Relationship between cachexia and perineural invasion in pancreatic adenocarcinoma
2019-12-14LiviaPetruselIoanaRusuDanielCorneliuLeucutaRaduSeiceanRamonaSuharoschiPaulaZamfirAndradaSeicean
Livia Petrusel,Ioana Rusu,Daniel Corneliu Leucuta,Radu Seicean,Ramona Suharoschi,Paula Zamfir,Andrada Seicean
Abstract
Key words: Pancreatic adenocarcinoma; Cachexia; Perineural invasion; Activin; Midkine;Biomarker; Survival; Metastases; Endosonography; Surgery
INTRODUCTION
Pancreatic cancer is currently ranked on the 3rdplace in cancer mortality,but is expected to reach the 2ndleading cause of cancer mortality in 2030[1,2].The unfavorable prognosis of PDAC can be attributed to late diagnosis and aggressive tumor biology,with a very low survival rate (< 6%)[2-4].A major factor in the low survival rate is the presence of cachexia,which is seen in over 85% of patients with pancreatic cancer[5-7]constituting the highest rate of cachexia in all malignancies.
Cancer cachexia is a complex syndrome,characterized by body-weight loss;muscle,skeleton and adipose tissue wasting; and inflammation,which is often associated with anorexia[8,9].
Cachexia has become an obstacle for the successful treatment of cancer and may significantly contribute to cancer-related death.
The mechanism of cachexia involves the interaction host- tumor through TGFβviathe SMAD2/3 pathway which can stimulate tumour growth and inhibits the muscle growth followed by myopenia through myostatin or activin pathway[9,10]or insulingrowth factor binding protein as part of IGF-1/PI3K/Akt signalling pathway with the inhibition of myogenesis and enhancing myotubule protein degradation[9].Another mechanism is related to hypercatabolism connected to the production of proinflammatory cytokines by the tumor,such as interleukin (IL)-1b,IL-6 and the tumor necrosis factor-α,both of which are involved in lipolysis with white adipose tissue wasting and early occurrence of brown fat tissue with high energy expenditure[11,12],in muscle catabolism too[9].
Astrocyte activation in the spinal cord induces lipolysis in the adipose tissue[13]and muscle atrophy[14,15]and is also involved in cachexia occurrence; this activation may result from the damage to peripheral nerves during perineural invasion in PDAC,with the hypertrophy and thickening of the nerve branches[16,17],but their relationship is not completely understood.The cachexia was associated with the degree of neural invasion,known as responsible for the aggressive behaviour of pancreatic cancer[18,19],with the involvement of the neurotrophic factors[20],such as midkine.It promotes neuronal differentiation and cell migration in peripheral invasion of pancreatic cancer[21].Midkine was found to be overexpressed in many pancreatic cancers and may represent a target for chemoresistant patients[22],but its relationship with cachexia has not been studied.
The main aim of this study was to determine the relationship between cachexia and perineural invasion in patients with PDAC by using clinico-pathological features and the protein expression levels of Activin and Midkine in plasma and tissue of patients compared to healthy patients.The secondary purpose was to assess the prognostic role of Activin and Midkine in survival and metastasis.
MATERIALS AND METHODS
Patients and sample collection
This study was performed at the Regional Institute of Gastroenterology and Hepatology “O.Fodor” in Cluj-Napoca,Romania.This study was prospectively performed and was approved by the Ethics Committee of the hospital and was registered at clinicaltrials.gov (NCT03042442).
Eligibility criteria
Subjects of the study group were at least 18-years-old,with no previous history of any other cancer in the last 5 years.Written consent was given prior to entry into the study.Patients with pancreatic ductal adenocarcinoma,based on the results of endoscopic ultrasonography (EUS),biopsy,or surgery,were enrolled at the time of diagnosis,before any therapeutic intervention had been given,from January 2015 to September 2017.
