Synthesis of Fine AlN Powders by Foamed Precursor-assisted Carbothermal Reduction-nitridation Method
2019-12-24MAOXiXiXUYongGangMAOXiaoJianZHANGHaiLongLIJunWANGShiWei
MAO Xi-Xi, XU Yong-Gang, MAO Xiao-Jian, ZHANG Hai-Long, LI Jun, WANG Shi-Wei
Synthesis of Fine AlN Powders by Foamed Precursor-assisted Carbothermal Reduction-nitridation Method
MAO Xi-Xi1,2,3, XU Yong-Gang1,2,3, MAO Xiao-Jian1,2, ZHANG Hai-Long2, LI Jun1, WANG Shi-Wei1,2
(1. State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China; 2. CAS Key Laboratory of Transparent and Opto-functional Advanced Inorganic Materials, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China; 3. University of Chinese Academy of Sciences, Beijing 100049, China)
A modified carbothermal reduction-nitridation (CRN) method was proposed to synthesize fine AlN particles using-Al2O3and sucrose as raw materials which were pre-treated as cellular foams. The reaction procedure and the produced AlN were investigated by XRD, SEM and TEM. XRD result suggests that-Al2O3transferred into AlN without transformation into-Al2O3. HRTEM shows that-Al2O3particles are covered by amorphous carbon from sucrose inhibiting the transformation of-Al2O3from-Al2O3in the synthesis procedure. The foamed structure is of benefit to the diffusion of N2and the produced CO. The minimum reaction temperature is 1450 ℃ for full conversion of Al2O3to AlN. SEM photographs show the particle size of synthesized AlN is 50 nm. This study demonstrated an efficient way to synthesize fine AlN powders which are urgently required for the manufacture of advanced AlN ceramics.
aluminum nitride; foams; particles; synthesis
Aluminum nitride (AlN) is one of the most important advanced ceramics and an ideal substrate material for semiconductor package because of the remarkably high thermal conductivity, low dielectric constant, high electrical resistivity, high mechanical strength, and low thermal expansion coefficient that matches well with silicon[1-4]. As well known, the property of the advanced ceramics are critically influenced by the characterization of raw powders. The manufacture of advanced AlN ceramics is the same case. It is still a challenge to fabricate high quality AlN powders with efficient sintering activity. Presently, the most practical method to synthesize AlN ceramic powders is the carbothermal reduction-nitridation (CRN) of Al2O3[5]. Compared with direct nitridation from aluminum[6], the CRN process is more appropriate to synthesize AlN powder, since it produces powders with high purity, high uniformity, high yield and low cost[7-10]. However, the CRN process requires high synthesis temperature[11], which leads to particle growth and agglutination. Furthermore, it is important to mix carbon and alumina particles in a very small scale, because the CRN reaction is the reaction involved two solid phases. Otherwise, much higher temperature and longer time are necessary in order to convert all Al2O3into AlN[12-14].
In order to decrease the synthesis temperature and time, much attention was attracted on using fine alumina and carbon particles or precursors. For example,-Al2O3[15], Al(OH)3[16], and-AlOOH[16]were used instead of-Al2O3. And nano-sized carbon particle was used to replace graphite particles. However, their effects are very limited in the respect of synthesis temperature and particle size. Other research work focused on applying soluble precursors which may transform into alumina and carbon afterwards. The idea is that the mixture of solution could reach atom level, compared with the mixture of solid particles. Mylinh,[17]prepared AlN particles using phenol resin as precursor at a temperature above 1700 ℃. Baik,[18]employed sucrose as carbon source and converted Al2O3fully to AlN at 1600 ℃. Chu,[19]prepared Al/C precursor by combustion synthesis using a mixed solution of aluminum nitrate, glucose, and urea, which was then nitrided into pure AlN at 1500 ℃. Jung,[20]obtained AlN particles by calcining basic dicarboxylate Al(III) complexes under flowing nitrogen at 1450 ℃ for 15 h. These efforts focused on reactants, but didn’t effectively decrease the synthesis temperature. The reason is that it is difficult for nitrogen to diffuse into the interior of the reactant through the gap between the sub-micron particles, as well as for CO escaping as by-product. Besides, the using of aluminum organics lead to much higher cost which will, of course, restrict the application of obtained AlN powders.
