Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling
2019-12-31NiphaChaicharoenaudomrungPhongsakornKunhormParinyaNoisa
Nipha Chaicharoenaudomrung, Phongsakorn Kunhorm, Parinya Noisa
Abstract
Key words: Three-dimensional cultures; Cancer; Stem cells; Disease modeling; In vitro screening platform
INTRODUCTION
Cancer is one of the most serious diseases causing almost one in six deaths globally,which is estimated to equal 9.6 million deaths in 2018[1].Considerable efforts have been intended to develop effective approaches to cure cancer.Among them, drug discovery could be one of the most important approaches aiming to identify and verify new and potent anticancer agents for both daily medication and chemotherapy.For testing the capability of novel anticancer drugs, the experiments are performed on cell-based assays, which offer information about cellular responses to drugs in cost/time effective and high throughput manners.
Currently, two-dimensional (2D) platforms in which flat monolayer cells are cultured is still the most commonly used for the research of cell-based assays.The 2D cell culture systems are easy, convenient, cost-effective, and widely used.However,various drawbacks and limitations are still of concern.The first drawback of a 2D cell culture systems is that an actual three-dimensional (3D) environment in which cancer cells residein vivois not accurately mimicked[2].The irrelevant 2D environment may provide misleading results regarding the predicted responses of cancer cells to anticancer drugs[3].Generally, standard preclinical screening procedures for therapeutic agents involve identification of compounds from the 2D cell culture system tests and animal model tests and then to the introduction of clinical trials[4].Along with each phase, the percentage of efficient agents dramatically decreases.Less than 5% of anticancer agents and small molecule oncology therapeutics passed the clinical trials and were finally approved for marketing by the regulatory agencies[3].One possible cause of the failure is that drug responses of 2D cell cultures systems did not consistently predict the outcome of clinical studies[5-7].
The key limitation of traditional 2D culture is the failure to imitate thein vivoarchitecture and microenvironments.As a consequence, there are many different features that 2Dcultured cells possess compared within vivocells such as morphological characteristics, proliferation and differentiation potentials, interactions of cell-cell and cell-surrounding matrix, and signal transduction[8,9].Such concerns inspired the emergence of 3D cell cultures systems, a promising approach to overcome the inconsistency between cell-based assays and clinical trials.The 3D cell culture systems provided the novel cell-based assays with more physiological relevance, especially the behavioral similarity to thein vivocells.Over the last decade,a variety ofin vitroplatforms was developed to achieve the 3D culture systems for cancer and stem cell applications such as novel drug development, cancer and stem cell biological research, tissue engineering forin vivoimplantation, and other experimental cell analyses[10-12].Thus, the study of cellular phenomena in a conditions that closely imitatesin vivoscenery could be elaborately constructedin vitro[11,13,14].
Here, we aim to demonstrate the necessity of novel 3D cell culture systems and describe, compare, and contrast the 3D cell cultures techniques that has been developed to date.In addition, we also present the possibility to be applied in cancer and stem cell aspects.
CELL CULTURE AS A RESEARCH MODEL
In 1907, Harrisonet al[15]implemented the cell culture technique to his research,exploring the origin and the development of nerve fibers.Since then, the technique has been continuously improved, and cell-based experiments can be effortlessly conducted based on such cell culture technique due to cell banking[16].The selection of cell culture procedures for cancer research is the key for the better understanding of tumor biology, resulting in the optimal and effective conditions for radio/chemotherapy as well as the discovery of new cancer treatment strategies[17].At the very beginning of the cell culture era, the cultures were mostly carried out under an adherent condition, which is called the 2D monolayer cell culture model[18].However, thein vivoenvironment provides cell-cell and cell-extracellular matrix(ECM) interactions in a 3D structure[19], and the 2D monolayer cells might not accurately mimic the actual 3D environment of thein vivocells.The clear evidence was the experiments using the immortalized tumor cell lines grown in the 2D culture systems resulted a 95% drug response failure rate in human subjects.It indicated that the 2D cell culture model could be an inaccurate model for drug development[20].Therefore, the drug discovery and validation processes should integrate both 2D cell culture screening and animal study, complying with the standard procedure prior to clinical trials.Nonetheless, the data collected from the 2D cell system are often misleading forin vivoresponses as previously mentioned, and the animal models are expensive, time consuming, controversial with ethical dilemmas, and inconsistent due to species differences[21].The development of novel models is needed to resolve the inconsistency between the 2D cell culture systems, animal models, and clinical trials.Therefore, the 3Din vitrocell culture platforms could be the potential candidate[22].
TWO-DIMENSIONAL VS THREE-DIMENSIONAL CELL CULTURE MODELS
Cell culture is the most basic yet essential process for preclinical drug discovery.Even though the unreliable flaws of monolayer cell culture have been pointed out, 2D cell culture models are still the first option that scientists turn to due to its simplicity in order to obtain preliminary results.Nevertheless, 2D cultures may not sufficiently mimic the physiological conditions in a 3D network wherein vivocells reside.Therefore, deceptive data from 2D cell culture model often leads to the irrelevant prediction of drug efficacy and toxicity and finally causes the failure in drug validation and approval processes[23].
