Silver nanoparticles embedded temperature-sensitive nanofibrous membrane as a smart free-standing SERS substrate
2020-01-14XioyunLiuLiyingWngSiyunChenLiushengZh
Xioyun Liu,Liying Wng,Siyun Chen,Liusheng Zh,*
a Research Center for Analysis and Measurement, Donghua University, Shanghai 201620, China
b State Key Laboratory for Modification of Chemical Fibers and Polymer Materials & College of Materials Science and Engineering, Donghua University,Shanghai 201620, China
Keywords:
Nanofibrous membrane
Silver nanoparticles
Electrospinning
Temperature-sensitiveness
SERS substrate
ABSTRACT
To develop a smart free-standing surface enhanced Raman scattering (SERS) substrate, silver nanoparticles (AgNPs) embedded temperature-sensitive nanofibrous membrane was fabricated by electrospinning their aqueous solution containing the copolymer poly(N-isopropylacrylamide-co-Nhydroxymethylacrylamide), followed by heat treatment to form crosslinking structure within its constituent nanofibers.To avoid negative effect of the additive like stabilizer and the reactant like reductant on their SERS efficiency, the AgNPs were in-situ synthesized through reducing Ag+ ions dissolved in the polymer solution by ultraviolet irradiation.The prepared hybrid nanofibrous membrane with high stability in aqueous medium can reach its swelling or deswelling equilibrium state within 15 seconds with the medium temperature changing between 25°C and 50°C alternately.When it was used as a free-standing SERS substrate,10-12 mol/L of 4-nitrothiophenol in aqueous solution can be detected at room temperature,and elevating detection temperature can further lower its low detection limit.Since its generated SERS signal has desirable reproducibility,it can be used as SERS substrate for quantitative analysis.Moreover, the hybrid membrane as SERS substrate is capable of real-time monitoring the reduction of 4-nitrothiophenol into 4-aminothiophenol catalyzed by its embedded AgNPs, and the detected intermediate indicates that the reaction proceeds via a condensation route.
As we know, silver nanoparticles (AgNPs) have dominant localized surface plasmon resonance(LSPR)optical property due to their very high extinction coefficient[1].The coupling between the oscillating electromagnetic fields on their surfaces originating from plasmon resonance provides huge enhancement of the local electromagnetic fields and makes the noble metal nanoparticles powerful for plasmonic sensing [2].Furthermore, the greatly strong surface electromagnetic fields resulting from the coupling make AgNPs very efficient in their use as substrate for surface enhanced Raman scattering(SERS)sensing[3].However,like other colloidal metal nanoparticles, colloidal AgNPs dispersed in aqueous medium are prone to self-aggregation and settling down owing to their high surface energy and great density, resulting in loss of their SERS activity.One of the attractive strategies to overcome the shortage is immobilization of AgNPs within the polymer network of a hydrogel [4,5].The analyte molecules in aqueous solution can easily diffuse into its water-swollen network to contact the AgNPs to produce SERS signal.Additionally, this strategy offers the possibility of protecting the AgNPs from oxidation.Further, if the hydrogel holds stimulus-responsive property, which is usually called smart hydrogel, the SERS efficiency of its embedded AgNPs are capable of being tuned by external stimulus thanks to the stimulus induced variation of their surrounding refractive index and distances[6].On the other hand,external stimulus can be sensed by detecting variation of their properties [7,8].
The previously reported smart hydrogels for carrying silver nanoparticles are stimulus-responsive bulky hydrogel and microgel [9-11], which can undergo phase transition and alter their physicochemical properties, such as volume, water content,refractive index, permeability and hydrophilicity-hydrophobicity,upon exposure to an external stimulus like pH,temperature,light or specific molecule [12].Compared to smart bulky hydrogel, its microscopic analogue has much faster stimulus-response rate,since the rate is inversely correlated to their size[13].It is known that rapid stimulus-responsiveness of smart hydrogels is important to their applications, such as tunable catalysis, sensing or detection,actuation,drug delivery,etc.[14].However,since smart microgels of microscopic size usually exist as colloidal particles in aqueous solution, their composites with metal nanoparticles also suffer from poor colloidal stability owing to introducing metal component with high density[15].Moreover,they are not able to be used as free-standing material for some application due to no macroscopic shape and strength.
