Coarctation of the aorta in twins with severe hypertension
2019-12-31GuoHuaZHUYanLingWANGHuanHuanWANGJingLIJingGAOHaiYingWUJunCAIQiHUA
Guo-Hua ZHU, Yan-Ling WANG, Huan-Huan WANG, Jing LI, Jing GAO, Hai-Ying WU, Jun CAI,#, Qi HUA,#
1Department of Cardiology, Xuanwu Hospital Capital Medical University, Beijing, China
2Department of Hypertension, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Keywords: Aortic coarctation; Gene mutation; Hypertension
Coarctation of the aorta (CoA) refers to the congenital coarctation near the isthmus, ductus arteriosus or ligamentum arteriosus, with an incidence of 5%–10% in children with congenital heart defects.[1]It can be isolated or coexisted with ventricular septal defects, subaortic stenosis, patent ductus arteriosus, and mitral aortic valve.[2]CoA has a poor prognosis. Without intervention, the median age of death with aortic coarctation is 38 years[3]and 75% death of those patients is at the age of 46 years.[4]Causes of death included congestive heart failure (26%), aortic rupture (21%), bacterial endocarditis (18%), and intracranial hemorrhage (12%).[5]Therefore, early diagnosis and optimal treatment are the key to improve the prognosis of this disease. The aim of this report is underlining the importance of early and accurate diagnose of CoA as a cause of systemic hypertension in young patients and also emphasizing the genetic factors of CoA in twins.
A 38-year-old man was presented to cardiology outpatient department complaining of uncontrolled hypertension, even under treatment with calcium channel blocker (CCB), angiotensin receptor inhibitors (ACEI) and diuretics. He was first diagnosed with hypertension at the age of 17 and the blood pressure (BP) was controlled at 180/90 mmHg (1 mmHg = 0.133 kPa) at ordinary times. The physical examination showed a normal body development, the BP of 185/82 mmHg in the right arm, 180/82 mmHg in the left arm, 100/61 mmHg in the right lower limb and 96/69 mmHg in the left lower limb. Heart rate was 72 beats per minute with regular rhythm and a systolic murmur was audible at scapular, left subclavian and supraclavicular fossa area. The arteries of the extremities pulsate forcefully and symmetrically. Blood routine, urine routine, serum potassium, liver, kidney, thyroid function and immune indexes were in normal range. The electrocardiogram revealed a sinus rhythm without criteria for ventricular hypertrophy and myocardial strain. Abnormality in heart structure and function also was not found in echocardiography. His father and younger brother had no hypertension and mother was diagnosed as middle-aged onset hypertension with the highest BP of 160/90 mmHg. Whereas, there was a twin brother who was also diagnosed as uncontrolled hypertension in the same year. The highest BP was 200/100 mmHg, while BP under combination treatment was about 180/90 mmHg.
Considering that the refractory hypertension might be due to gene variation, the patient was transferred to Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Serum renin, angiotensin, aldosterone, cortisol, norepinephrine, adrenaline, dopamine, 24-hour urine sodium and 24-hour urinary potassium were within the normal range (Table 1). Computed tomography angiography (CTA) of aorta was performed and it revealed a hypoplastic aorta and a severe coarctation in the descending aorta. The diameter of the aortic arch was about 15 mm, and the descending aorta was stenosis immediately close to the left subclavian artery. The diameter of the narrowest lesion was about 4 mm, and the dilation after the stenosis was seen from a distance, with a diameter of about 33 mm. The compensatory bilateral internal mammary arteries were widened, and there were a large number of collateral vessels with tortuous dilated vessels in the mediastinum (Figure 1).
His identical twin brother underwent the CTA as well and the result revealed that both of them suffered from CoA, while he had no family history of that (Figure 2). Our hypothesis was to evaluate whether any genetic variation contribute to the development of the twinborn CoA. Therefore, genetic screening was performed using whole-exome sequencing to detect gene mutation. Of all candidate loci identified related with aorta development, none mutated loci was attributed to CoA in this clinical case.