To accelerate the reaction, the diffusion of N2into the interphase draws our attention. Recently, we employed a slurry foaming method, which is normally used to make ceramic foams[21-22], to prepare porous-Al2O3/carbon foam, where plenty of connected pores act as channels for N2transportation during the following CRN procedure[23]. In this method, Al2O3starts to transfer to AlN at 1400 ℃, and is completely converted at 1550 ℃ for only 2 h.
In the present work, we combined the foaming technology with sucrose as carbon source to ensure all-Al2O3particles are covered by the resulting carbon. Excessive sucrose was used in order to form residual carbon to avoid the particle growth and agglutination of AlN powders. The effects of reaction temperature and sucrose/Al2O3ratio on the nitridation rate and morphology of AlN powder were investigated.
1 Experimental
Commercial-Al2O3with the average particle size of 13 nm (purity 99.99%, specific surface area 120 m2/g, Dalian Hiland Photoelectric Material Co., Dalian, China), and sucrose (C12H22O11, purity 99.5%; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were used as starting raw materials. The weight ratio of sucrose to alumina was 2.0. The corresponding carbon addition is 1.2 times of that required for the reduction of alumina. The existence of excess carbon, which originally covered on alumina particles, is expected to separate the formed AlN particles to avoid the agglutination. The forming procedure was described elsewhere[23]. Sucrose,-Al2O3, and copolymers of isobuthylene and maletic anhydride[24-25](Isobam104# and Isobam600AF, Kuraray; Osaka, Japan) were dispersed in deionized water. Then, Emal TD (triethanolamine laurylsulfate, with 40% active content, Kao Chemical Co., Tokyo, Japan) was added into the mixed slurries, which were then vigorously stirred for 3 min to generate foams. After gelation and drying, the sucrose/Al2O3precursor foams were pyrolysed for 1 h at 1000 ℃ with a heating rate of 10 ℃/min in flowing N2in a graphite furnace (High-Multi 10000, Fujidempa Kogyo Co., Tokyo, Japan). Following CRN reaction was carried out at 1250–1600 ℃ for 2 h. After synthesis, residual carbon in the nitridation product was removed by firing at 650 ℃ in air for 6 h.
The crystalline phase of the synthesized products was examined by X-ray diffraction (XRD, D8-Advance A25, Bruker Co., Karlsruhe, Germany) with Cu Kα radiation and a scan speed of 0.1 (°)/s. The AlN conversion fraction was determined on the basis of the relatively peak intensity of the AlN phase in XRD patterns. The morphologies of the products were observed by scanning electron microscopy (SEM, Hitachi, S-4800, Tokyo, Japan). A field-emission transmission electron microscope (TEM, JEM-2100F, JEOL, Japan) was used for high resolution transmission electron microscopy (HRTEM). Differential thermal analysis/thermo gravimetric analysis of sucrose was studied in N2using a thermal analyzer (TG/DTA, Netzsch, STA449C, Germany). The oxygen content of the resultant AlN powder was measured by Oxygen-Azote mensuration equipment (TC600C, Leco Co., Chicago, America).
2 Results and discussions
In order to confirm the carbonization of sucrose, the TG/DTA curves of sucrose pyrolysis procedure from room temperature to 1000 ℃ in N2atmosphere is presented in Fig. 1. Two endothermic peaks take place at 172.9 and 219.4 ℃, revealing the carbonization of sucrose with a carbon yield of 21.41wt% according to the TG curve. The corresponding carbon addition is about 1.2 times of that required for the reduction of alumina, which agrees well with the design.