The exclusion criteria were obvious malabsorption,major depression,artificial nutrition,hyperthyroidism,and other causes of malnutrition.The final diagnosis was based on the histologic results from endoscopic ultrasonography fine needle aspiration (EUS-FNA) or surgery.
The subjects of the control groups were healthy people who were at least 18-yearsold,with no previous history of any cancer and other chronic diseases.For the most part,controls were matched to cases for sex and age (plus/minus 5 years).Age,sex,tumor stage,tumor differentiation,body-mass index (BMI),smoking,and the presence of diabetes were noted.
Nutritional and functional assessment
Current body weight and height were measured at the time of inclusion.Diabetes was diagnosed if fasting glucose values met the ADA criteria[23]and the duration since diabetes onset was recorded.
Cachexia was defined as an involuntary weight loss of more than 5% or a weight loss of more than 2% in individuals with a BMI of less than 20 kg/m2over the past 6 mo[24].
Blood sampling
Blood samples were collected at the time of diagnosis.Peripheral venous blood was drawn into a tube containing ethylenediaminetetraacetic acid and was prepared by centrifugation at 5000 × g for 5 min.The plasma samples were stored at -80 °C until use.
Selected proteins were quantified in the plasma using Western blot analyses.
Antibodies
The following antibodies were obtained from Abcam (Cambridge,United Kingdom):rabbit polyclonal antibody to ACV Receptor Type IIB antibody (cat.no.ab128544),rabbit polyclonal antibody to GAPDH (glyceraldehyde 3-phosphate dehydrogenase)(cat.no.ab37168),and horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG H.L antibody (cat.no.ab97051).The antibodies against midkine,mouse monoclonal IgG1 MK antibody (sc-46701,Santa Cruz Biotechnology,Santa Cruz,CA,United States) and goat anti-mouse IgG-HRP for MK (sc-2005; Santa Cruz Biotechnology,Santa Cruz,CA,United States) were obtained from Santa Cruz.
Western blot analyses
Protein concentration was determined using a protein assay kit,Quick Start Bradford Protein Assay (BioRad Laboratories,Inc.).A total of 50 μg of total protein from each plasma sample was loaded per lane onto a 12% polyacrylamide gel.Electrophoresis was performed at 120 mV,and the protein fractions were electrotransferred onto a nitrocellulose membrane at 100 mV for 1 h.The membranes were blocked for 3 h with nonfat dry milk powder (BioRad Laboratories,Inc.) in Trisbuffered saline containing 0.1% Tween20 (TBST) under constant agitation.Subsequently,the membranes were incubated overnight at 4 °C with a rabbit polyclonal antibody to ACV Receptor Type IIB antibody (cat.no.ab128544AbCam) diluted 1: 1000 in TBST,with nonfat dry milk powder and a mouse monoclonal antibody to IgG1 MK antibody (sc-46701; Santa Cruz Biotechnology,Santa Cruz,CA,United States) diluted 1: 100 in TBST with nonfat dry milk powder.For the loading control,a rabbit polyclonal antibody to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (cat.no.ab37168 AbCam) was used at a concentration of 0.8 µg/mL in nonfat dry milk powder in TBST.Membranes were washed with TBST and incubated at room temperature for 1 h with a horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG H.L antibody (cat.no.ab97051,Abcam) diluted 10000fold in TBST and a goat anti-mouse IgG-HRP antibody for MK (sc-2005; Santa Cruz Biotechnology,Santa Cruz,CA,United States) diluted 5000fold in TBST,with an additional washing step performed prior to detection.For GAPDH determination,an HRPconjugated goat IgG antirabbit IgG antibody (cat.no.ab97051,Abcam) diluted 10000fold in TBST was used.Total protein expression was normalized by dividing each of the protein units by those for GAPDH.
Proteins were detected by using Clarity™ Western ECL (BioRad Laboratories,Inc.)and the membranes were imaged in a Chemidoc Imaging System (BioRad Laboratories,Inc.) and analyzed using ImageLab Software version 5.2.1 for Windows(BioRad Laboratories,Inc.).