In recent years, stimulus-responsive nanofibrous membrane consisting of randomly or orderly aligned hydrogel nanofibers with the diameters of less than 1000 nm has attracted more and more attention,which overcome the above-mentioned disadvantages of smart bulky hydrogels and microgels [16].Firstly, it is able to respond rapidly to external stimulus due to very small diameters of it constituent hydrogel nanofibers and its porous structure,which facilitates the transfer of the stimulus to the whole material[17,18].Secondly, the reported applications have demonstrated that the smart membrane can be used as the free-standing material in a device because of their macroscopic strength and flexible morphology [19,20].The smart nanofibrous membranes are usually fabricated by electrospinning technique, which features simplicity, flexible morphology tuning and wide variety in usable polymers [21,22].More interestingly, electrospinning shows potential applications in assembly of nanoscale building blocks for generating new functions [23].
Since temperature change is a common stimulus in chemical or biological system, the smart material with temperature-sensitive property has been paid considerable attention due to its potential applications in sensor, actuator,drug delivery,cell culture,and so on [14].Poly(N-isopropylacrylamide) (PNIPAM), with a lower critical solution temperature (LCST) at ca.32°C close to human body temperature, has been the most attractive temperaturesensitive polymer over past 20 years [12].Below the LCST, the polymer easily dissolves in water;while at temperatures above the LCST, it becomes insoluble and precipitates out of aqueous solution.The temperature-sensitive bulky hydrogels or microgels based on PNIPAM have been exploited as the carriers of silver nanoparticles for acquiring their temperature-tunable properties by many researchers [24,25].For example, Ballauff et al.reported on a switchable catalyst that was made by synthesizing AgNPs inside a PNIPAM shell that had been grafted to the surface of a colloidal poly(styrene) particle.Temperature-dependent swelling and shrinking of the shell were used to alternatively expose and hide the AgNPs on its surface, thereby modulating their catalytic activity [26].In our group, AgNPs loaded pH/temperature dually responsive microgels with inter-penetrating polymer network structure based on PNIPAM and poly(acrylic acid)were fabricated and used as smart SERS substrates with both pH and temperature tunable efficiency [5].
Recently, we fabricated AgNPs embedded temperature-sensitive nanofibrous membrane by electrospinning the aqueous solution containing the metal nanoparticles and PNIPAM copolymer with heat-curable groups,followed by heat treatment,and the hybrid nanofibrous membrane has successfully been used as a recyclable dip-catalyst with temperature-tunable catalytic activity[27].In this study, based on the previous work, we utilized the hybrid nanofibrous membrane as a smart free-standing SERS substrate.Since its produced SERS signal has excellent reproducibility,it can be used for quantitative analysis.In addition,its SERS efficiency can be increased by elevating detection temperature.Interestingly,it was found that it can be used as the SERS substrate for real-time monitoring the chemical reaction catalyzed by its embedded AgNPs.
N-Isopropylacrylamide (NIPAM, 99%) was purchased from J&K Chemical Co.and purified by recrystallization from a mixture of toluene and hexane (60:40, v/v).N-Hydroxymethylacrylamide(NMA,98%),4-nitrothiophenol(4-NTP,99%), 2,2'-azobis(isobutyronitrile) (AIBN, 99%), sodium borohydride (NaBH4, 96%), silver nitrate (AgNO3, 99.9%) and anhydrous ethanol (analytical purity)were purchased from Shanghai Chemical Reagent Co., and used as received.Deionized water used in the synthesis and characterization was made using a Millipore Direct-Q system.
Poly(N-isopropylacrylamide-co- N-hydroxymethylacrylamide)(PNN) was firstly synthesized via AIBN initiated free radical copolymerization of NIPAM and NMA in ethanol solvent according to the reference[28].The molar ratio of NIPAM,NMA and AIBN in the feed recipe is 1000:100:1.Then,12 mg AgNO3dissolved in 1 mL water was added into 2 mL of 37.5 wt%PNN aqueous solution.After stirring for 30 min under dark condition, the mixed solution was exposed to ultraviolet(UV)irradiation of 365 nm wavelength with power density of 300 mW/cm2(UV lamp: WFH-203B) for 60 min.Next, the prepared electrospinning solution was loaded into a 10 mL plastic syringe and electrospun at positive voltage of 13 kV,20 cm working distance(distance between the needle tip and the grounded metal plate), and 0.9 mL/h flow rate at room temperature.Finally, the electrospun nanofiber membrane was heated at 110°C under N2purge for 5 h to produce AgNPs embedded temperature-sensitive nanofibrous membrane(AgNPs/PNN hybrid nanofibrous membrane).