The SEM images of the foam heated at 1000 ℃ in N2are shown in Fig. 2(a). The pyrolysis foam sustains the cellular structure of the precursor foam, where large number of spherical cells and interconnected windows act as the transportation channels for N2and the by- product CO. Fig. 2(b) shows the microstructure of the produced foam after CRN reaction at 1550℃. It could be observed that the struts and walls of the cells become loose with more interconnected windows.
Fig. 3 shows X-ray diffraction patterns of the products from the foam with sucrose/Al2O3ratio of 2.0. It can be clearly seen that only-Al2O3phase is detected in the sample heated at 1300 ℃, which implies that the produced carbon is amorphous and the nitridation reaction did not occurr at this temperature. The diffraction peaks of AlN start appearing at 1350 ℃, and become stronger as the heating temperature increases. When the heating temperature is higher than 1450 ℃, only AlN diffraction peaks are detected, which infers that all alumina is transferred to AlN. It is notable that in the temperature range of 1300–1400 ℃, the unreacted alumina exists as-Al2O3instead of-Al2O3. It suggests that in the present case-Al2O3is transferred into AlN without any transformation into-Al2O3. However, in our previous work where nano-sized carbon black was used, all-Al2O3was changed to-Al2O3at 1300 ℃, which is consistent with the thermal stability of-Al2O3in literature that-Al2O3is transferred to-Al2O3at around 1200 ℃[26]. This phenomenon probably originates from the C/-Al2O3core shell structure.

Fig. 1 DTA and TG analysis of sucrose pyrolysis procedure from room temperature to 1000℃ in N2 atmosphere
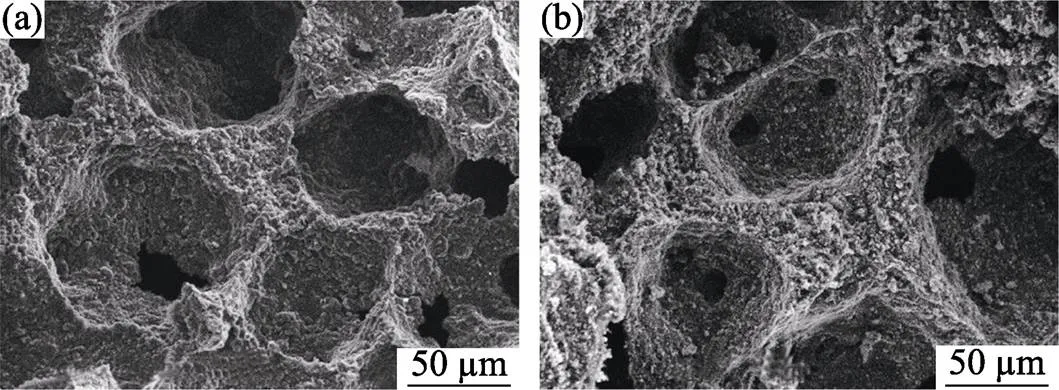
Fig. 2 SEM images of the foam heated at (a) 1000 and (b) 1550 ℃ with sucrose/Al2O3 ratio of 2.0
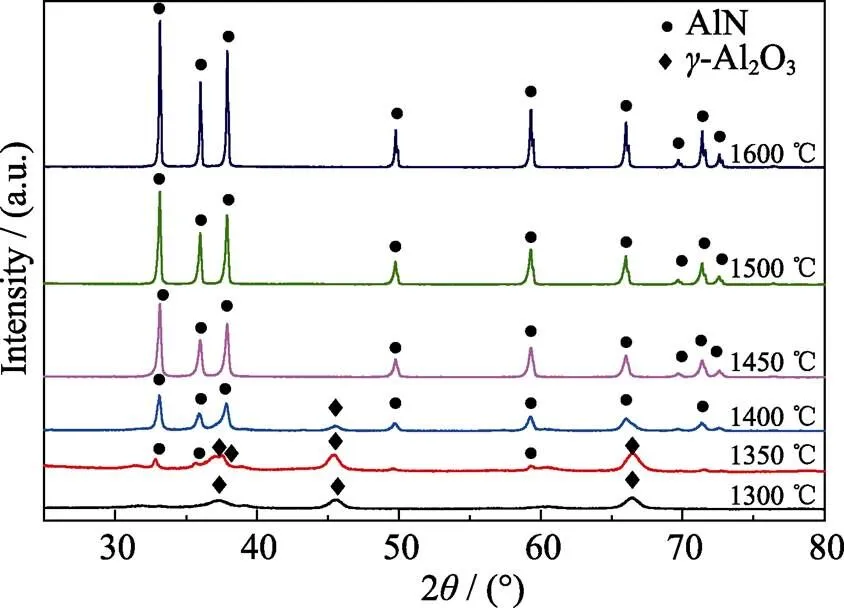
