Trends in treatment and overall survival among patients with proximal esophageal cancer
2019-12-31JudithdeVosGeelenSandraMEGeurtsMargreetvanPuttenLiselotBJValkenburgvanIerselHeikeGrabschNadiaHajMohammadFrankJPHoebersChantalHogePaulJeeneEvelienJMdeJongHannekeWMvanLaarhovenTomRozemaMarijeSlingerlandVivianneCGTjan
Judith de Vos-Geelen, Sandra ME Geurts, Margreet van Putten, Liselot BJ Valkenburg-van Iersel,Heike I Grabsch, Nadia Haj Mohammad, Frank JP Hoebers, Chantal V Hoge, Paul M Jeene,Evelien JM de Jong, Hanneke WM van Laarhoven, Tom Rozema, Marije Slingerland,Vivianne CG Tjan-Heijnen, Grard AP Nieuwenhuijzen, Valery EPP Lemmens
Abstract BACKGROUND The management of proximal esophageal cancer differs from that of tumors located in the mid and lower part of the esophagus due to the close vicinity of vital structures. Non-surgical treatment options like radiotherapy and definitive chemoradiation (CRT) have been implemented. The trends in (non-)surgical treatment and its impact on overall survival (OS) in patients with proximal esophageal cancer are unclear, related to its rare disease status. To optimize treatment strategies and counseling of patients with proximal esophageal cancer,it is therefore essential to gain more insight through real-life studies.AIM To establish trends in treatment and OS in patients with proximal esophageal cancer.METHODS In this population-based study, patients with proximal esophageal cancer diagnosed between 1989 and 2014 were identified in the Netherlands Cancer Registry. The proximal esophagus consists of the cervical esophagus and the upper thoracic section, extending to 24 cm from the incisors. Trends in radiotherapy, chemotherapy, and surgery, and OS were assessed. Analyses were stratified by presence of distant metastasis. Multivariable Cox proportional hazards regression analyses was performed to assess the effect of period of diagnosis on OS, adjusted for patient, tumor, and treatment characteristics.RESULTS In total, 2783 patients were included. Over the study period, the use of radiotherapy, resection, and CRT in non-metastatic disease changed from 53%,23%, and 1% in 1989-1994 to 21%, 9%, and 49% in 2010-2014, respectively. In metastatic disease, the use of chemotherapy and radiotherapy increased over time. Median OS of the total population increased from 7.3 mo [95% confidence interval (CI): 6.4-8.1] in 1989-1994 to 9.5 mo (95%CI: 8.1-10.8) in 2010-2014(logrank P < 0.001). In non-metastatic disease, 5-year OS rates improved from 5%(95%CI: 3%-7%) in 1989-1994 to 13% (95%CI: 9%-17%) in 2010-2014 (logrank P <0.001). Multivariable regression analysis demonstrated a significant treatment effect over time on survival. In metastatic disease, median OS was 3.8 mo (95%CI:2.5-5.1) in 1989-1994, and 5.1 mo (95%CI: 4.3-5.9) in 2010-2014 (logrank P = 0.26).CONCLUSION OS significantly improved in non-metastatic proximal esophageal cancer, likely to be associated with an increased use of CRT. Patterns in metastatic disease did not change significantly over time.
Key words: Esophagus; Esophageal cancer; Proximal; Cervical; Upper thoracic; Trends;Treatment; Survival; Outcome
INTRODUCTION
Esophageal cancer is the seventh most common cancer worldwide[1]. Although the absolute number of deaths has decreased, esophageal cancer is still the sixth leading cause of cancer-related mortality globally[1]. Surgical treatment of patients with esophageal cancer, and in particular treatment of cancer located in the proximal part of the esophagus, is challenging because of the close proximity to vital structures. The proximal part of the esophagus consists of the cervical and the upper thoracic segment. Proximal esophageal cancer is relatively uncommon, accounting for 10% of all esophageal cancer cases[2].