1H NMR spectrum of PNN was measured by an AV 400 nuclear magnetic resonance (NMR) spectrometer (Bruker, Switzerland)based on the residual proton resonance of deuterateddimethylsulfoxide.The number-average molecular weight of PNN was determined by a BI-MWA gel permeation chromatography (GPC)equipped with light scattering detector (Brookhaven, USA) using HPLC-grade tetrahydrofuran as eluent.The temperature dependent absorbance(at 500 nm)of diluted PNN aqueous solution was measured by a Lambda 35 ultraviolet visible(UV- vis)spectrometer(PerkinElmer, USA) using a quartz cell with 1 cm optical path length, and the temperature of the solution was controlled by a Peltier temperature controller.TEM images of AgNPs/PNN hybrid nanofibrous membrane were taken with a 2100 F transmission electron microscope(TEM)(JOEL,Japan)at a voltage of 200 kV.The samples were collected on copper grids during electrospinning,followed by heating at 110°C under N2purge for 5 h.SEM images of AgNPs/PNN hybrid nanofibrous membrane were obtained with an S-4800 field emission scanning electron microscope (SEM)(Hitachi,Japan)at a voltage of 5 kV after the sample was sputtered with gold.
SERS measurements were performed using a micro-Raman system (inVia-Reflex, Renishaw) equipped with a multi-channel charge-coupled device detector and a confocal microscope(DM2500 M, Leica) upon excitation by 532, 633 or 785 nm laser source.All SERS spectra were the results of 10 s accumulations.The SERS efficiency of AgNPs/PNN hybrid nanofibrous membrane was assessed by putting the membrane sample of 0.7 cm×0.7 cm size into the quartz cell, then injecting 200 μL of 4-NTP aqueous solution( of different concentrations)into it,followed by recording SERS spectrum using Raman microscope with 50X objective.Temperature dependent SERS spectra were measured on the membrane(HNM:0.7 cm×0.7 cm)immersed in 10-4mol/L 4-NTP aqueous solution in a THMS600 temperature-controllable cell(Linkam,USA),which was equilibrated thermally for at least 5 min before each measurement.For real-time monitoring the catalytic reaction by SERS, the membrane (HNM: 0.7 cm×0.7 cm) was immersed in 100 μL of 10-4mol/L 4-NTP aqueous solution in the sample cell,and then 100 μL of 0.1 mol/L NaBH4aqueous solution was injected into it,keeping its temperature at 25°C.The reaction progress was monitored in real-time by recording SERS spectra of the reaction solution every 3 min interval.
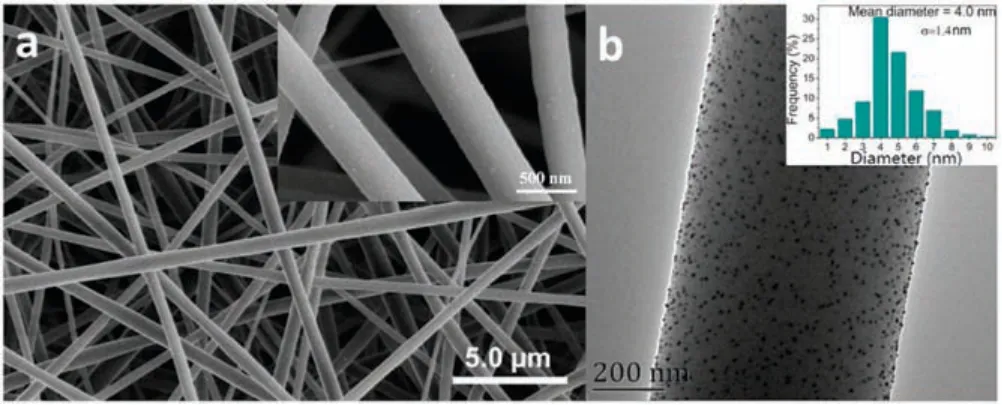
Fig.1.SEM image (a) and TEM image (b) of AgNPs/PNN hybrid nanofibrous membrane (Inset of (b) is the particle diameter distribution histogram of its embedded AgNPs).