Fig. 3 XRD patterns of the sucrose/Al2O3 powders synthesized at different temperatures
Fig. 4 shows morphologies of the corresponding particles synthesized at different temperatures. Fig. 4(a) is the microstructure of products after pyrolysis where the CRN reaction didn’t happen yet as indicated by the XRD results in Fig. 3. There are shuttle-like dark particles which are surrounded by the gray carbon decomposed from sucrose. The dark particles should be-Al2O3which remains until 1300 ℃ as shown in Fig. 3. In Fig. 4(b), however, it can be found that the dark particles have spherical shape with a diameter about 20 nm. The change of particle morphology relates to the transformation of Al2O3to AlN, which is confirmed by XRD results. As the synthesis temperature increases to 1450 and 1550 ℃, the diameters of the spherical particles increase to about 50 and 200 nm, as shown in Fig. 4(c, d). It is noted that the AlN particles are separated by the residual carbon, which could avoid the growth of AlN, because carbon are very stable in the synthesis temperature range.
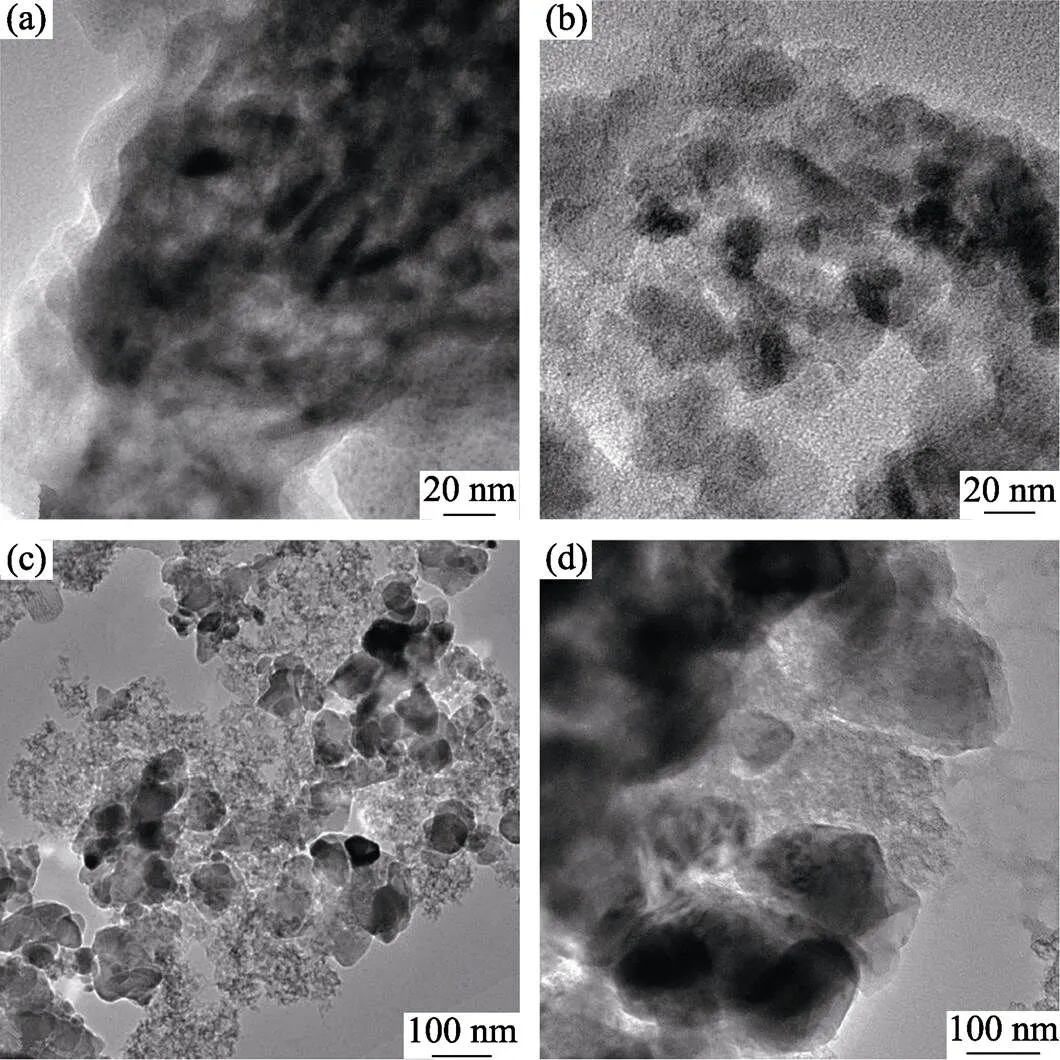
Fig. 4 TEM images of particles heated at different temperatures
(a) 1250 ℃; (b) 1350 ℃; (c) 1450 ℃; (d) 1550 ℃
The HRTEM image of the particles after pyrolysis at 1250 ℃ is shown in Fig. 5(a) with a selective electron diffraction spot pattern indicated in Fig. 5(b). The periodic lattice in Fig. 5(a) has a periodic distance of 0.458 nm, which is proximate to the interplanar distance of {111} plane of-Al2O3. The diffraction spots in Fig. 5(b) is consistent with the diffraction pattern of the-Al2O3phase along the [011] zone axis, with spots corresponding to crystal plane {111} and {400}. It is obvious that the inside dark particles are-Al2O3, which is consistent with the XRD results (Fig. 3). The-Al2O3particles are covered by amorphous carbon with the boundary appearing a quasi-ordered structure, with