The management of proximal esophageal cancer differs from that of tumors located in the mid and lower part of the esophagus. Patients with proximal esophageal cancer often present with locally advanced disease, for which potentially curative surgery would require extensive mutilating resections, with a high risk of major complications and a significant impact on patients quality of life. To prolong survival and improve quality of life, non-surgical treatment options like radiotherapy and definitive chemoradiation (CRT) have been explored since the 1990s, following promising treatment results of cancers in the thoracic esophagus, hypopharynx, and non-smallcell lung cancer[3-6]. In a meta-analysis in 2006, Wonget al. showed that the addition of chemotherapy to radiotherapy for the definitive treatment of esophageal cancer significantly increased response and overall survival (OS) rates[7].
Therefore, definitive CRT is recommended as treatment modality for patients with non-metastatic proximal esophageal cancer[8,9]. However, only four of the 19 studies in the aforementioned meta-analysis incorporated patients with proximal esophageal cancers, limiting the extrapolation of these findings to the proximal esophagus.
Separate OS rates for patients with proximal esophageal cancer are largely lacking from clinical trials, due to exclusion of this subpopulation or related to its rare disease status. To optimize treatment strategies and counseling of patients with proximal esophageal cancer, it is therefore essential to gain more insight in patient characteristics, provided therapies and OS through real-life studies.
The aim of this population-based cohort study was to establish the trends in treatment and OS in patients diagnosed with non-metastatic or metastatic proximal esophageal cancer in a nationwide registry between 1989 and 2014.
MATERIALS AND METHODS
Patients
All patients with a tumor located in the cervical or upper thoracic esophagus diagnosed between 1989 and 2014 were identified in the Netherlands Cancer Registry(NCR). The NCR is a population-based cancer registry of all residents of the Netherlands. The NCR is linked to the national automated pathological archive,which leads to the automatic inclusion of all newly diagnosed malignancies in the Netherlands. Additional data sources linked to the NCR are the national hospital discharge register and registers of radiotherapy institutions. Information on vital status was obtained through annual linkage with the Municipal Administrative Database, in which all deceased or emigrated individuals in the Netherlands are registered. This study was approved by the Privacy Review Board of the NCR and the need for a separate approval from an ethics committee in the Netherlands was waived.
Definitions
Topography and histolo gy were coded according to the International Classification of Diseases for Oncology (ICD-O)[10]. ICD-O histology codes were used to classify tumors as squamous cell carcinoma (SCC), adenocarcinoma, and other origin. Cancers of the proximal esophagus can be subdivided in cancers originating in the cervical esophagus (CEC, ICD-O C15.0), commencing at the lower border of the cricoid cartilage and ending at the thoracic inlet, approximately 18 cm from the incisors, and cancers in the upper thoracic section (UTEC, ICD-O C15.3), extending from the thoracic inlet to the level of the tracheal bifurcation, which is approximately 24 cm from the incisors[11].
Tumor staging was registered according to the Union for International Cancer Control TNM classification that was valid at the time of diagnosis. As the classification of tumor stage (cT) was reasonably comparable from the TNM-4 to -6,but changed with the introduction of the 7thedition in 2010, we converted all tumor and lymph node stages according to TNM-6thedition. Patients with a cM1a tumor according to TNM-6thedition, defined as cervical lymph node involvement, were categorized as having a positive lymph node status (cN+). Patients with unknown metastatic status (cMx) were included in the non-metastatic group.
All treatments for the primary disease stage were registered. Treatment categories included resection, neoadjuvant treatment and resection, radiotherapy,chemotherapy, radiotherapy and chemotherapy, other treatment, and no (anti-cancer)treatment. Resection included patients who received a surgical resection or an endoscopic excision (n= 20). The group of “neoadjuvant and resection” comprised patients who underwent a resection, preceded by radiotherapy, chemotherapy or with concurrent CRT. The group “radiotherapy and chemotherapy” included patients who were treated with sequential or concurrent radiotherapy and chemotherapy,without any resection. Other treatments were not otherwise specified (palliative)treatments. “Other treatment” and “no (anti-cancer) treatment” were summarized as“no localized treatment”. Type of surgical treatment and details on chemotherapy or radiotherapy were not collected by the data clerks of the NCR.
Five-year periods of diagnosis were defined: 1989-1994, 1995-1999, 2000-2004, 2005-2009, and 2010-2014.