Measurement and confirmation of the ACV and MK protein levels is often performed with normalization against “housekeeping proteins”,such as glyceraldehyde-3-phosphate dehydrogenase,to correct for protein loading and other factors,such as transfer efficiency.
For protein expression,after densitometry,the integrated density value (IDV) for each protein band (ACV and MK) was determined,and the normalized levels of ACV and MK were calculated by dividing the IDV of a protein band by the IDV of the GAPDH band (arbitrarily assigned a value of 100) in the same sample,thus quantifying the expression of the proteins as high or low expression.
Tissue samples
Tumor tissue samples from endoscopic ultrasound-fine needle aspiration and surgery were fixed with 10% formalin for pathology studies.
In addition,there was a supplementary group of 14 samples containing normal pancreatic tissues from patients who received partial pancreatectomy for benign tumors that were used as normal controls for the immunohistochemical interpretation.
Analysis of perineural invasion
The perineural invasion was assessed only on surgical tissues.The associations of perineural invasion with characteristics of PDAC were assessed on slides stained with hematoxylin-eosin.The presence of perineural invasion was defined by the infiltration of cancer cells into the perineurium or neural fascicles[25].The evaluation of perineural invasion was assessed mainly in tissue obtained from surgery after curative treatment.The degree of perineural invasion was defined as follows: 0- less than one occurrence per slide; 1 -two to four occurrences per slide; and 2 - more than four occurrences per slide or intraneural invasion.
Immunohistochemistry
For each case a paraffin-embedded block was selected.3-micron-thick sections were obtained for immunohistochemistry investigation.The primary antibodies used were the following: ACV Receptor Type IIB antibody (cat.no.ab128544) diluted 1: 150 and mouse monoclonal IgG1 MK antibody (sc-46701,Santa Cruz Biotechnology,Santa Cruz,CA,United States) diluted 1: 200.The BONDIII staining instrument (Leica Biosystems) and Bond Polymer Refine Detection Kit (Leica Biosystems) were used for all antibodies.This analysis was performed with pancreatic tumor and normal tissue samples.
All slides were scored by a pathologist (I.R.) who was blinded to all clinical data.Finally,the tissues were evaluated under a microscope.The intensity of the staining was scored as negative,weak,moderate,or strong (scores of 0,1,2,or 3,respectively).
Statistical analysis
Chi square test or Fisher's exact test were used for categorical data.Comparisons between two groups of continuous data were performed with at-test for independent samples for data with a normal distribution or a Wilcoxon rank-sum test otherwise.Univariate and,multivariate Cox proportional hazard models with each protein expression variable adjusted for age ≥ 50 years,gender,stage (T4vsT1-T3),metastasis,tumor size ≥ 3 cm,and diabetes were built.The Cox proportional hazard assumption and multicollinearity assumptions were checked.Similarly,univariate and multivariate logistic regression models were built to predict metastasis.We checked the models for multicollinearity,misspecification and the goodness of fit.
For all statistical tests,a two-tailedPvalue was used,along with a 0.05 significance level.All analyses were performed in the R environment for statistical computing and graphics,version 3.4.4.
RESULTS
Patient characteristics
A total of 114 patients with PDAC and 125 controls with no tumor were enrolled in this study (Table 1).
The mean age of the population was 62.41 years (SD 11.56,range: 27-88 years).There were more males than females (61%vs39%).
A total of 49 (42.98%) patients with PDAC reported a history of diabetes,including new-onset diabetes for 24 (21.05%) patients.
The most common localization of PDAC was in the pancreatic head (66 patients-49%),followed by a localization in the body (33 patients-24%),the isthmus (17 patients-12%),the pancreatic tail (13 patients-10%) and the uncinated process (7 patients-12%).
Nutritional and functional characteristics
Cachexia was more common in women (P= 0.033) and in patients with metastasis (P= 0.011).The presence of cachexia was unrelated to age (P= 0.389),cancer site (P=0.611),tumor staging (P= 0.148) and perineural invasion (P= 0.12).
