0.180 h-1when pH increased from 5.0 to 6.0,due to more negative chargers at pH 6.0 and was not favorable for99TcO4-adsorption.In addition,Eqs.(5)and(7)indicated that higher concentration of H+also facilitate formation of Tc(IV)O2(s).However,the kobsvalue then increased to 0.201 h-1at pH 8.0,and it is mainly due to higher corrosion rate of Fe0at lower pH.He and Zhao[12]reported that the corrosion rate of CMC-nZVI increased by~31.4 times from pH 8.2 to 6.0.Therefore, higher corrosion rate at lower pH results in the partial loss of reactive sites of nZVI, leading to lower99Tc(VII) removal efficiency.
The inhibition effect of HA on CMC-nZVI reactivity for reduction of99Tc(VII) contains various mechanisms [33].First, the negative charged HA will compete surface reactive sites of CMC-nZVI with99TcO4-, resulting in a protective and nonconductive layer, which cut off the electron transfer and thus inhibited99Tc(VII)reduction[34].Secondly, HA can not only be adsorbed on the surface of particles but also react with Tc(VI)O2·H2O colloids.Boggs et al.[35]reported that the presence of HA may re-dissolve the reduced Tc(IV) species due to the formation of [Tc(OH)2]-HA complexes,resulting in remobilization of99Tc(VII).Thirdly,HA can also easily bond with99TcO4-in solution and form stable HA-Tc complex[35],which is hard to reduction.Similarly, complexation between HA and Fe2+or Fe3+will inhibit the electron transfer and then affect the reduction reaction [36].
In the presence of bicarbonate,the corrosion of Fe0by HCO3-will occur [37].The added bicarbonate can be sorbed onto CMC-nZVI surface and react as oxidant following the reaction(Eq.8)[38]:
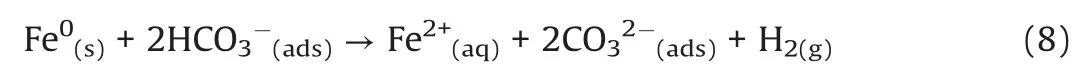
The precipitated carbonate can also coat on the iron surface thereby inhibit the further reaction [38,39].Severe corrosion of nZVI generally occurs at low pH [38,39], so the reduction of99Tc(VII)was only slightly inhibited in this study when pH varied from 6.0 to 8.0.
With rapid rate and high remove efficiency in the short term,the CMC-stabilized nZVI is considered as a promising reductive agent for remediation of99Tc contaminated groundwater.However,the factor of oxygen cannot be ignored or completely excluded from the99Tc(VII) reduction in natural environment or artificial perturbations.To simulate this process,a set of batch experiments regarding long-term monitor on reduction stability and reoxidation fraction were carried out.
Fig.5 shows the redissolution or remobilization of99Tc(VII)after complete reduction of99Tc(VII)by CMC-nZVI for 120 d under both anoxic and oxic conditions.Without air involved, which simulate typical subsurface redox environment,no Tc remobilization was detected during the 120 d of aging.This observation suggested that the immobilized99Tc(IV) remained highly stable over prolonged period of time under anoxic condition.However,with air involved and exposed to the atmospheric condition, the immobilized Tc was partially remobilized,with a peak of soluble Tc concentration accounting to 29.1%of the total Tc in the system.The peak of dissolved Tc concentration occurred at day 7, and then gradually decreased to a steady concentration (21.5% of total Tc)after 20 d,which can be attributed to the slow adsorption by iron oxyhydroxides resulting from oxidation of Fe0[20]and radiolytic auto-reduction of99TcO4-to99TcO2.The largely irreversible reduction of99Tc(VII) by CMC-nZVI is desirable in practical applications,and it can be attributed to:(1)The structural barrier effect of the nanoparticles on Tc that is situated in the pores[40],and (2) reoxidation of the immobilized Tc(IV)O2(s)in the solid phase needs much more activation energy than the reductive sequestration of Tc(VII).In addition, the protective effect will be addressed due to the formation of oxidized layer[41],such as iron(hydr)oxides.
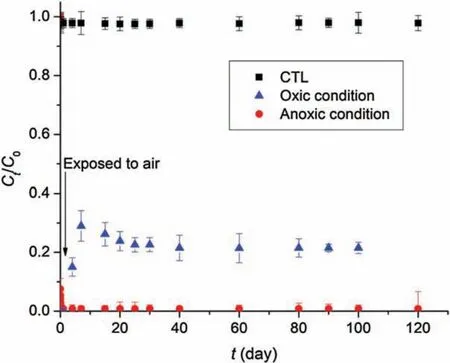
Fig.5.Long term evaluation on stability of 99Tc(VII) under anoxic and oxic conditions.(Initial 99Tc(VII)=12 mg/L; nZVI concentration=60 mg/L as Fe;CMC=0.2 wt%;initial pH 8.0;CTL:control text with CMC and 99Tc(VII)but without material addition).
This study investigated the feasibility of reductive immobilization99TcO4-using stabilized nZVI nanoparticles in water.CMCstabilized nZVI performed much faster kinetics of99Tc(VII)reduction than starch-stabilized particles, especially when the nZVI dosage was <60 mg/L, mainly due to the extra charges carried by CMC molecules minimized the diffusion barrier as to accelerate effective contacts between CMC-nZVI and99TcO4-.XRD and XPS analyses demonstrated formation of99TcO2(s)after reduction.The reductive reaction of99Tc(VII) was a pH-independent process, the co-existing HA inhibited the reaction while bicarbonate showed minor effect.Extremely stable immobilized Tc was observed under anoxic condition even after 120 d,and only~21% reoxidation of aged99Tc(IV) was found after ingress of atmospheric O2,which further supported that the CMC-nZVI was an innovative alternative for radionuclides groundwater remediation.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China(No.41230638),and a grant from the USDA AAES 2015 Hatch and Multistate funding program.
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.06.004.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