Statistical analysis
OS was calculated by period of diagnosis using the Kaplan-Meier method and a comparison between groups was made using the log-rank test. OS was defined as the time from diagnosis to death from any cause, censored at last follow-up date or untill February 1, 2017. The median follow-up time was calculated using the reverse Kaplan Meier method (death censored). Multivariable Cox proportional hazards regression analyses were performed to assess the effect of period of diagnosis on OS, adjusted for age, histological type, tumor location, cT category, cN category, and treatment modality. Variance inflation factors were calculated to assess the degree of multicollinearity among the independent variables in the Cox proportional hazard model. Analyses were stratified by the presence of metastatic disease (cM0vscM1),tumor location (CECvsUTEC), and histological type (SCCvsadenocarcinoma). As the interaction analysis did not show any difference in OS between tumor location,i.e., cervical or upper thoracic site, and histology, results are presented by presence or absence of metastatic disease.
The statistical review of the study was performed by two senior epidemiologists.
RESULTS
Study population
We identified 2783 patients diagnosed with proximal esophageal cancer in the Netherlands between 1989 and 2014 (Table 1). The median follow-up time of all patients was 103 mo [95% confidence interval (CI): 91-117 mo]. Fifty-six percent of patients were male, and 47% were between 60 and 74 years old at the time of diagnosis. In total, 81% of cancers were SCC. Two percent of the patients were diagnosed with clinical stage 1, 20% with stage 2, 28% with stage 3, 21% with stage 4,and 29% with unknown stage disease. The number of patients with unknown stage disease decreased over time. In 2010-2014, 27% of patients had been diagnosed with another malignancy prior to the diagnosis of proximal esophageal cancer (data not shown).
Trends in treatment in patients with proximal esophageal cancer
In patients with non-metastatic disease, the proportion of patients treated with CRT alone increased from 1% in 1989-1994 to 49% in 2010-2014 (Figure 1A). Resection without neoadjuvant treatment was performed in 17% of patients in 1989-1994 and in 2% of patients in 2010-2014. The proportion of patients treated with neoadjuvant therapy and resection was relatively constant over time, varying between 3% and 7%.The proportion of patients with non-metastatic proximal esophageal cancer that did not undergo any form of treatment varied between 15% and 22%, without a cleartrend over time.
For patients with metastatic disease, only minor variations in treatment were observed (Figure 1B). Fourty-four percent of patients were treated with radiotherapy alone in 1989-1994, which slightly decreased to 37% in 2010-2014. Over time,multimodal treatment of chemotherapy and radiotherapy, concurrent or sequential,was administered more frequently: In 3% of patients in 1989-1994 and 23% of patients in 2010-2014. Chemotherapy alone was given to 7%-12% of patients in all time periods. The proportion of patients diagnosed with metastatic proximal esophageal cancer who did not undergo any form of anti-cancer treatment decreased from 33% in 1989-2004 to 24% in 2010-2014.
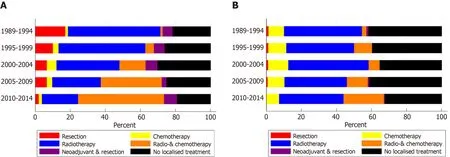
Figure 1 Treatment of patients with proximal esophageal cancer in the Netherlands between 1989 and 2014. A: Patients with non-metastatic proximal esophageal cancer; B: Patients with metastatic proximal esophageal cancer.
Trends in survival in patients with proximal esophageal cancer
The median OS of the total population of patients with proximal esophageal cancer was 8.0 mo (95%CI: 7.6-8.5 mo). Median OS increased over the study period, from 7.3 mo (95%CI: 6.4-8.1 mo) in 1989-1994, to 9.5 mo (95%CI: 8.1-10.8 mo) in 2010-2014(logrankP< 0.001) (Figure 2). In patients with non-metastatic proximal esophageal cancer, 1- and 5-year OS rates improved from 30% (95%CI: 26%-34%) and 5% (95%CI:3%-7%) in 1989-1994, to 44% (95%CI: 40%-48%) and 13% (95%CI: 9%-17%) in 2010-2014, respectively (logrankP< 0.001) (Figure 3A). Median OS of patients with nonmetastatic proximal esophageal cancer was 8.0 mo (95%CI: 7.0-8.9 mo) in 1989-1994 and 13.3 mo (95%CI: 11.1-15.5 mo) in 2010-2014. Patients with stage 1 disease showed the most favorable outcome with a 1- and 5-year OS rate of 70% (95%CI: 57%-80%)and 22% (95%CI 13%-34%), compared with 50% (95%CI: 46%-54%) and 15% (95%CI:12%-18%) in stage 2, and 35% (95%CI: 32%-38%) and 10% (95%CI: 8%-13%) in stage 3 disease, respectively (logrankP< 0.001) (Supplementary Figure 1).