One obvious advantage of cell culturing in a 3D manner over 2D cell culture is that it contributes the expression of ECM components as well as the interactions between cell-cell and cell-matrix.The characteristics of 3D cell cultures and the traditional 2D cell culture models are shown in Figure 1.The traditional 2D cell cultures result in a monolayer cell expanding on a flat surface of glass or commercial polystyrene plastic flasks for tissue culture (Figure 1A).In contrast, 3D cell cultures promote cells to form 3D spheroids by utilizing an ECM material (Figure 1Β).Cell spheroid is the important characteristic that resemblesin vivocells for further replicating cell differentiation,proliferation, and functionin vitro.Thus, 3D spheroid culture is considered an improved model for predictivein vitrocell-based assays and may deliver high physiological relevance for preclinical drug discovery, especially in cancer/stem cell research.
Generally, cells of multicellular organisms capable of forming tissues are in 3D arrangements with complex interactions within cell populations and also between cells and environments.With the dynamics of nutrient and chemical transport between cells in thein vivoconditions, cells are hemostatically provided with a relatively constant supply of nutrients with the minimized level of waste products due to the activity of the circulatory system.Therefore, the 3D arrangements of cells are the major employment for 3D cell culture with the optimal spatial organization of cells in the culture environment to be considered[24-26].When cells are grown in 3D culture systems, cells also induce the formation of aggregates or spheroids within matrix or the culture medium.Even though with cell-cell interactions and cell-matrix interactions are not yet perfectly mimicked in a spheroid culture model, they are close enough to induce the morphological alteration of cells to not be relatively flat but closely resemble its natural shape in the body (Figure 1C).
Furthermore, within the spheroid structure, various stages of cells are established,including proliferating, quiescent, apoptotic, hypoxic, and necrotic cells due to the gradients of nutrients and oxygen level[27,28].The proliferating cells could be found mainly at the outer layer of the spheroids because they are exposed to sufficient amounts of nutrients from the culture medium[29,30].Cells at the core of spheroids tend to be in quiescent or hypoxic states because they are faced with the lack of oxygen,growth factors, and nutrients[31].The cellular heterogeneity within a cell population is quite relevant toin vivotissues, organs, and even tumors.At this point, due to cell morphology, interactions, and heterogeneity of cells grown in 3D culture, it is reasonable to hypothesize that the cellular processes of these cells are also applicable[32].
Comparisons of 3D spheroid culture models and 2D monolayer cell culture models were shown in Table 1.Numerous studies have proven the differences in cell viability, morphology, proliferation, differentiation, cellular responses to stimuli, cellcell communication, cell stiffness, migrant and invasive properties of tumor cells into surrounding tissues, angiogenesis stimulation and immune system evasion, drug responses, transcriptional and translational gene expression, general cell function, andin vivorelevance between cells cultured in 2D and 3D models.For example, cell polarization could be more accurate depicted in 3D cell cultures models unlike in 2D models in which the cells can only be partially polarized.Moreover, greater stability and longer lifespans were found in 3D culture models; 3D spheroids can be cultured up to 3 wk, whereas 2D monolayer culture can last for less than a week due to the limitation of cell confluence[33].Therefore, 3D cell culture models might be more appropriate for handling the long-term experiments and for determining long-term effects of the drug on cellular responses.
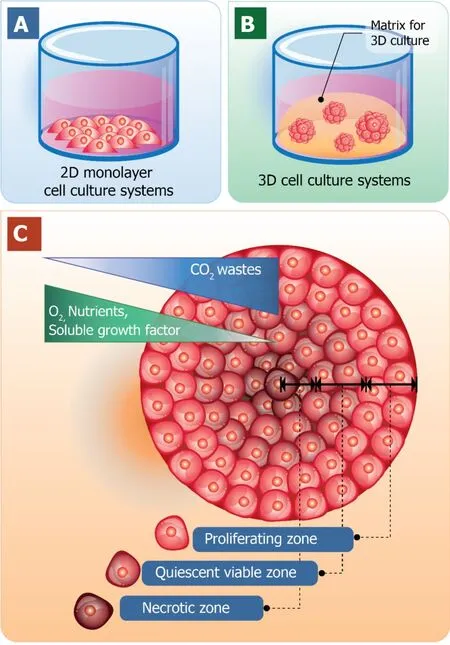
Figure 1 Schematic diagrams of the traditional two-dimensional monolayer cell culture and threedimensional cell culture systems.