Expression of plasma and tissue MK and ACV in pancreatic cancer
MK was expressed in plasma from 54 (47.37%) PDAC patients compared to 20 (16%)controls (P< 0.001).Immunohistochemical staining in PDAC tissue was positive in 56(49.12%) patients,including weak in 34 (29.82%),moderate in 16 (14.04%),and strong in 6 (5.26%),compared to none of the tissue from the supplementary controls (P=0.012) (Figure 1).
ACV was expressed in plasma from 72 (63.16%) PDAC patients compared to 39(31.2%) controls (P< 0.001).Immunohistochemical staining in PDAC tissue was positive in 59 patients (51.8%),including weak in 53 (46.49%) and moderate in 6(5.26%) compared to none of the tissue from the control group (P= 0.001) (Figure 2).
Both proteins were detected in the tumor tissue by immunohistochemistry,and the tissue expression was significantly correlated with the plasma expression (P= 0.002 for Activin; andP= 0.046 for Midkine).
Relationships of ACV and MK expression with clinicopathological features
Table 2 summarizes the associations of MK and ACV expression with clinicopathological features in pancreatic cancer.
MK was highly expressed in the plasma of patients with an advanced T tumor stage (P= 0.006),metastases (P= 0.046),long-term diabetes (P= 0.002) and nonsmokers (P= 0.032).Nevertheless,there was no relationship between clinicopathological factors and high ACV expression.The association between ACV expression and cachexia was not statistically significant (P= 0.5).
Relationship between perineural invasion and expression of MK and ACV
The presence of perineural invasion was observed in only 61 PDAC specimens.The MK expression levels in PDAC were significantly higher in patients with perineural invasion than in those without perineural invasion (P= 0.033).The ACV expression level was not significantly associated with perineural invasion (P= 0.5).

Table 1 Patient characteristics in the adenocarcinoma and control groups,n (%)
Prediction of metastasis
To predict the presence of metastasis in patients with adenocarcinoma,we performed uni- and multivariate analysis by creating regression models,where each expression variable (MK and ACV high expressionvslow expression) in the model was adjusted by age > 50 years,sex,diabetes,stage T4vsT1-3,stage N1vsN0 (Table 3).There were no statistically significant factors.
Survival in PDAC patients
Using data from the 114 patients with PDAC,we further evaluated whether the overexpression of ACV and MK was correlated with patient survival.
The univariate and multivariate Cox proportional survival analysis showed that after adjusting for age,gender,adenopathy,tumor size ≥ 3 cm,and metastases,tumor size > 3 cm,metastasis and ACV expression (Table 4) were independent predictors of poor survival (Figure 3).The survival of patients with high or low levels of MK expression was similar (Figure 4).
DISCUSSION
Our work aimed to study the relationship between perineural invasion and cachexia and their biomarkers,such as MK and ACV,respectively,and their prognostic value in patients with pancreatic cancer.
The survival in PDAC is very low,approximately 6% in 5 years,and one of the reasons is the rich stroma in the tumor tissue and the perineural invasion that extends into the pancreatic nerve plexus[26].One of the promoters of perineural invasion is a neurite growth factor,MK,which is present on the surface of cells and extracellular matrix that facilitates neural differentiation,cell migration,perineural invasion,neuritis out-growth[27,28],neuronal survival[29,30],carcinogenesis and tumor progression[31,32].During carcinogenesis in PDAC,it appears that the pancreatic cells bind at the level of the nerve gap or perineurium,then MK binds its receptor through an interaction that involves chondroitin sulfate,which promotes cancer infiltration,migration and the rapid development of peripheral invasion.During the repair of damage to the perineurium,more cells expressing MK are attracted,and a vicious circle is formed[21].MK expression was not inducible in GEM-treated chemosensitive PDAC cell lines,suggesting that MK is necessary to promote survival during chemotherapy by triggering Notch-2 pathway activation[22].