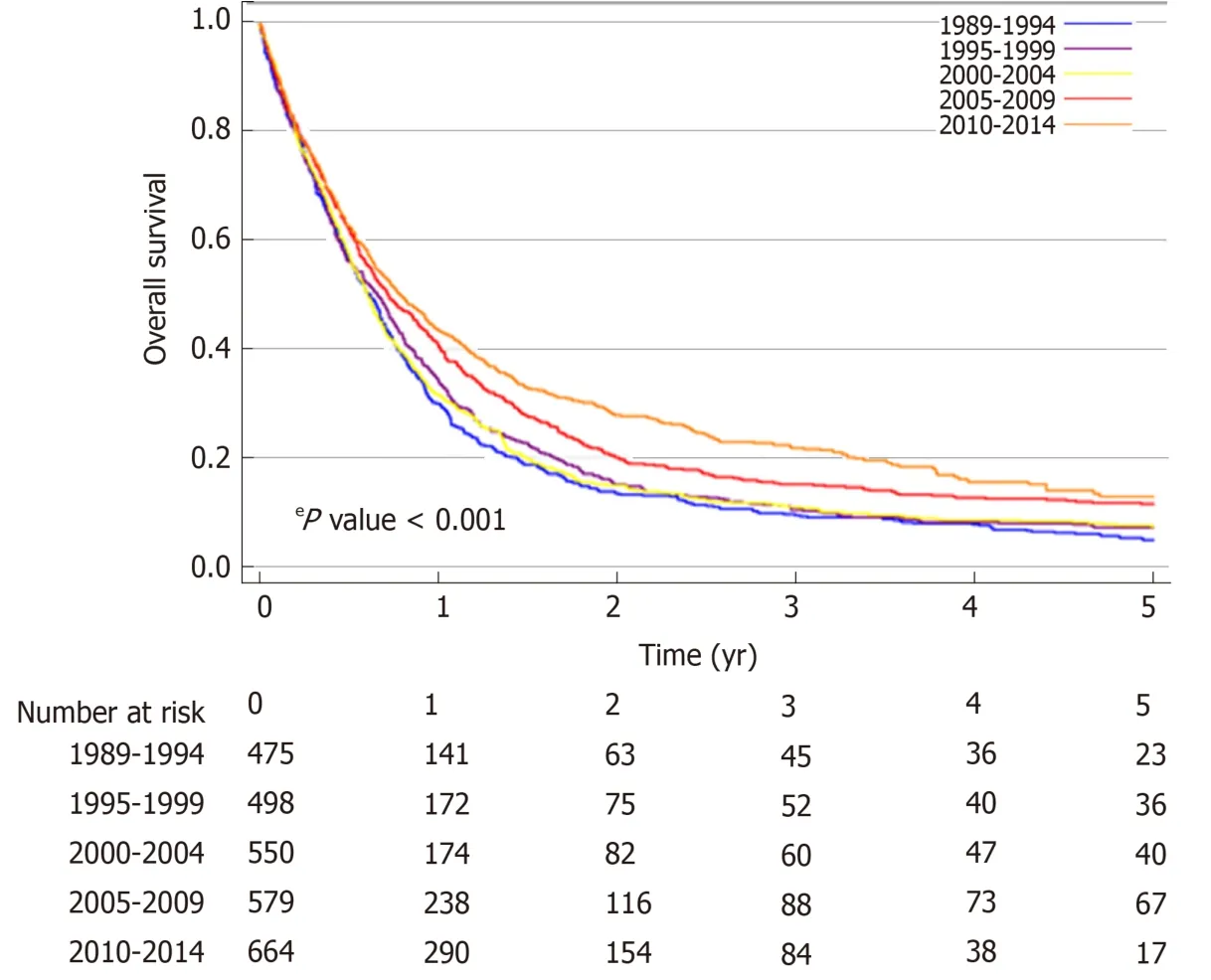
Figure 2 Overall survival by 5-year period of diagnosis of patients with proximal esophageal cancer in the Netherlands between 1989 and 2014, irrespective of stage at diagnosis.