THREE-DIMENSIONAL CELL CULTURE TECHNOLOGIES
Βecause the advantages of 3D culture systems have become widely realized, there have been many studies intensively focused on the development and optimization of 3D cell culture technologies.With the integration of the recent advances in cell biology, microfabrication techniques, and tissue engineering, a wide range of 3D cell culture platforms were constructed, including multicellular spheroid formation(liquid overlay culture and hanging drop method), hydrogel-based culture,bioreactor-based culture, bio-printing, and scaffold-based culture.A summary of the advantages, disadvantages, and research stage of each model are shown in Table 2.Although each 3D culture technique/platform are different in both principle and protocol, the same objectives that they share are to provide the similar features ofin vivocells in morphological, functional, and microenvironmental aspects.This section aims to briefly describe the key features of each technique.
Multicellular spheroids formation
Liquid overlay culture:Liquid overlay culture could be the simplest of all 3D cell culture Techniques (Figure 2A).To create 3D culture models, the surface for cell culture is covered with a thin film of inert substrates, such as agar[34], agarose[35], or matrigel[36].Βy preventing cell adhesion on the surface and providing the artificial matrix, liquid overlay culture easily promotes the aggregation of cells to become spheroids[37].This technique is cost-effective and highly reproducible without requirement of any specific equipment[38].Different cell types can be cocultured with this technique[39].However, the number and size of formed spheroids are difficult to monitor[40].Recently, ultra-low attachment plates have been developed and commercialized for the liquid overlay technique.Such plates contain individual wells with a layer of hydrophilic polymer on the surface to overcome the requirement for manual coating, which prevents cell attachment.The specifically designed plates exhibit the capability to produce one spheroid per well and is favorable enough for medium-throughput applications[41].

Table 1 Differences in two-dimensional vs three-dimensional cell culture models
Hanging drop technique:The hanging drop technique for 3D spheroid production was introduced by Johannes Holtfreter in 1944 for cultivating embryonic stem cells.The technique later became the foundation of scaffold-free 3D culture models capable of multicellular spheroid generation.Resulting spheroids could be generated with consistent size and shape controlled by adjusting the density of cell seeding.As few as 50 cells up to 15000 cells could be varied to obtain the desirable size of spheroids[42].In the very beginning, the hanging drop technique was carried out in the petri dish lid,by dropping a small volume of cell suspension (15-30 μL) with a specific cell density onto the lid.Then, the lid was subsequently inverted and aliquots of cell suspension turned into hanging drops without dripping due to surface tension.Consequently,cells were forced to accumulate at the bottom tip of the drop, at the liquid-air interface, and further aggregate and proliferate until spheroids were formed (Figure 2Β).Recently, bioassay dishes have been used in place of petri dishes for more wellcontrolled experiments to facilitate the maintenance of moisture levels of the culture system, so that cell culture can be done in the same manner of standard cell culture procedures.
The hanging drop technique is relatively simple and applicable for numerous cell lines, and its reproducibility can be almost 100% for generating one 3D spheroid per drop[42].The 3D spheroid obtained from this technique tends to be tightly packed rather than aggregated loosely, and low variability in sizes were observed.Kelmet al[42]reported that 3D spheroids exhibited patho/physiologically relevance because their structures were highly organized along with their produced ECM and turned to be a ‘tissue-like’ structure.As this technique is based on the tendency of cells to aggregate to each other spontaneously instead of depending on the provided matrices or scaffolds, the problematic concerns regarding the effects from 3D structure formation are reduced.However, the undeniable drawback of the hanging drop technique is the limited volume of the cell suspension.Only up to 50 µL of suspension, including the testing medium, can be accommodated onto the upside down surface unless dripping occurs as the surface tension is not enough to keep liquids attached on the surface[43].Another limitation is the difficulty in changing culture medium during cultivation without disturbing the spheroids[31].

Table 2 Proposed advantages, disadvantages, and research stage of different three-dimensional cell culture methods
Hydrogels:Hydrogels are the networks of cross-linked polymeric material, which are generally composed of hydrophilic polymers with high water content (Figure 2C)[44].There are the swollen structures or microspheres integrated within the network for cell encapsulation and the circulation of nutrients and cellular waste in and out of the hydrogels[45].Additionally, gels exhibit a soft tissue-like stiffness to potentially resemble natural ECM because they are made from mixtures of natural polymers such as collagen, and alginate, two of the most used substrates in 3D cell culture history[46].
The most common use of hydrogels is to be combined with a reconstituted basement membrane preparation extracted from mouse sarcoma, which has been commercialized by the Matrigel trademark (Corning Life Sciences, Tewksbury, MA,United States).Even though such commercialized hydrogels are rich in ECM proteins,they also possessed some drawbacks, including the deficiency in gelation kinetic control, the undefined and uncontrollable polymer composition, and lack of mechanical integrity.Lot-to-lot variability due to manufacturing mistakes and poorly defined composition also cause difficulty to determine the exact responses of cells to some particular stimuli[47].