Figure 1 Immunohistochemical staining of Midkine in pancreatic adenocarcinomaand normal pancreatic tissue.
Increased levels of MK are found in 53% of PDAC,are related to venous invasion,microvessel density,and liver metastasis[22,33]and are involved in biological activities that favor cell growth,survival and angiogenesis[34].MK had a low expression in the primary tumor in 80% of patients and had moderate expression in 18% of patients[35],similar to our results in which the tissue expression was weak in almost 30%,moderate in 14% and strong in 6% of patients,and the plasma MK protein was strongly positive in PDAC patients compared to controls (47.37%) (Figure 5).
We found that high MK expression was closely correlated with advanced tumor stage,the presence of metastasis,diabetes and perineural invasion,but no relationship with cachexia was found.Similar to a previous study[20],we confirmed the association of the MK protein with the presence of perineural invasion but in a larger group of patients (61 patients with pancreatic adenocarcinoma) than was previously reported.
The high level of MK in plasma was more frequently observed in patients with metastasis,similar to other studies[33],but it did not predict the development of metastasis or survival in multivariate analysis.

Figure 2 Immunohistochemical staining of Activin in pancreatic adenocarcinoma and normal pancreatic tissue.
Activin A is a member of the TGFβ superfamily and is involved in many pathophysiological processes[36].ACV exerts most of its biological actions by binding to the membrane ACV type II receptor B[10],a receptor that is shared with Myostatin(another TGF-β superfamily member),and these proteins eventually activate the SMAD pathway,which is involved in pancreatic carcinogenesis[37-39].ACV receptor B seems to be involved in cancer cachexia[40,41],and its blockade by a soluble form prevents muscle atrophy and increases survival without affecting tumor growth[42,43].
The overexpression of ACV in the tumor tissues[44]and elevated blood ACV levels were found in 38 patients with PDAC,sustaining a non-SMAD (MAPK,PI3K/AKT)pathway[45],which is contradictory to the suppressive role of ACV (by activating the SMAD pathway) that has been previously reported.Further studies are required to confirm the mechanism of ACV in pancreatic cancer.
In our study,the plasma ACV level in PDAC patients was higher than that in controls (63%vs32%) (Figure 5),and the immunohistochemistry was positive in 51%of patients,most of whom had a low level of expression.
Despite the previous association of ACV and cachexia in patients with lung and colorectal cancer[46],in our study,high expression of plasma ACV was not associated with cachexia,but this may be related to the small number of cachectic patients (n=22).Additionally,in the multivariate analysis,ACV had no role in metastasis.The PDAC patients with high levels of ACV expression had lower survival rates than those of patients with low levels of ACV expression,supporting the prognostic role of ACV,as reported elsewhere[45],together with tumor size and metastatic status.
Cachexia is associated with decreased visceral body fat and is associated with a lower survival in diabetic patients than in nondiabetic patients[47].In our study,we found no difference regarding survival in patients with or without diabetes.
Also,in our study we did not find a correlation between the presence of the cachexia and the perineural invasion.This may be due to the small number of patients who have undergone surgery (only in these patients it was possible to evaluate perineural invasion).
Patients with newly diagnosed diabetes have a 5-8 fold higher risk of being diagnosed with PDAC in the first 3 years after the development of diabetes,probably due to the direct action of insulin,which facilitates malignant transformation[48].Diabetes can also be a secondary phenomenon that is induced by PDAC through the destruction of beta cells[49],but in our study,no correlation was noted between newonset diabetes and the level of ACV or MK.In contrast,the MK level was higher in patients with long-term diabetes,but diabetes and MK again had no prognostic role.Hyperglycemia favors perineural invasion in PDAC by two mechanisms.First,hyperglycemia increases the proliferation of cancer cells,which increases theexpression of cytokines such as the neurotrophic factor (NGF).The overexpression of NGF may increase the interaction between nerve cells and cancerous cells and neurotropism.The second mechanism is that hyperglycemia causes demyelination and axonal nerve degeneration,allowing cancer cells to invade nerve structures.These two mechanisms promote hyperglycemia or diabetes and may have a role in perineural invasion in pancreatic cancer[50].The intervention of hyperglycemia and signaling between neurons and cancer cells was associated with a higher expression of NGF in PDAC cells and the p75 neurotrophin receptor in nerve fibers in an experimental study[51].