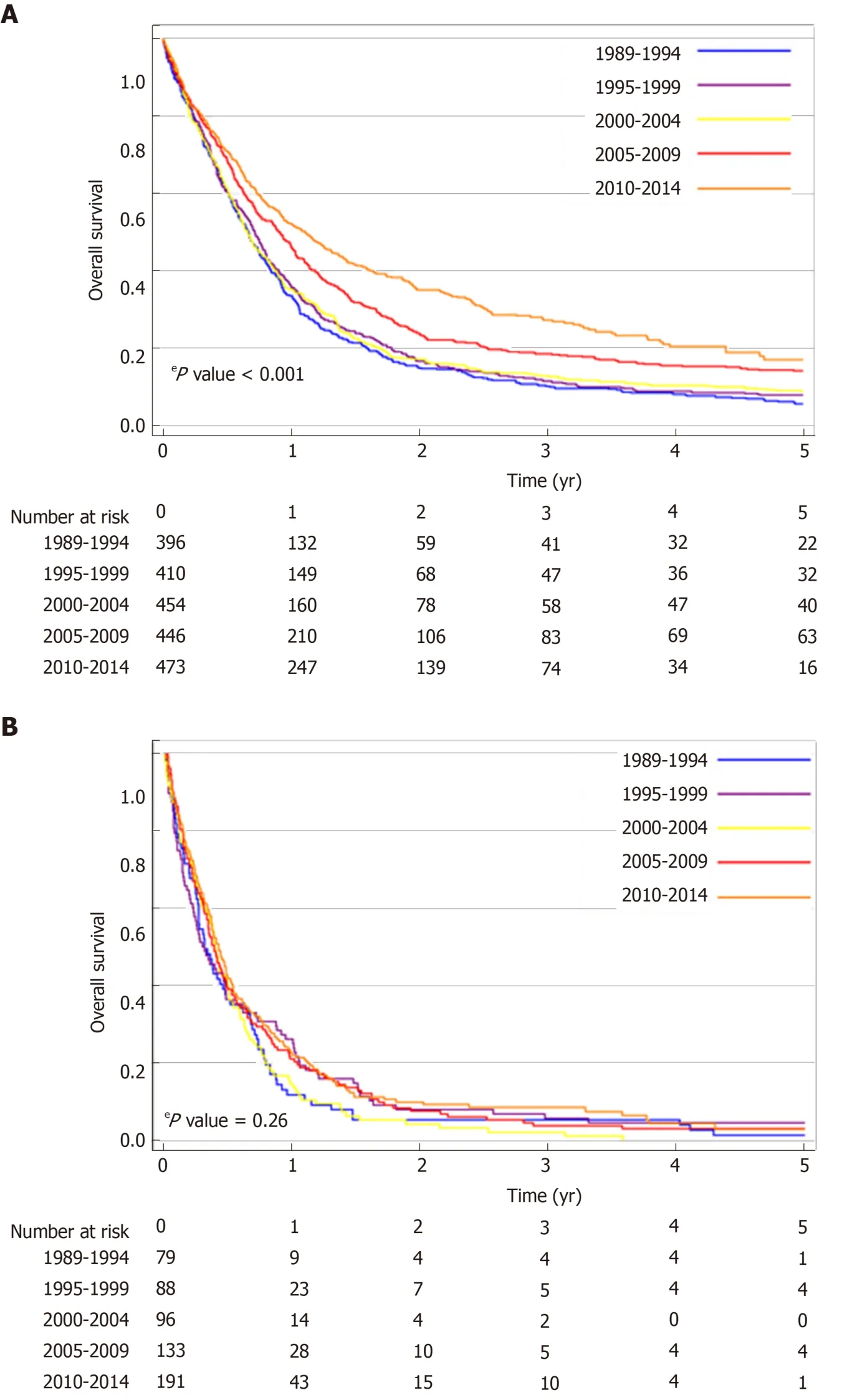
Figure 3 Overall survival by 5-year period of diagnosis of patients with proximal esophageal cancer in theNetherlands between 1989 and 2014. A: Patients with non-metastatic proximal esophageal cancer; B: Patients with metastatic proximal esophageal cancer.