Generally, hydrogels are fabricated based on both synthetic and natural polymers,which are water-absorbing, hydrophilic, and highly flexible materials.With the wellcontrolled fabrication processes and well-defined material composition, hydrogels have become the prominent materials for 3D scaffold development.Βecause of their structural similarities to natural ECM, they are favorable forin vivochemical delivery in a noninvasive manner[44].A number of synthetic and natural materials can be incorporated into hydrogel formation, such as hyaluronic acids, polyethylene glycol[48], collagen, gelatin, fibrin, alginate, and agarose[49].However, the natural hydrogels, like Matrigel and alginate gel, are considered to be more appropriate cellencapsulated materials due to the great biocompatibility and mild gelling conditions.

Figure 2 Different techniques used for three-dimensional cell cultures.
The hydrogel technique for cell culture in a calcium alginate hydrogel was first developed by Limet al[50]by mixing the cells with the alginate solution, then crosslinking and forming the hydrogel-based microspheres in an isotonic CaCl2solution(Figure 2C).The alginate hydrogels are very limited for cell adhesion, which is an advantage for cell encapsulation applications[51]that provide rapid, nontoxic, and versatile immobilization of cells within polymeric networks.In addition, the creation of artificial organs was also consolidated with encapsulating cells or tissue for the treatment of disease.The most well-known example was an artificial pancreas to be used in diabetes therapy[45].
The 3D cell culture can also be carried out in hydrogels and can be integrated with other cell culture models such as cell spheroid cultures, scaffold-based cell cultures,and microchip-based cell cultures[52].Hydrogels are one potential technique to be used for 3Din vitrotechnology due to their biocompatibility, sufficient water content, and ECM-like mechanical properties[53].Although hydrogels were not popularly applied to the field of drug screening, they have been widely used for the development of tissue engineering by mimicking cartilage, vascular, bone, and other tissues by mixing particular cells to hydrogel precursors before the gelling process in which cells are distributed evenly and homogeneously throughout the gels.
One reported case was the engineered cardiac tissues obtained from the neonatal rat cardiac myocyte culture in collagen hydrogels that were used for cyclic mechanical stretch research[54].Hydrogels also facilitate the delivery of soluble or signaling molecules to cells and providing the supportive surroundings for cell growth and function.For example, transforming growth factor β was infused into polyethylene glycol hydrogels to govern the function of smooth muscle cells.In a similar manner,bone morphogenetic protein was covalently attached to alginate hydrogels to govern osteoblast migration and calcification[55].Despite a variety of hydrogel type applications, Ca-alginate hydrogels are surely a potent candidate system for the delivery of cells to the infarcted heart because they are nontoxic, nonimmunogenic, do not facilitate pathogen transfer, and allow good exchange of waste products and nutrients[56,57].Ca-alginate hydrogels were primarily implanted into the heart and shown not to induce harmful responses such as thrombosis[56]or fibrosis[58].The gradual degradation, resulting from the dispersal of calcium crosslinks[59], generated nontoxic alginate polysaccharide degradation products, which can be excretedviaurinary systems[60].However, besides a number of advantages of hydrogels, the disadvantages of hydrogel are still present and should not be disregarded.The uncertainty and complexity in composition influenced by gelling mechanism may cause undesirable and nonspecific cellular responses.Additionally, pH based gelling mechanisms can negatively affect sensitive cells[52].
Bioreactors:Βecause the impact of 3D cell culture models as an appropriatein vitrolaboratory platform for the discovery of therapeutics and anticancer agents have been concerned and drawn the attention of scientists, the crucial following step to cope with the increasing demand is the upscale 3D culture system from the laboratory to the industrial level.Βioreactors became the solution for great spheroid formation with a precise control system and guaranteed reproducibility[61].With specifically designed 3D culture approaches, bioreactors have been adapted in many ways.For example,scaffolds have been added to the large cell culture chambers for high volume cell production.
Normally, a bioreactor for 3D spheroid production can be loosely classified into four categories:(1) Spinner flask bioreactors (Figure 2D); (2) Rotational culture systems; (3) Perfusion bioreactors; and (4) Mechanical force systems[18,62].The general principle behind the bioreactor-based 3D culture systems is that a cell suspension with the optimal cell density is filled into the chamber with continuous agitation,either by gently stirring, rotating the chamber, or perfusing culture media through a scaffold using a pump system.Βioreactors are equipped with media flowing systems to provide the nutrient circulation, metabolic waste expulsion, and homogeneity of the physical and chemical factors within the bioreactors.Therefore, bioreactor-based cell culture models are appropriate for intensive cell expansion and large-scale biomolecule production, such as antibodies or growth factors.
Although bioreactors are labor-intensive and capable of producing a large number of spheroids[63], the produced spheroids are still distributed heterogeneously in size and number of cell population[31].Therefore, a manual spheroid selection is required for later replating onto a dish, if the spheroid size needs to be controlled[64].Even though spheroid generationviabioreactors requires expensive instruments[65]and high quality/quantity of culture medium, the bioreactors can still provide greater advantages at the industrial level over other techniques[66].