To fabricate AgNPs/PNN hybrid nanofibrous membrane, the temperature-sensitive polymer, i.e., PNN, was firstly synthesized.Its NIPAM/NMA molar ratio determined from its1H NMR spectrum is 100:12.3, close to the feeding one of 100:10 in its synthesis recipe,and its number-average molecular weight estimated by GPC is 2.27×105g/mol with polydispersity index of 1.18.From the temperature dependent absorbance (at 500 nm) of diluted PNN aqueous solution, its lower critical solubility temperature (LCST)was determined to be around 37.5°C.Then, AgNPs were in-situ synthesized via reducing the AgNO3dissolved in PNN aqueous solution by UV irradiation,which is a green synthesis process due to no use of reductant, stabilizer and organic solvent.With extending UV irradiation time, the color of the reaction solution changed from colorless transparency to dark brown,implying that the Ag+ions in the solution were reduced into AgNPs.By measuring time dependent UV- vis spectra of the solution,most of the added Ag+ions were reduced to AgNPs within 1 h.Next, the produced AgNPs/PNN blend aqueous solution was put into the syringe of electrospinning setup and electrospun under certain conditions(as described in Experimental section) to form a nanofibrous membrane of ca.420 μm thickness.Finally, the membrane was heated at 110°C under N2purge for 5 h to induce the crosslinking reaction between the polymer chains inside its constituent nanofibers,producing AgNPs/PNN hybrid nanofibrous membrane.
Figs.1a and b provide SEM and TEM image of AgNPs/PNN hybrid nanofibrous membrane.From the SEM image we can note that the fibers inside the membrane are homogeneous in their diameters,without beaded morphology.There are some white spots appear on their surfaces,which were demonstrated to be AgNPs by energy dispersive X-ray spectroscopy.Their average diameter was statistically determined to be 452±154 nm by randomly measuring 100 fibers within the membrane.From the TEM image in Fig.1b, we can note that the AgNPs observed as dark spherical spots are evenly distributed within the nanofiber matrixes.Their average particle diameter obtained by randomly measuring 100 particles from the TEM image is ca.4.0 nm.
To evaluate the stability of AgNPs/PNN hybrid nanofibrous membrane in water medium, about 25 mg of the sample was immersed in 20 mL water and the solution was oscillated at 400 rpm for 2 h.Fig.S1(Supporting information) shows their optical images before and after oscillation.It can be seen that,after the harsh oscillation, the hybrid nanofibrous membrane was left intact.In addition, through weighing the dry samples before and after oscillation, it was found that its weight loss percentage of HNM is 1.56%.The results indicate that AgNPs/PNN hybrid nanofibrous membrane has high stability in water.Its excellent stability is resultant from the crosslinking structure between the polymer chains inside its constituent nanofibers or between the nanofibers, which was formed by the condensation reaction between the hydroxymethyl groups of NMA units after heat treatment, as described above.
The solid UV- vis spectrum of the membranes were measured using a UV- vis spectrometer with an integrating sphere(diameter 50 mm) and the result are shown in Fig.S2 (Supporting information).There is no absorption peak on the spectrum of blank PNN membrane,but an absorption peak at about 425 nm on the one of AgNPs/PNN hybrid nanofibrous membrane,which is due to the localized surface plasmon resonance (LSPR) effect of the loaded AgNPs inside the hybrid membrane.

Fig.2.(a)Typical optical images of AgNPs/PNN hybrid nanofibrous hydrogel membrane immersed in 25°C or 50°C water;(b)Temperature dependent area of AgNPs/PNN hybrid nanofibrous membrane in water measured at a heating rate of 0.1°C/min;(c)Time dependent area of AgNPs/PNN hybrid nanofibrous membrane immersed alternately into the water of 25°C or 50°C after the interval of 2 min.