In patients with non-metastatic proximal esophageal cancer, univariable analysis showed that period of diagnosis, age, histological type, cT, cN, and treatment were all associated with OS (Table 2). OS was similar for patients diagnosed with CEC or UTEC. Multivariable Cox regression analysis adjusted for age, histological type,tumor location, cT, and cN demonstrated an OS benefit for patients diagnosed in 2005-2009 [Hazard ratio (HR) = 0.77,P< 0.001] or 2010-2014 (HR = 0.72,P< 0.001)when compared with patients diagnosed in 1989-1994. However, the time period effect dissapeared after additional inclusion of treatment modality in the multivariable model. All treatment modalities had a statistically significant effect on OS compared with no localized treatment (P< 0.001). Patients with non-metastatic proximal esophageal cancer treated with surgery with or without neoadjuvant therapy or treated with definitive CRT showed 5-year OS rates of 31% (95%CI: 23%-40%), 21% (95%CI: 16%-28%), and 22% (95%CI: 19%-26%), respectively (logrankP=0.32) (Supplementary Figure 2).
In patients with metastatic disease, OS did not change significantly over time(logrankP= 0.26) (Figure 3B). Median OS was 3.8 mo (95%CI: 2.5-5.1 mo) in 1989-1994 and 5.1 mo (95%CI: 4.3-5.9 mo) in 2010-2014. One-year OS rate was 12% (95%CI: 6%-20%) in 1989-1994 and 23% (95%CI: 17%-29%) in 2010-2014.
DISCUSSION
In the Netherlands, median OS of patients with proximal esophageal cancer significantly increased by approximately two mo between 1989 and 2014. In patients with non-metastatic proximal esophageal cancer, 5-year OS almost tripled to 13% in 2010-2014, although the absolute longterm outcome remains poor. Multivariable analysis showed that improvements in treatment over time might have led to this survival benefit. The improvement is likely to be attributable to the implementation of CRT in the late nineties, accounting for almost 50% of treatment choices in nonmetastatic proximal esophageal cancer nowadays. The proportion of patients who did not receive any anti-cancer treatment remained remarkably high, being one in five patients with non-metastatic and one in four patients with metastatic proximal esophageal cancer, which may be a reflection of the poor performance status of these patients.
We observed that in the patients with non-metastatic proximal esophageal cancer(n= 2194), the median OS improved from 8 mo in 1989-1994 to 13 mo in 2010-2014,with comparable OS between CEC and UTEC. Considering OS in patients with metastatic disease did not improve significantly over time, stage migration was not expected to be a major contributor to the improved survival in the non-metastatic group. A Surveillance, Epidemiology, and End Results (SEER) data-based study in 362 patients with non-metastatic CEC diagnosed between 1998 and 2008 showed a longer median OS,i.e., 14 mo[12]. The shorter median survival observed in our study may partly be explained by the inclusion of patients with a history of previous malignancies, whereas the SEER data-based study excluded these patients. In addition, we included patients with unknown metastatic status in the group of patients with non-metastatic disease, which could have lead to an underestimation of the OS in the non-metastatic patient group.
Our study showed a reduction of surgical approaches from 23% in the earliest time period to 10% in the most recent period. The aforementioned SEER population-based study showed similar results, where only 11% of patients with cervical esophageal cancer underwent surgery and 79% radiotherapy (chemotherapy data were not available)[12]. These findings confirm a different approach in the management of proximal esophageal cancer in specific as compared with cancers from all sites of the esophagus. In the latter group the proportion of patients treated with surgery remained relatively stable over time, from 25% between 1989 and 2004, to 29%between 2010 and 2014[2].