an interplanar distance thickness of 0.395 nm. The boundary layer is considered as the critical factor for the existence of-Al2O3at high temperature. Confined by the boundary layer, the growth of-Al2O3is prevented even the temperature is over 1200 ℃. Hence, the nucleation of-Al2O3becomes difficult. Another reason might be attributed to the surface energy barrier from the interlayer.

Fig. 5 (a) HRTEM image of particles synthesized at 1250 ℃ and (b) selective electron diffraction pattern
The formation of AlN is directly from-Al2O3in the present study, which has much larger specific surface area than that of-Al2O3. Compared with our previous study[23], this transformation procedure is beneficial to the CRN reaction. Fig. 6 shows the microstructures of the AlN particles synthesized at different temperatures after de-carbon calcination. The particle size increases from about 50 nm to 200 nm as the reaction temperature raises from 1450 ℃ to 1600 ℃, which are much smaller than that reported in previous study[23], where the average AlN particles size is 300 nm when synthesized at 1450 ℃.
In an early study where sucrose was also used as carbon source[18], the minimum reaction temperature for full conversion of Al2O3to AlN is 1600 ℃, even a fine and reactive precursor boehmite was used. The high reaction temperature results in agglutination of the produced AlN particles. The lower minimum full conversion temperature at 1450 ℃ in our study, which could be attributed to porous structure of foamed precursor, is the key to gain fine particle size. The interconnected channels make it possible that the rapid diffusion of N2and easy removal of produced CO, eliminating the screening effect of CO[11]. Considering the chemical reaction formula of the CRN, lower pressure of CO corresponds to lower energy barrier for the AlN formation and thus higher reaction rate[27].