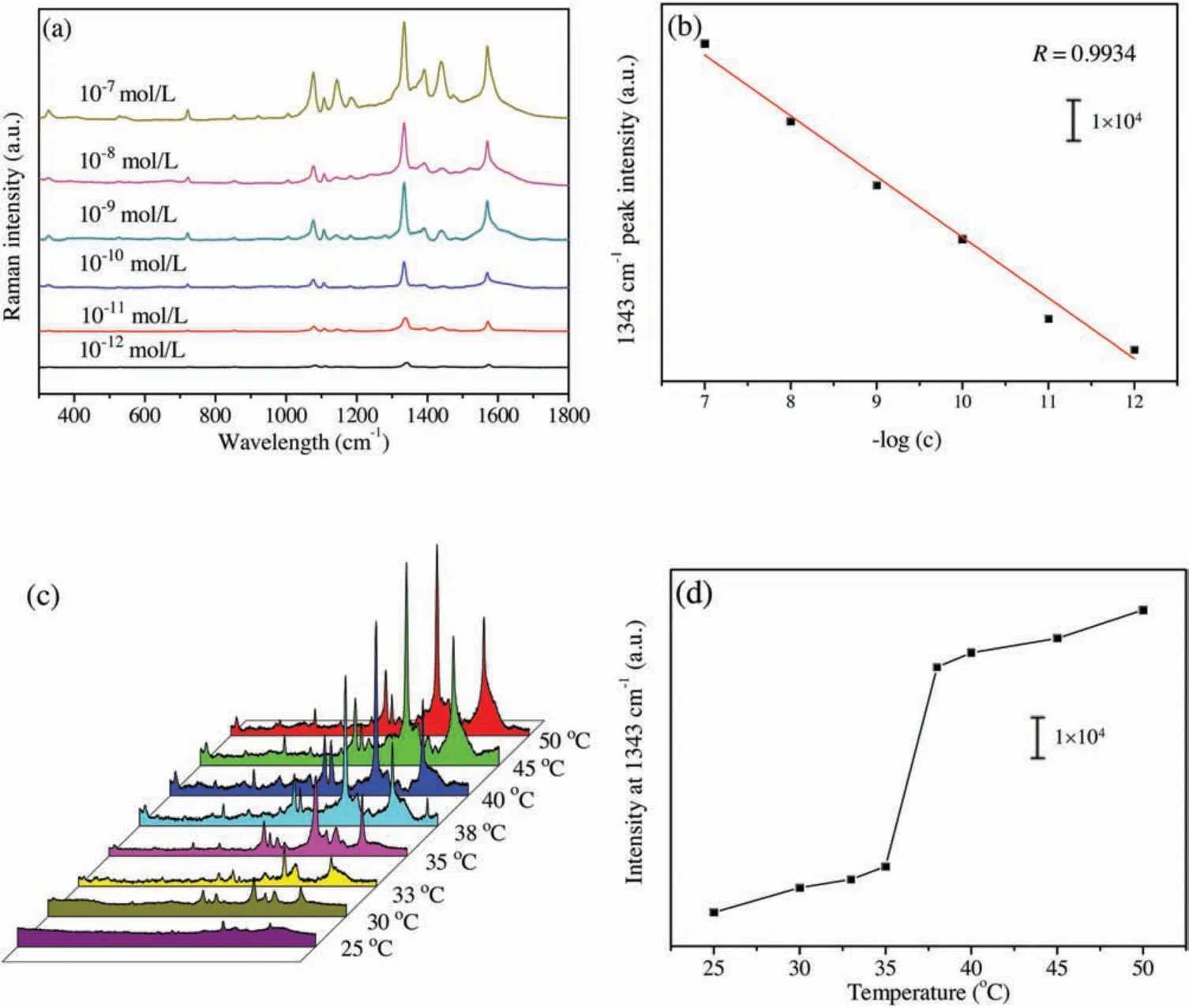
Fig.3.(a) SERS spectra of different concentrations of 4-NTP in aqueous solution based on AgNPs/PNN hybrid nanofibrous membrane as substrate with 532 nm laser excitation;(b)Plot of 1343 cm-1 peak intensity vs.log(c)of 4-NTP;(c)SERS spectra of 10-7 mol/L 4-NTP aqueous solution obtained on the membrane at different temperatures;(d) The 1343 cm-1 peak intensity as a function of temperature.
Typical optical images of AgNPs/PNN hybrid nanofibrous membrane immersed in 25°C or 50°C water are exhibited in Fig.2a,and it can be found that its area at 50°C is about half of the one at 25°C,exhibiting that it possesses remarkable temperaturesensitive property.To identify its phase transition temperature,temperature dependent area of AgNPs/PNN hybrid nanofibrous membrane in water was measured at a heating rate of 0.1°C/min,and the result is depicted in Fig.2b, one can note that it steeply shrunk with increasing the temperature from 35°C to 42°C,with the phase transition temperature at ca.38°C close to the LCST of PNN.Further investigation on its temperature-sensitive property is to assess its temperature change responding rate by measuring the time-dependent area of the membrane immersed alternately into the water of 25°C or 50°C after the interval of 2 min.As shown in Fig.2c, AgNPs/PNN hybrid nanofibrous membrane reached the deswelling equilibrium state within 10 s after a jump in temperature from 25°C to 50°C.On the other hand, a plunge in temperature from 50°C to 25°C brought about its swelling equilibrium within 15 s, and the reversible swelling-deswelling cycle was repeated at least 4 times.As mentioned previously, so fast temperature change responding rate of AgNPs/PNN hybrid nanofibrous membrane should be associated with very small diameters of its constituent nanofibers and porous structure[16].