Table 2 Protein plasma expression in adenocarcinoma patients and interaction with clinical and biological parameters,n (%)
The limitations of this study are that perineural invasion was only examined in patients who underwent surgery (and not in all patients) and survival analysis was performed only for cancer patients.Another limitation of the study is that clinicopathological associations were made based on the expression of plasma proteins rather than in tissue.Further studies are,therefore,warranted to explore the mechanism of ACV and MK function,to investigate their potential as therapeutic targets in PDAC progression and the relationship with diabetes and perineural invasion.
In conclusion,the present data suggest that MK is a useful biomarker for perineural invasion,being also correlated with advanced tumor stage,the presence of metastasis and diabetes.Unfortunately,the perineural invasion and the expression of activin was not correlated with casexia,but ACV could be an effective biomarker for predicting poor prognosis in PDAC patients,and it might be a novel therapeutic marker for selectively targeting cancer cells.
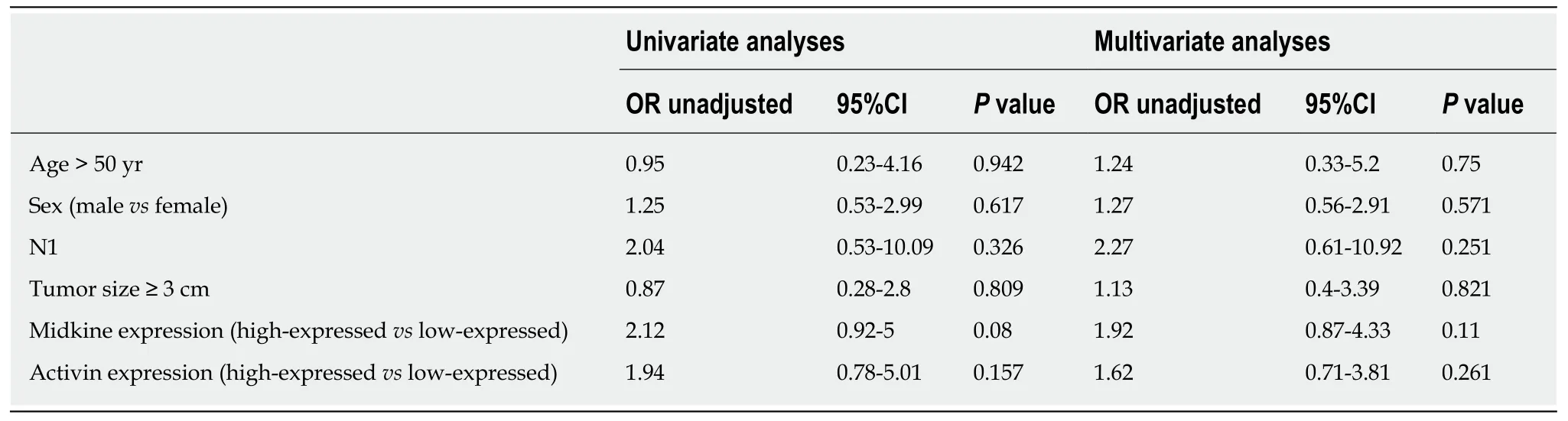
Table 3 Univariate and multivariate analysis to predict metastasis

Table 4 Univariate and multivariate analysis to predict survival

Figure 3 Overall survival comparison between high and low-expressed Activin stratified by tumoral stage T1-3 vs T4.