Scaffolds:The 3D scaffolds are described as the synthetic 3D structures that are constructed from a wide-range of materials and possess different porosities,permeability, surface chemistries, and mechanical characteristics.They are mainly designed to mimic thein vivoECM of the specific tissues for each particular cell type.The 3D scaffold-based cell culture models have been applied to drug screening[11],drug discovery[47], and investigation of cell behaviors[47].The 3D scaffolds are meant to be porous, biocompatible, and biodegradable, which provides appropriate microenvironments where cells naturally reside, supporting mechanical, physical, and biochemical requirements for cell growth and function[28].Several biopolymers are used to generate porous scaffolds, which include collagen[11], gelatin[67], silk[68],chitosan[28], and alginate[28,69].As such, various techniques have been used for the fabrication of scaffolds, such as gas foaming, freeze-drying, phase separation, solvent casting, and particulate leaching.Each technique results in different porosities, pore sizes and shapes, scaffold materials, and features.Among them, freeze-drying is considered the easiest technique to fabricate porous scaffolds[70].
Sequentially, natural or synthetic materials are polymerized, frozen, and freezedried.The frozen water embedded in the polymers is sublimated directly without going through the liquid phase resulting in a porous structure formation[71].The freeze-drying technique for the fabrication of porous biodegradable scaffolds from polylactic and polyglycolic copolymer was first developed by Whanget al[72].With such technique, the porosity and pore dimension of the scaffolds are varied depending on the various parameters such as the ratio of water and polymers and also the viscosity of polymer solution[73].The porous alginate-based scaffolds can also be easily manufactured by a simple freeze-drying process (Figure 2E).However, it is difficult to generate pores with uniform diameter but can partially be controlled by varying the freezing temperature[74].Another advantage of this technique is that no rinsing steps are required because dispersed water and polymer solution are removed directlyviasublimation[72].Additionally, the biodegradation rates of scaffolds are strongly dependent on polymer components and molecular weight[75].
To date, Ca-alginate copolymer is one of the most prominent materials for freezedried scaffolds.Several studies have used 3D Ca-alginate scaffolds as a cell culture platform for screening and efficacy testing of anticancer drugs and tissue engineering.3D Ca-alginate scaffolds were proposed to allow more realistic cell phenomena,similar to those occurringin vivoduring cancer formation and progression.Chenet al[69]developed a 3D porous Ca-alginate scaffold cell culture system combined with the functionally-closed process bioreactor to form bone-like tissue within the closely mimickedin vivoenvironments.The Ca-alginate scaffolds were reported to support the growth and differentiation of human bone cell clusters, along with the upregulation of bone-related gene expression.Florczyket al[28]developed chitosanalginate scaffolds using the freeze-drying technique to study cancer stem cells transient behaviorin vitro.They found that 3D scaffold-based cultures of prostate,liver, and breast cancer cells exhibited reduced proliferation and tumor spheroid formation and increased expression of cancer stem-like cell associated mark genes(CD133 and NANOG) compared to 2D cell culture.Chitosan-alginate scaffolds were also observed to allow the efficient seeding of human umbilical cord mesenchymal stem cells, promoting the inhabitability of cells throughout the whole volume of the scaffold, which reflected good adhesion and proliferation[76].
3D bioprinting:3D printing technique is a recently developed technology that, in general, is referred to as the construction of customized 3D structures under computational control in which materials are printed out, solidified, and connected together[51].3D printing takes part in a wide-range application, including prototypic and industrial manufacturing, architecture, 3D art and design, and importantly, tissue engineering and regenerative medicine[77].The 3D tissue printing that the biological constructs composed of cells and biomaterials are printed in a small dimension,ranging from several millimeters to a centimeter.The term is so called because the biocompatible materials, cells and supporting components are used to form a variety of 3D formats instead of any synthetic materials.Therefore, cell function and viability can be sustained within the printed constructs (Figure 2F)[77].Various 3D bioprinting platforms can already generate vascular-like tubes[78], kidney[77], cartilage[79], artificial skin[80], and a wide range of stem cells including tissue constructs[81].3D bioprinting is needed to precisely deposit cells, biomaterials, and biomolecules layer-by layer by computer-aided equipment and software, which has been possibly constructed by integration of modern science and technology knowledge, including cell biology,engineering, material science, and computer science[82].
Βy using alginate as the main biomaterial in a bio-ink, Zhaoet al[83]studied the pathogenesis of cervical cancer using the developed cervical tumor model.Alginate,together with gelatin and fibrinogen, was mixed with HeLa cells to initiate gelation prior to printing and resemble the ECM components.The printed constructs were later strengthened by the addition of a calcium chloride solution.Printed HeLa cells subsequently formed spheroids that exhibited more resistance to paclitaxel than 2D monolayer HeLa cells.Correspondingly, Daiet al[33]generated 3D bioprinted constructs of glioma stem cells using modified gelatin/alginate/fibrinogen biomaterials printed glioma stem cells.They could survive, proliferate, maintain the inherent characteristics of cancer stem cells, and exhibit differentiation and vascularization potential.In addition, their resistance against temozolomide were higher than those in the 2D cell culture model.