Compared to previously reported hybrid microgels [5,10],AgNPs/PNN hybrid nanofibrous membrane should be more suitable for the use as SERS substrate because of its free-standing and flexible features[29].We used 4-NTP as a Raman active probe molecule to assess its SERS efficiency.Figs.S3a and b(Supporting information) show the Raman spectra of pure 4-NTP and PNN membrane,respectively.In Fig.S3a,the three prominent peaks at 1099 cm-1, 1332 cm-1and 1574 cm-1are assigned to C-S stretching,O-N=O symmetric stretching and the phenyl ring stretching modes of 4-NTP[30].From Fig.S3b,we can see that there are only some very weak peaks on the spectrum of pure PNN membrane,so it will not interfere with the following SERS measurements.
Fig.S4a(Supporting information)shows the SERS spectra of 4-NTP aqueous solution with the same concentration based on the membrane as substrate, which were respectively obtained with three excitation wavelengths, i.e.,532 nm,633 nm and 785 nm.The above typical Raman peaks of 4-NTP are reflected in these spectra.From Fig.S4a we can see that the hybrid nanofibrous membrane as SERS substrate exhibits much stronger efficiency with the excitation wavelength of 532 nm than with others,due to the fact that it most matches the localized surface plasmon resonance wavelength of the AgNPs embedded inside the membrane,causing the strongest surface electric field enhancement effect.For evaluating the detection sensitivity based on the hybrid nanofibrous membrane as SERS substrate,the SERS spectra of different concentrations of 4-NTP aqueous solution from 10-12mol/L to 10-7mol/L were obtained on the membrane,and the results are given in Fig.3a.With the increase of 4-NTP concentration, its SERS signal intensity is progressively raised.Even at the concentration of 10-12mol/L, the signal is obviously observed,meaning that 4-NTP of so low concentration can be detected.The plot of the 1343 cm-1peak intensity in the SERS spectra versus the logarithmic concentration(log(c))of 4-NTP is illustrated in Fig.3b,which can be fitted into a line with the correlation coefficient of 0.9934 (n=6).This shows that there is a good linear relationship between the SERS intensity of the analyte and its logarithmic concentration.Fig.S4b (Supporting information) shows the SERS spectra acquired from 20 different spots on the membrane with 532 nm laser excitation,and 1343 cm-1peak intensities of these SERS spectra are given in Fig.S4c(Supporting information).It can be estimated from the data that their relative standard deviation(RSD)is 5.8%,indicating that the SERS signals from the substrate have excellent reproducibility, and the embedded AgNPs are homogeneously distributed wihin the membrane.These results imply that AgNPs/PNN hybrid nanofibrous membrane is able to be used as the SERS substrate for quantitative analysis.As illustrated in Fig.3c, the SERS spectra of 10-7mol/L 4-NTP aqueous solution based on AgNPs/PNN hybrid nanofibrous membrane as substrate were measured at different temperatures.All peaks in its SERS spectrum are remarkably enhanced with temperature rising from 20°C to 50°C.The plot of 1343 cm-1peak intensity vs.temperature is exhibited in Fig.3d,one can find that its sharp change occurs within the temperature range from 35°C to 38°C,in agreement with the one for the area change of the hybrid nanofibrous membrane with temperature( Fig.2b).The low detection limit of 4-NTP basing on the hybrid nanofibrous membrane as SERS substrate at 25°C and 50°C are 10-12mol/L and 10-13mol/L,respectively.It can be speculated from the phenomena that the thermo-driven SERS signal enhancement should result from the plasmonic coupling between the approached AgNPs after temperature-triggered shrinkage of the nanofibers within AgNPs/PNN hybrid nanofibrous membrane [5].This result indicates that the detection sensitivity based on the membrane as SERS substrate can be improved by elevating detection temperature over 40°C.As above demonstrated, the smart hybrid nanofibrous membrane has very fast temperature change responding rate,so that the measurement time at elevated temperature is greatly shortened since it can instantly reach swelling equilibrium state.