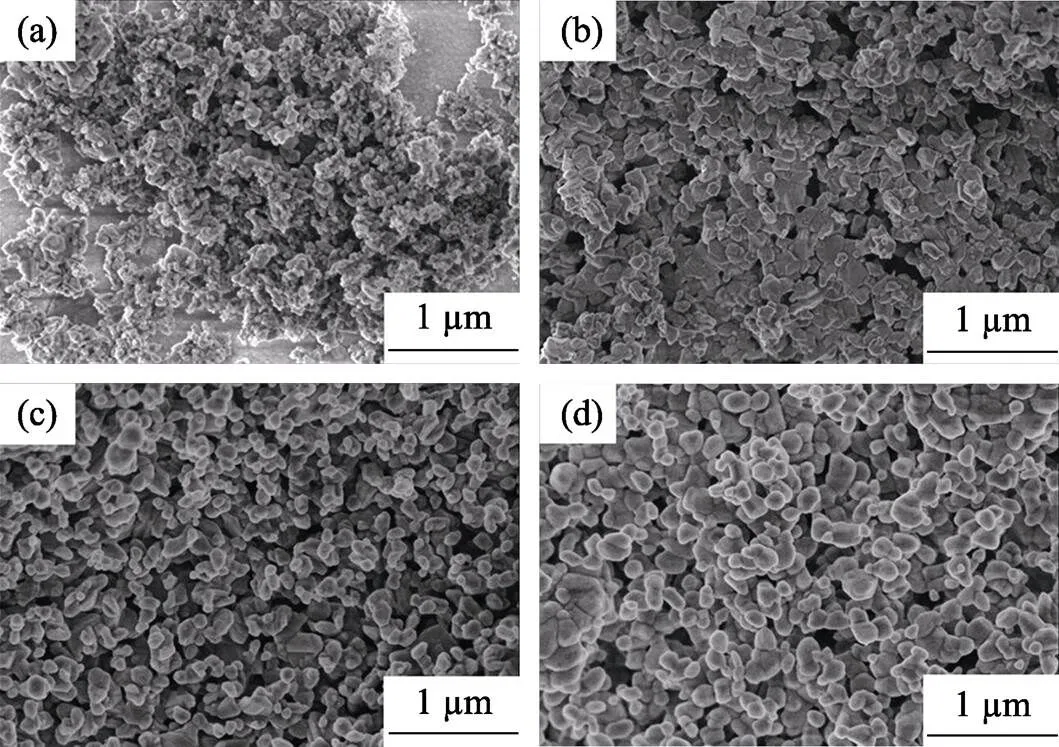
Fig. 6 SEM photographs of the AlN powders synthesized at different temperatures for 2 h
(a) 1450 ℃; (b) 1500 ℃; (c) 1550 ℃; (d) 1600 ℃

Table 1 Nitrogen and oxygen content of AlN powders with various sucrose/Al2O3 ratios and synthesizing at 1550℃
The nitrogen and oxygen content of the AlN powder synthesized at 1550 ℃ with different sucrose/Al2O3weight ratio are also investigated and are listed in Table 1. An increase of nitrogen from 29.3wt% to 30.2wt% can be seen with the oxygen decreasing from 2.4wt% to 1.6wt% when sucrose/Al2O3weight ratio increases from 2.0 to 3.0. Taken the low cost and non-pollution of sucroseand the simple starting materials mixing process into consideration, the current modified CRN process shows great potential for a convenient and efficient method to fabricated AlN powder.