Considering bias by indication, we hypothesized that patients with resectable tumors, undergoing surgery, might show a superior outcome when compared with CRT. However, in the current population-based study, we observed a comparable OS in patients treated with surgeryvsthose treated with definitive CRT which is consistent with a recent observational study in 148 patients with cervical esophageal cancer[13]. The current study showed that period effect in the multivariable model dissapeared after including treatment modality. These findings suggest that improvements in the (non-surgical) treatment had a substantial effect on the observed improvement in OS. However progress in OS may also have partly occurred due to advancements in the management of non-cancer related high mortality disorders,e.g.,cardiovascular disease[14]. Figures from Statistics Netherlands show that the remaining life expectancy for, for example, an average 65 year old person was 17 years in 1989 and 20 years in 2014[15]. Whether this increase in life expectancy is also seen in the high-risk population presented in our study is unknown.
In patients with metastatic proximal esophageal cancer, we did not observe any significant improvements in OS over time. These findings are in contrast to previous population-based studies, observing an increased survival over the years in the total group of patients with metastatic esophageal cancer patients, including 10% of cancers originating from the proximal esophagus[16,17]. This difference in the trend in OS may be explained by the more prominent increased use of systemic therapy in metastatic adenocarcinomas[2], which are more common in the distal part of the esophagus[18]. For example, in patients with HER2 amplified adenocarcinomas of the distal esophagus, HER2 directed therapies have led to a survival benefit[19]. In metastatic SCC, palliative systemic therapy is scarcely applied[2]. A recent metaanalysis, however, showed that systemic therapy in patients with metastatic SCC improved OS and quality of life, and is considered standard of care[20]. The outcomes of patients with metastatic SCC is expected to improve in the coming decades,because the pace of development of cancer immunotherapies is accelerating. Recent studies show clinical evidence of efficacy of immune checkpoint inhibitors in SCC of the esophagus[21,22], and are expected to be approved for implementation in clinical practice.
Furthermore, since proximal esophageal cancer is extremely rare, development of high-volume expert centers is challenging. Centralization of surgery in esophageal cancer has led to an increased survival in resectable esophageal cancer[23]. A recent Dutch study showed that center volume of palliative systemic therapy for metastatic esophagogastric cancer was associated with improved survival, suggesting a volumeoutcome relationship[24]. Giving the low incidence rate and the challenging performance status of these patients, this could be a plea for centralization of care for patients with proximal esophageal cancer.