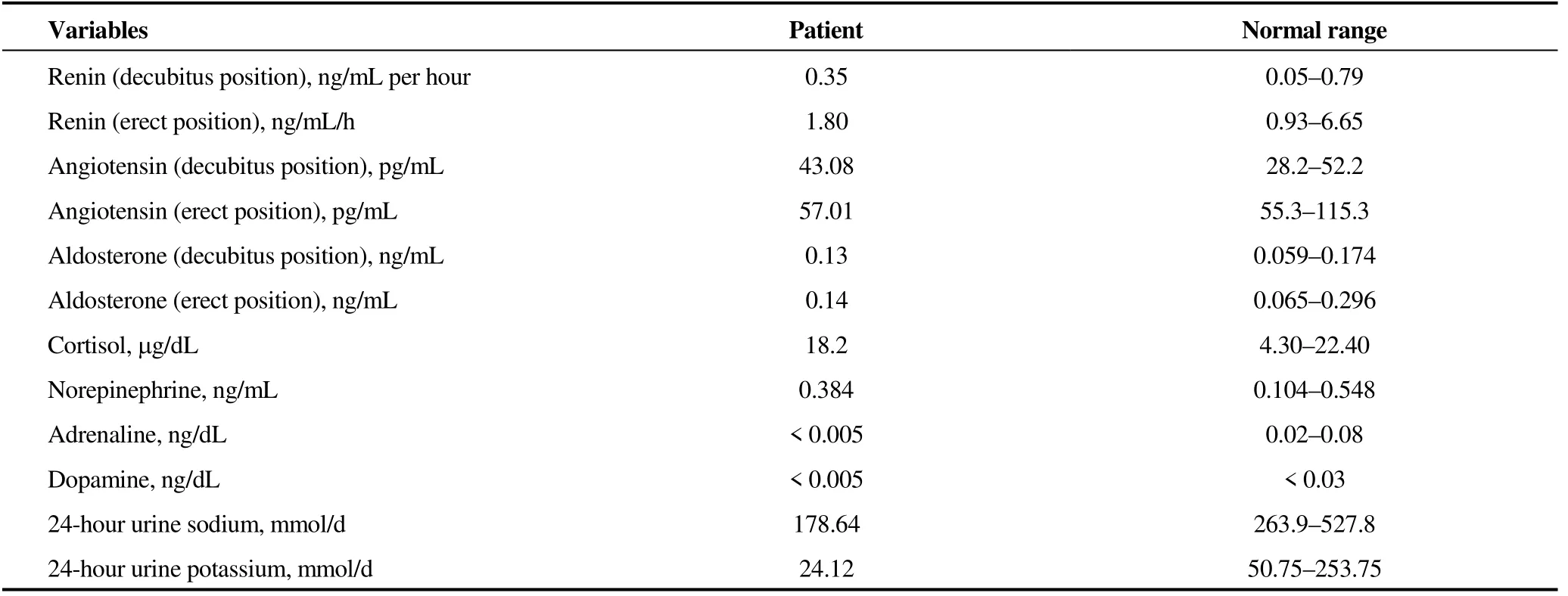
Table 1. Endocrine indexes.
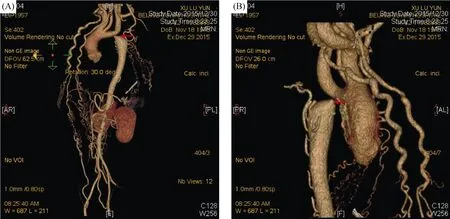
Figure 1. Aortic computed tomography angiography. (A): From anterior right position view, the diameter of the aortic arch was about 15 mm, and the descending aorta was stenosis immediately close to the left subclavian artery (arrow); and (B): from posterior left position view, the narrowest part of coarctation (arrow) was about 4 mm in diameter, and the dilation after the stenosis was seen in the distance.

Figure 2. Pedigree of the patient. The figure showed the family members of three generations of the patient, and no coarctation of the aorta was found except for the patient and his twin brother.
Interventional or surgical treatment was recommended. However, the patient refused due to certain reasons. Increasing dosage of CCB, ACEI and diuretic were prescribed for uncontrolled BP and the BP was reduced to about 160/90 mmHg.