3 Conclusions
AlN powders were synthesized by the novel sucrose/-Al2O3foam assisted CRN method. The inter-connected channels in the foam structure facilities N2and CO diffusion and supplies larger effective reaction area. The-Al2O3particles, covered by the pyrolytic carbon from sucrose, convert directly to AlN without the appearance of-Al2O3. The residual carbon from extra sucrose can effectively avoid the growth of AlN particles. Size controllable AlN particles from 50 nm to 200 nm can be obtained by correspondingly varying the synthesis temperature from 1450 ℃ to 1600 ℃.
[1] TAYLOR K M, LENIE CAMILLE. Some properties of aluminum nitride., 1960, 107(4): 308–314.
[2] VIRKAR ANIL-V, JACKSON T-BARRETT, CUTLER RAYMOND-A. Thermodynamic and kinetic effects of oxygen removal on the thermal-conductivity of aluminum nitride., 1989, 72(11): 2031–2042.
[3] BAIK Y, DREW R A W, Aluminum nitride: processing and applications., 1996, 122: 553–570.
[4] JACKSON T-BARRETT, VIRKAR ANIL-V, MORE KARREN-L,High-thermal-conductivity aluminum nitride ceramics: the effect of thermodynamic, kinetic, and microstructural factors., 1997, 80(6): 1421–1435.
[5] SELVADURAY G, SHEET L. Aluminium nitride: review of synthesis methods., 1993, 9(6): 463–473.
[6] TAJIKA MASAHIKO, RAFANIELLO WILLIAM, NIIHARA KOICHI. Sintering behavior of direct nitrided AlN powder., 2000, 46(23): 98–104.
[7] HASHIMOTO NOBORU, YODEN HIROYOSHI, DEKI SHIGEHITO. Effect of milling treatment on the particle size in the preparation of AIN powder from aluminum poly-nuclear complexes., 1993, 76(2): 438–442.
[8] PATHAK LOKESH-CHANDRA, RAY AJOY-KUMAR, DAS SAMAR,Carbothermal synthesis of nanocrystalline aluminum nitride powders., 1999, 82(1): 257–260.
[9] GAO ZHI-FANG, WAN YI-ZAO, XIONG GUANG-YAO,Synthesis of aluminum nitride nanoparticles by a facile urea glass route and influence of urea/metal molar ratio., 2013, 280: 42–49.
[10] NIU JING, SUZUKI SHOTA, YI XUEMEI,Fabrication of AlN particles and whiskerssalt-assisted combustion synthesis., 2015, 41(3): 4438–4443.
[11] FORSLUND B, ZHENG J. Carbothermal synthesis of aluminium nitride at elevated nitrogen pressures., 1993, 28: 3125–3131.
[12] QIN MING-LI, DU XUE-LI, LI ZI-XI,Synthesis of aluminum nitride powder by carbothermal reduction of a combustion synthesis precursor., 2008, 43(11): 2954–2960.
[13] JUNG WOO-SIK. Synthesis of aluminum nitride powder from-alumina nanopowders under a mixed gas flow of nitrogen and hydrogen., 2012, 38(1): 871–874.
[14] KIM KYUNG-IN, CHOI SUNG-CHURL, KIM JIN-HO,Synthesis and characterization of high-purity aluminum nitride nanopowder by RF induction thermal plasma., 2014, 40(6): 8117–8123.
[15] TSUGE A, INOUE H, KASORI M,Raw material effect on AlN powder synthesis from Al2O3carbothermal reduction., 1990, 25: 2359–2361.
[16] CHO Y W, CHARLES J A. Synthesis of nitrogen ceramic powder by carbothermal reduction-nitridation Part 3 aluminium nitride., 1991, 7(6): 495–504.
[17] MYLINH DANG-THY, YOON DAE-HO, KIM CHANG-YEOUL. Aluminum nitride formation from aluminum oxide/phenol resin solid-gel mixture by carbothermal reduction nitridation method., 2015, 60(2): 1551–1555.