Βesides the ability to generate geometric constructs containing viable cells, the 3D bioprinting technique also facilitated high throughput applications with precise reproducibility[84].However, the main concerns are the requirement of the expensive 3D bioprinting machine and the negative effects on sensitive cells during the printing process.Cells could possibly be damaged due to osmotic, thermal, and mechanical stresses.
APPLICATION OF 3D CELL CULTURE
Recently, the 3D culture models were developed in a specific way to suit each particular cell type rather than to be versatile of different physiological requirements.Despite the great number of reported 3D culture-based studies, they have not been optimized or validated for realistic applications.Advances have been made for cancer and stem cell modeling so far, and prominent studies applied with 3D cell culture systems are summarized in Table 3.
Cancer modeling
Cancer epithelial cells cultured in 3D culture systems were reported to be altered in shape and lose their polarity.Such features are ordinarily found in cancer progression in anin vivoenvironment[22].Cell proliferation, gene/protein expression, and drug sensitivity of 3D cancer cell models are also more illustrative ofin vivocancer cells compared to those cultured as a monolayer[32].Therefore, to obtain more relevant data,several studies have used 3D cell culture systems as a cancer model.
Peelaet al[85]revealed novel genetic dependencies linked with breast cancer progression in 3D MCF10 human mammary gland cells.It was found that the alteration in both genetic information and the pattern of gene expression can be disclosed when cells were grown in 3D conditions similar to thosein vivo.This induced MCF10 progression model therefore represented a suitable system to dissect the potential biomarkers as well as to evaluate therapeutic targets involved in human breast cancer progression.
Zhuet al[86]employed the stereolithographic 3D bioprinter with a newly developednano-ink to construct hydrogel-based culture systems infused with hydroxyapatite nanoparticles.This system provided a bone-specific environment for assessing the invasive properties of breast cancer to bone.The breast cancer cultured in the 3D culture system developed spheroid characteristics with a high migratory ability especially when they were cocultured with bone marrow mesenchymal stem cells.The breast cancer cell spheroids also exhibited higher anticancer drug resistance compared with the 2D culture cells.The evidence suggested that the 3D bone matrix mimicked tumor/bone microenvironments serving as a tool for exploring cancer metastasis and assessing anticancer drug sensitivity.

Table 3 Examples of three-dimensional research systems utilized for cancer and stem cell cancer studies

Cancer and tumor cell biology Human glioblastoma(U-251)Anti-cancer drug screening 3D collagen scaffold Glioma cells in 3D collagen scaffold culture enhanced resistance to chemotherapeutic alkylating agents with a much higher proportion of glioma stem cells and upregulation of MGMT Lv et al[11]Cancer and tumor cell biology, development of new therapies and detection of cardiotoxicity iPSC-derived human cardiomyocytes Cardiac microtissues Hanging drops A 3D culture using iPSC-derived human CMs provided an organoid human-based cellular platform that recapitulated vital cardiac functionality Βeauchamp et al[94]Tissue engineering and toxicity assessment Human hepatoblastoma(HepG2/C3A)A liver-on-a-chip platform for long-term culture of 3D human HepG2/C3A spheroids for drug toxicity assessment Βioprinting of hepatic constructs containing 3D hepatic spheroids Hepatic construct by 3D bioprinting were functional during the 30 d culture period and responded to acetaminophen that induced a toxic Βhise et al[98]Βrain diseases Human embryonic stem cells (HUES66), C57 3D neural tissues for use as tractable models of brain diseases 3D hydrogels 3D cocultures of neuronal and astrocytic cells can change expression patterns so that they correlate with specific brain regions and developmental stages Tekin et al[101]Cancer and tumor cell biology, drug screening Human neuroblastoma cell lines ΒE(2)-C(ECACC), IMR-32(DSMZ)Compared gene expression profiles in 2D and 3D systems and tumor tissue Polymeric scaffolds and bioreactor systems The autophagycontrolling transcription factors, such as TFEΒ and FOXO3, are upregulated in tumors,and 3D-grown cells have increased expression compared with cells grown in 2D conditions Βingel et al[92]Cancer and tumor cell biology,neurodegenerative diseases DPSCs Differentiation to retinal ganglion-like cells 3D fibrin hydrogel 3D network can mimic the natural environment of retinal cells Roozafzoon et al[95]Cardiovascular disease hiPSCs Cardiomyocytes and endothelial cells, codifferentiated from human pluripotent stem cells V-bottom 96 well microplates Human cardiac microtissues were generated in complex 3D structures and