Fig.4.(a) Time dependent SERS spectra for monitoring the reduction of 4-NTP catalyzed by AgNPs/PNN hybrid nanofibrous membrane.(b) Kinetic trace of the SERS intensities of 1570 cm-1 peak (4-NTP),1140 cm-1 peak(4,4'-DMAB) and 1590 cm-1 peak (4-ATP).
It is well known that AgNPs of less than 10 nm particle diameter possess SERS activity as well as catalytic activity for many organic reactions,such as the reduction reactions of nitro compounds and organic dyes, ethylene epoxidation and dehydrogenation reaction[31].Therefore,AgNPs/PNN hybrid nanofibrous membrane has the possibility of being used to real-time SERS monitor the chemical reaction catalyzed by its embedded AgNPs,such as reduction of 4-NTP to 4-aminothiophenol (4-ATP) by NaBH4.This reaction has been intensively investigated by SERS technique, but its mechanism still remains ambiguous.Generally,it is accepted that 4-NTP is reduced to 4-ATP through a two-step consecutive reaction route,as illustrated in Fig.S5(Supporting information),either by a direct one via the intermediate hydroxylamine or a condensation one via the intermediate 4,4'-dimercaptoazobenzene(4,4'-DMAB)[30,32].Therefore, the real-time detection of 4,4'-DMAB during the reaction is crucial to identifying its mechanism.Fig.4a displays the time dependent SERS spectra,which was recorded every 3 min interval after adding NaBH4to 10-4mol/L 4-NTP solution containing AgNPs/PNN hybrid nanofibrous membrane at 25°C.Note that the intensities of the peaks associated with 4-NTP decrease gradually and three new peaks at 1140 cm-1, 1385 cm-1and 1434 cm-1appear accordingly, which are assigned to the vibrational modes of 4,4'-dimercapto-azobenzene(4,4'-DMAB),i.e., the C-N symmetric stretching, N =N stretching, and C-H inplane bending ones, respectively [33].Their intensities initially increase with reaction time and then gradually decrease up to near-zero,further corroborating its character as an intermediate.In addition to the peaks of 4,4'-DMAB,another new peak at 1590 cm-1shows up nearly simultaneously and gradually dominates the spectrum,which is attributed to the amino vibrational mode of the product 4-ATP.The results indicate that the reduction of 4-NTP into 4-ATP by NaBH4, catalyzed by AgNPs/PNN hybrid nanofibrous membrane, proceeds via a condensation route.The SERS intensities of three characteristic peaks at 1570 cm-1, 1140 cm-1and 1590 cm-1against reaction time,which are respectively associated with 4-NTP, 4,4'-DMAB and 4-ATP,are plotted in Fig.4b.It can be found that the reduction of 4-NTP into 4-ATP was completed within 30 min, as indicated by complete disappearance of the peaks of 4-NTP and 4,4'-DMAB.In spite of the difference in their scattering cross sections,it can be estimated that the concentration of 4,4'-DMAB remains very low during the whole reaction process,implying that the intermediate reacts very rapidly.This shows that the reaction rate of the second step is highly faster than that of the first, and producing intermediate should be a rate determining step.
In conclusion, AgNPs embedded temperature-sensitive nanofibrous membrane with desirable stability in aqueous medium was fabricated by electrospinning the solution containing temperature-sensitive polymer with heat curable groups and the metal nanoparticles, followed by heat treatment to form crosslinking structure with its constituent nanofibers.To avoid negative effect of the additive like stabilizer and the reactant like reductant on their SERS efficiency,the AgNPs were in-situ synthesized through reducing Ag+ions dissolved in the spinning solution by UV irradiation.The produced hybrid nanofibrous membrane is able to be used as a free-standing SERS substrate for quantitative analysis,and its efficiency can be easily tuned by temperature because of its fast temperature change responding rate.Additionally,the hybrid membrane as SERS substrate has the ability of real-time monitoring the reduction of 4-nitrothiophenol into 4-aminothiophenol catalyzed by its embedded AgNPs, and the detected intermediate indicates that the reaction proceeds via a condensation route.
Acknowledgment
The authors are grateful to the National Natural Science Foundation of China ( Nos.51503033, 51373030).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.04.031.
杂志排行
Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