In young adults presenting with severe upper extremity hypertension, secondary causes should be excluded. In clinic practices, the etiologic factors of secondary hypertension in young patients mostly include renal-vascular diseases, cardiovascular factors, and endocrine problems.[6]Hypertension caused by CoA is mediated by decreased arterial and left ventricular wall compliance due to increased afterload and it is seen in 30%–50% of patients starting in adolescence.[7]Clinical manifestations of CoA vary with different segment and severity of lesions.[6]Most patients present with refractory hypertension, excessive BP difference between upper and lower limbs and systolic continuous murmur,[8,9]just as the patient mentioned above. Imaging is reliable diagnostic basis. A computed tomography (CT) scan or magnetic resonance imaging (MRI) can provide detailed anatomic information of the coarctation and cardiac abnormalities, and these modalities are commonly used to create three-dimensional images for interventional planning.[10]
About 90% congenital CoA presents as a sporadic and non-syndromic congenital malformation,[11]while it can also occur as a part of a recognized genetic syndrome, mainly the Ullrich-Turner syndrome, Noonan syndrome, DiGeorge syndrome, and Williams-Beuren syndrome.[12]The Mendelian inheritance of CoA is 1%–2% of all cases,[12]and it is not well understood about genetic causes of sporadic CoA. Although previous cases of familial and genetic mutations have been published, such as NOTCH1,[13,14]MYH6,[15]MATR3,[16]SEMA3D mutation encoding,[17]which play important roles in abnormality of aorta, the underlying mechanism has not been clearly understood yet. In this case, both the patient and the twin brother suffered from CoA, while the parents and the younger brother had no such disease. Hence gene mutation should be taken into consideration besides a developmental defect during the embryonic period. However, after gene detection, there is no known mutation gene related to this disease. Therefore, it is unclear whether the cause of CoA in twin brothers is gene mutation or malformation in embryonic development. Although no known gene mutation was detected in this case, it is undeniable that gene diagnosis provides a new idea for the early embryo screening and pathogenesis of CoA.
It is a correctable cause of hypertension. Therapies for CoA include balloon or stent angioplasty and prosthetic vessel replacement, regardless of the etiology.[9]Both transcatheter and surgery therapy can reduce systemic BP and persistent antihypertensive medication use, at least in the short term and midterm.[18]The patient presented here just accepted the modification of antihypertensive drugs to lower BP rather than transcatheter or surgery therapy for personal reasons. However, even undergoing coarctation repair, treated patients remain at a higher risk of death compared with the general population.[19]
Most of the adverse sequelae of CoA are from refractory hypertension, including accelerated atherosclerosis, aortic dissection, congestive heart failure, claudication and stroke.[20]Patients with other cardiac defects in addition to CoA tend to have worse prognoses.[21]In the second year of follow-up, this patient developed acute cerebral infarction, while the whole brain angiography showed occlusion of right middle cerebral artery and severe stenosis of left vertebral artery, because of significant hemodynamic changes of intracranial vessels, subject to cerebral arteriosclerosis.[22]
In conclusion, CoA, a cause of secondary hypertension, requires careful attention to physical findings to make a diagnosis. It is recommended for adult patients with suspicious CoA to receive CT or MRI to screen possible lesions, providing guidance for further treatment according. To date, the pathogenesis of CoA is unclear and it may be partly attributed to genetics. The possibility of gene mutation should be considered in multiple patients of the same family. Both lethality and disability rates of CoA are high, and the poor outcome of coarctation is improved with surgical repair, but is not still normal.
Acknowledgments
All authors had no conflicts of interest to disclose.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Depression and cardiovascular diseases among Canadian older adults: a cross- sectional analysis of baseline data from the CLSA Comprehensive Cohort
- Hypoxia promotes pulmonary vascular remodeling via HIF-1α to regulate mitochondrial dynamics
- Irregular surface of carotid atherosclerotic plaque is associated with ischemic stroke: a magnetic resonance imaging study
- Pacemaker therapy in very elderly patients: survival and prognostic parameters of single center experience
- Risks of incident heart failure with preserved ejection fraction in Chinese patients hospitalized for cardiovascular diseases
- Delayed cardiac tamponade after simultaneous transcatheter atrial septal defect closure and left atrial appendage closure device implantation: a particular case report