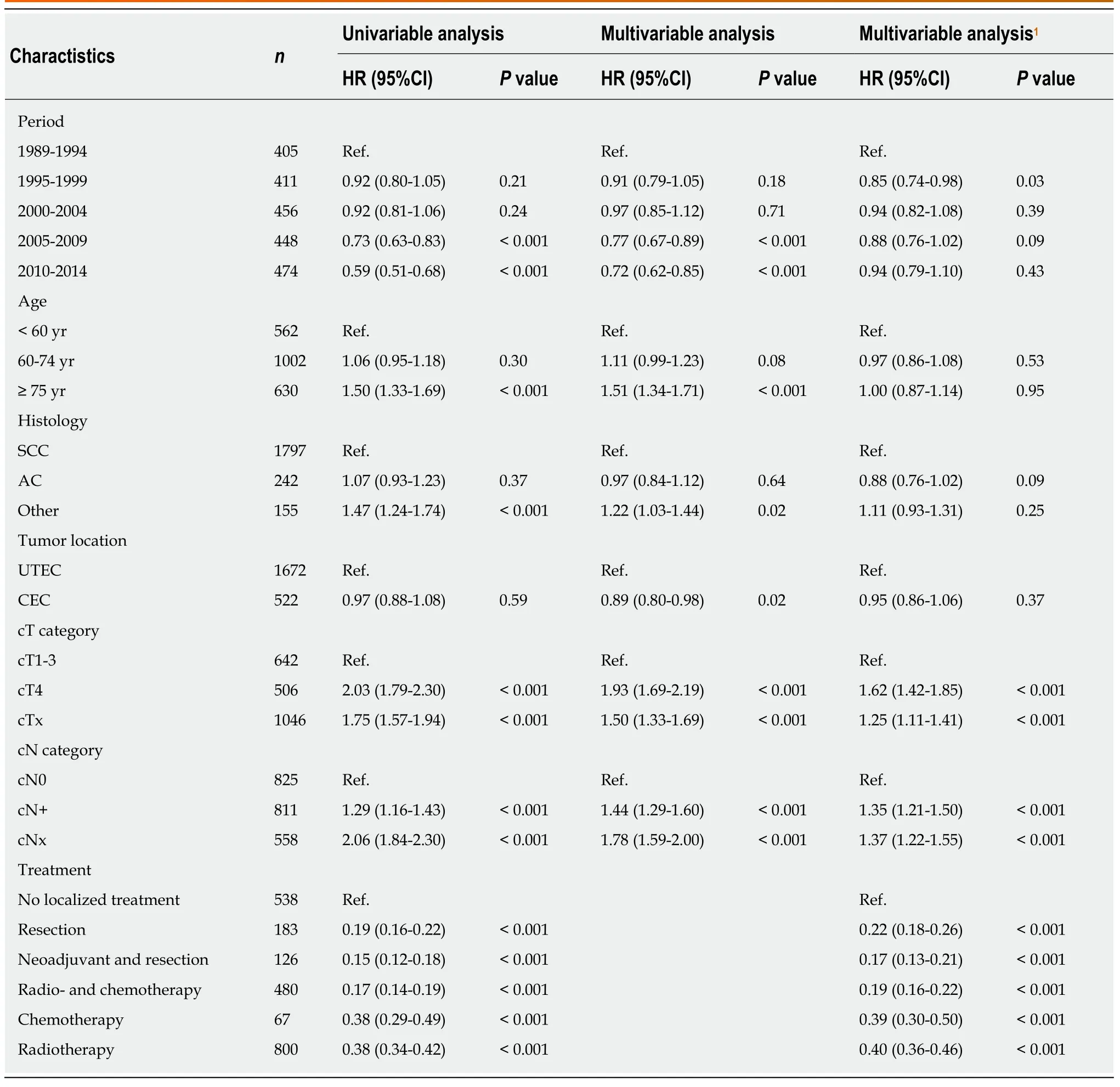
Table 2 Univariable and multivariable hazard ratios for overall survival of patients diagnosed with non-metastatic proximal esophageal cancer (n = 2194)
The retrospective nature of this study is inherent with some limitations mainly attributable to the availability of information. Coding of the tumor was being performed on the basis of topography, extracted from the medical records depending on input of physicians and interpretation of administrators, posing a risk of misclassification. The NCR does not include information on treatment techniques,schedules, and its related toxicities, causing interpretation adversity. Furthermore,data regarding risk factors,e.g., smoking behaviour and alcohol consumption,comorbidity, performance status, and disease specific cause of death were not available, resulting in a risk of residual confounding. However, our multivariable model showed that the period effect almost completely dissapeared after including treatment modalities to the multivariable model, implicating that there are no major confounders missing.
The strength of our study is that it is a large population-based cohort. This nationwide cohort of patients with proximal esophageal cancer in the Netherlands represents daily clinical practice, reflecting real-life treatment and survival. Moreover,the follow-up period can be considered long, given the relatively short survival time of patients with proximal esophageal cancer.
In conclusion, this nationwide study in patients with proximal esophageal cancer showed an increasing use of definitive CRT over the study period, with improved survival in non-metastatic disease, although long-term result is still rather poor.
ARTICLE HIGHLIGHTS
ACKNOWLEDGEMENTS
The authors would like to thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry.
杂志排行
World Journal of Gastroenterology的其它文章
- Epidemiology of inflammatory bowel disease in South America: A systematic review
- Direct costs of carcinoid syndrome diarrhea among adults in the United States
- Influence of bile contamination for patients who undergo pancreaticoduodenectomy after biliary drainage
- MiR-96-5p inhibition induces cell apoptosis in gastric adenocarcinoma
- Non-SMC condensin I complex subunit D2 and non-SMC condensin II complex subunit D3 induces inflammation via the IKK/NF-κB pathway in ulcerative colitis
- Expanding the donor pool: Hepatitis C, hepatitis B and human immunodeficiency virus-positive donors in liver transplantation