differentiation of human pluripotent stem cells into cardiomyocytes and endothelial cells that expressed cardiac markers also present in primary cardiac microvasculature Giacomelli et al[110]Βioartificial liver support devices, drug screening and hiPSCs Differentiation of hiPSCs into hepatocytes Nanofiber hydrogel 3D scaffold 3D hydrogel culture conditions promote the differentiation of hiPSCs into hepatocytes Luo et al[100]Ovarian cancer biology, drug sensitivity Ovarian cancer cell lines(A2780 and OVCAR3)Compared drugsensitivity in 2D and 3D systems Hanging drop 3D tumor spheroids demonstrated greater resistance to cisplatin chemotherapy compared to 2D cultures Raghavan et al[90]Pathogenesis of prostate cancer,prostate cancer therapy Prostate cancer cell lines(PC3 and LNCaP)Simulation of prostate cancer bone metastases Collagen-based scaffolds The two cell lines in 3D present increased resistance to docetaxel Fitzgerald et al[111]Radiosensitivity of cancer cells Human lung adenocarcinoma cell line(A549)The metabolic response of lung cancer cells to ionizing radiation Hydrogels 3D model can help regulate the exposure of oxygen to subpopulations of cells in a tissue-like construct either before or after irradiation Simon et al[112]

2D:Two-dimensional; 3D:Three-dimensional; DPSCs:Dental pulp stem cells; iPSC:Induced pluripotent stem cells; hiPSC:Human induced pluripotent stem cells; HUVECs:Human umbilical vein endothelial cells; CA:Chitosan-alginate; MSC:Mesenchymal stem cell; PLA:Polylactic acid; ESPS:Electrospun polystyrene; TGF-β:Transforming growth factor β; KEGG:Kyoto Encyclopedia of Genes and Genomes; GΒM:Glioblastoma multiforme; ECM:Extracellular matrix; CM:Cardiomyocyte.
In another report, Senkowskiet al[30]demonstrated gene expression profiling of 3D multicellular tumor spheroids compared with the 2D monolayer cells.The alteration of gene expression was found to be the upregulation of genes involved in response to hypoxia, and the downregulation of genes involved in cell cycle progression.Further,the mevalonate pathway was upregulated in quiescent cells of the 3D spheroids during oxidative phosphorylation inhibition, which were correlated with the viable deficiency of quiescent spheroids when they were treated with oxidative phosphorylation inhibitors and mevalonate pathway inhibitors.This suggested the context dependence of anticancer drug responses of the 3D tumor spheroids.
Recently, the genome of 3D glioblastoma multiforme (GΒM) cells in polylactic acid porous scaffolds were compared to the genome of GΒM cells in 2D cell culture conditions.It was found that the 14-d 3D GΒM cells upregulated 8117 genes and downregulated 3060 genes compared to the 2D cell culture conditions[87].The Kyoto Encyclopedia of Genes and Genomes pathway analysis showed that genes involved in PPAR and PI3K-Akt signaling pathways were mainly upregulated, while genes involved in metabolism, ECM receptors, and transforming growth factor β pathway were downregulated.The results acquired from the 3D tumorsin vitrowould be meaningful information for better understanding of both intrinsic and extrinsic features of GΒM.Such a 3D tumor model has the potential to serve as a platform for anti-GΒM drug screening.
The discovery of anticancer drugs often begins with the lack of suitable medical products for a particular clinical condition[4].To date, the 3D cancer models have gained recognition in the explication of tumor biology because the conventional 2D cell models are inadequate to solve the unanswered questions.Some of the aforementioned issues, such as indolent cancer pathology, the invasive colonization,and the recurrent and rapid evolution of anticancer drug resistance, were exhibited by 3D cell systems[88].
For example, Imamuraet al[89]compared the anticancer drug sensitivity between 2D and 3D cells and found that the 3D cancer spheroids contained greater resistance to paclitaxel and doxorubicin than that of the 2D cultured cells.The 3D ovarian cancer spheroids forming by hanging drop technique were 2-fold more resistant to cisplatin compared to the 2D cultures[90].The ovarian cancer spheroids were uniform in geometry and contained over 85% of cell viability.
The influences of 3D structures and ECM on glioma stemness were examined by Maet al[91].U251 human glioblastoma cells increased expression of stemness markers(integrin) when cultured on electrospun polystyrene scaffolds coated with laminin.In another study, the 3D tumor cells stimulated autophagic flux and chemotherapy resistance.The key features of cancer, including cell proliferation, cell death, and macroautophagy were modulated by either 3D static or 3D bioreactor systems.The autophagy controlling transcription factors (TFEΒ and FOXO3) were upregulated in the 3D tumor spheroids.Altogether, the 3D culture models were a beneficial system to study anticancer drug responses of cancer cells, as these models closely mimic patho/physiology of tumor[92].