[18] BAIK YOUNGMIN, SHANKER KARTIK, MCDERMID JOSEPH-R,Carbothermal synthesis of aluminum nitride using sucrose., 1994, 77(8): 2165–2172.
[19] CHU AI-MIN, QIN MINGLI, DIN RAFIUD,Effect of urea on the size and morphology of AlN nanoparticles synthesized from combustion synthesis precursors., 2012, 530: 144–151.
[20] JUNG WOO-SIK, AHN SANG-KYEUNG. Synthesis of aluminum nitride by a modified carbothermal reduction and nitridation method using basic dicarboxylate Al (III) complexes Al(OH)(C+2H2nO4)∙H2O (=3,6,8)., 2001, 21: 79–85.
[21] YANG YAN, SHIMAI SHUNZO, SUN YI,Fabrication of porous Al2O3ceramics by rapid gelation and mechanical foaming., 2013, 28(15): 2012–2016.
[22] ZHANG XIAO-QIANG, SUN YI, SHIMAI SHUNZO Z,Effect of water-soluble epoxy resin on microstructure and properties of porous alumina ceramics by gel-casting., 2015, 30(10): 1085–1088.
[23] MAO XI-XI, LI JUN, ZHANG HAI-LONG,Synthesis of AlN powder by carbothermal reduction-nitridation of alumina/ carbon black foam., 2017, 32(10): 1115–1120.
[24] YANG YAN, SHIMAI SHUNZO, WANG SHI-WEI. Room- temperature gelcasting of alumina with a water-soluble copolymer., 2013, 28(11): 1512–1516.
[25] SUN YI, SHIMAI SHUNZO, PENG XIANG,A method for gelcasting high-strength alumina ceramics with low shrinkage., 2014, 29(2): 247–251.
[26] LEVIN IGOR, BRANDON DAVID. Metastable alumina polymorphs: crystal structures and transition sequences., 1998, 81(8): 1995–2012.
[27] Wang QI, Cui WEI, Ge YI-YAO,. Preparation of spherical AlN granules directly by carbothermal reduction-nitridation method., 2015,98(2): 392–397.
碳热还原氮化法结合泡沫前驱体制备超细氮化铝粉体
茅茜茜1,2,3, 徐勇刚1,2,3, 毛小建1,2, 张海龙2, 李军1, 王士维1,2
(1. 中国科学院 上海硅酸盐研究所, 高性能陶瓷和超微结构国家重点实验室, 上海 200050; 2. 中国科学院上海硅酸盐研究所, 中国科学院光功能无机材料重点实验室, 上海 200050;3. 中国科学院大学, 北京 100049)
本研究使用改良的碳热还原氮化法合成超细氮化铝粉体。以氧化铝和蔗糖作为铝源和碳源, 先预处理制备成多孔泡沫, 再通过碳热还原氮化法合成氮化铝粉体。反应过程和产物通过X射线衍射分析、SEM和TEM确定。X射线衍射分析表明整个反应过程不存在氧化铝的相转变。高分辨透射电子显微镜显示-Al2O3颗粒被无定型碳包裹, 从而抑制了-Al2O3到-Al2O3的相转变。泡沫的多孔结构促进了氮气的扩散和反应副产物的释放, 使得最低反应温度降低至1450 ℃。SEM结果表明得到的氮化铝颗粒粒径大约为50 nm。本研究合成的氮化铝粉体可用于制备高热导氮化铝陶瓷。
氮化铝; 泡沫; 粉体; 合成
TQ174
A
2019-01-28;
2019-05-12
National Key R&D Program of China (2017YFB0310500); National Natural Science Foundation of China (51772309)
MAO Xi-Xi (1991−), female, candidate of Master degree. E-mail: 112113192@qq.com
MAO Xiao-Jian, professor. E-mail: maoxiaojian@mail.sic.ac.cn; WANG Shi-Wei, professor. E-mail: swwang51@ mail.sic.ac.cn
1000-324X(2019)10-1123-05
10.15541/jim20190055