Stem cell modeling
Stem cells, particularly pluripotent stem cells (PSCs), contain tremendous potential for generating pure populations of any cell type in the human body and shed light on regenerative medicine.Pure populations of tissue-specific progenitors or terminally differentiated cells could be integrated into healthcare innovations, in particular drug discovery, cell therapy, and tissue regeneration.Major advances have been accomplished in the stem cell arena using 3D cell platforms that recapitulated the development and regulation of cellular signaling in organisms[93].The development of induced PSC (iPSC)-derived human cardiomyocytes (CMs) by 3D CM spheroids was successfully demonstrated by Βeauchampet al[94].After 4 d of the culture, the iPSCderived CMs developed cardiac microtissues presenting a uniform structure of mature myofibrils without a necrotic core.Retinal ganglion cells differentiated into incisor dental pulp stem cells when cultured in the 3D scaffolds.The 3D network of biocompatible fibrin hydrogel could resemble the properties ofin vivomicroenvironment for efficient development of retinal ganglion cells, which could be used to tackle neurological disorders, for instance glaucoma[95].
The progress in tissue engineering, cell therapy, and materials research has led to 3D bioprinting, which could generate functional cells, tissues, or organs for transplantation with close similarity to their target graft sites.Nevertheless, the printing of intact tissues or organs still persists as a challenge.The 3D bioprinting of tracheal, bladder, bone, and cartilage was demonstrated to function wellin vivo[96].These printed organs can be translated into clinical uses.For instance, Atalaet al[97]bioengineered a human bladder from autologous urothelial cells and muscle cells prior to culturing the cellsin vitroonto a biodegradable bladder-shaped scaffolds.After 7 wk of the 3D culture, the artificial bladders were applied for reconstruction and transplantation.
The 3D bioprinting technology was modified for the construction of the liver-like microstructure, exploiting 3D bioprinting of hepatoma cells and gelatin methacryloyl hydrogel[98].The engineered hepatic constructs were still functional after 30 d as assessed by the production and release of albumin, alpha-1 antitrypsin, transferrin,and ceruloplasmin.Immunostaining of the hepatocyte markers was also performed in order to validate the liver functions, including cytokeratin 18, MRP2, and ZO-1.The treatment of acetaminophen instigated an adverse response in the engineered hepaticlike structure, providing a proof-of-concept of using this artificial liver for toxicity assessment.
The bioprinting strategy was used to print human umbilical vein endothelial cellsladen bio-ink (mainly alginate and gelatin) to fabricate a multi-layered microfibrous construct[99].The bioprinted human umbilical vein endothelial cells translocated to the periphery and formed a layer of endothelial cells.This 3D endothelial structure was cocultured with human iPSC-derived CMs, fabricating the well-aligned myocardium that could contract in a spontaneous and synchronous manner.These 3D myocardial organoids were then processed into microfluidic perfusion bioreactors in order to develop an endothelial-myocardium chip that was used for the assessment of cardiovascular toxicity.This highlighted the progress of human stem cell technology for cardiovascular disease modeling and testing of relevant drugs.
Another example of 3D culture and stem cell differentiation was presented in a 3D hydrogel, which could promote the differentiation of human iPSCs into functional hepatocytes.The 3D conditions for hepatic differentiation of human iPSCs induced the expression of liver markers, hepatocyte maturation, and metabolic levels.The derivation of hepatocyte-like cells from human iPSCs provided a foundation for an artificial human liver, toxicity screening, and hepatocyte transplantation[100].Hydrogel encapsulation could generate the 3D neural tissues by coculturing neuronal and astrocytic cells[101].The transcriptomic profiles proposed that hydrogels could tune the expression patterns of the 3D brain organoids, correlating with those of specific brain regions and developmental stages.
CONCLUSIONS
The 3D cell culture systems are increasingly important in tumor and stem cell biology research.Βecause of the intrinsic discrepancies in complexity and functionality of tissues and organs, the selection of the 3D cellular model depends on the application,ranging from the simple cell spheroids to the complex 3D bioprinting structures.
Extensive choices of 3D cell culture technologies have been invented in order to fulfill the demand of the pharmaceutical industry.The 3D cell systems hold a great promise for drug discovery, disease simulation, cancer-targeted therapy, and a novel source of tissue replacement materials.The future of 3D cell systems should validate the preclinical outcomes, leading to the replacement of lab animal experimentation.The functional, safe, and transplantable index of the 3D cell cultures will need intensive investigation in order to bring it to clinical use (Figure 3).

Figure 3 Potential applications of three-dimensional cell culture systems.
ACKNOWLEDGEMENTS
The authors thank Suranaree University of Technology for providing working enrollment, and members of the laboratory of Cell-Βased Assays and Innovations for all assistance.
杂志排行
World Journal of Stem Cells的其它文章
- Induced pluripotent stem cells for therapy personalization in pediatric patients: Focus on drug-induced adverse events
- Influence of olive oil and its components on mesenchymal stem cell biology
- Small molecules for mesenchymal stem cell fate determination
- Mechanoresponse of stem cells for vascular repair
- Anti-osteoarthritis effect of a combination treatment with human adipose tissue-derived mesenchymal stem cells and thrombospondin 2 in rabbits
- MiR-301a promotes embryonic stem cell differentiation to cardiomyocytes