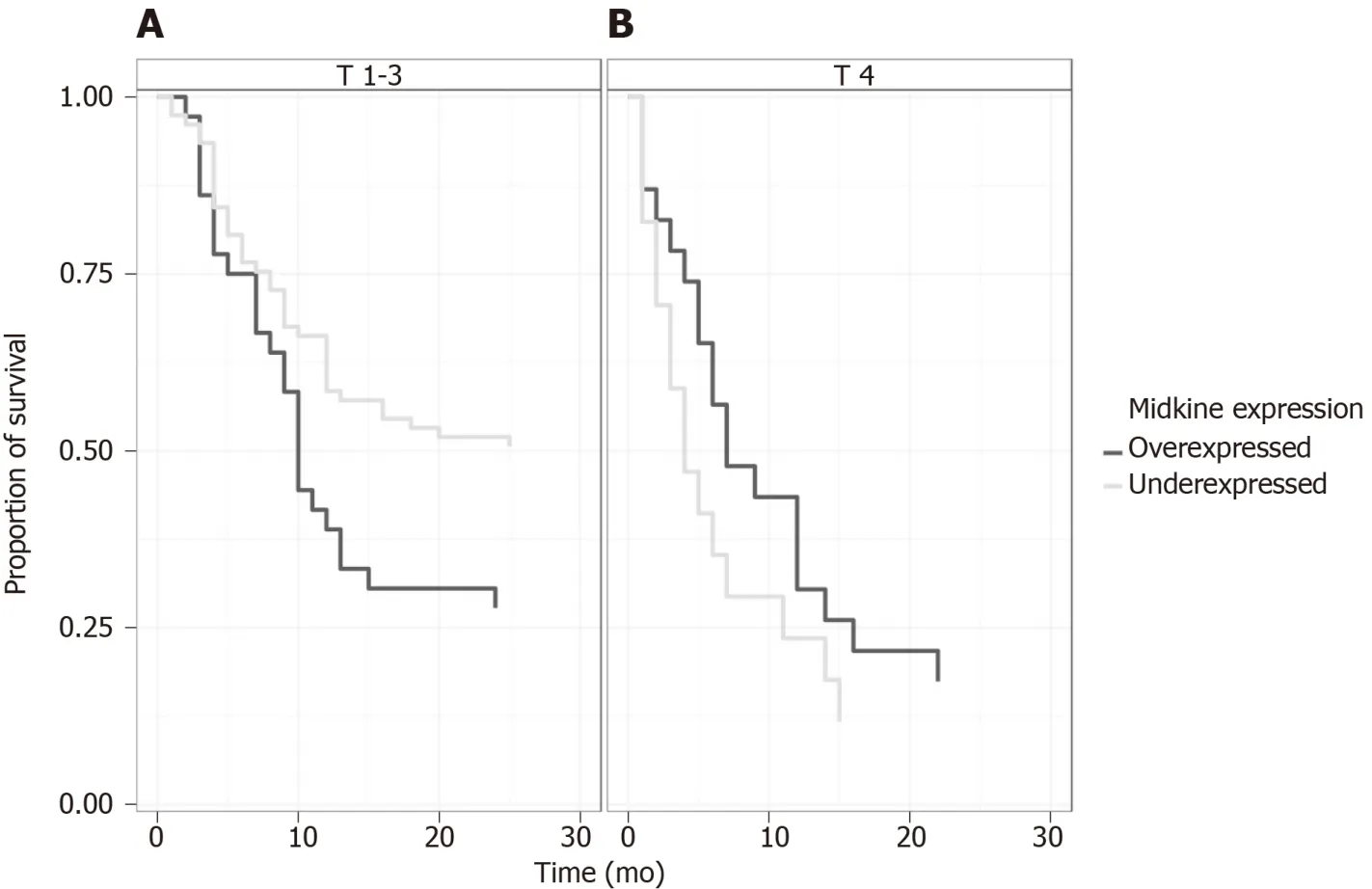
Figure 4 Overall survival comparison between high and low-expressed Midkine stratified by tumoral stage T1-3 vs T4.

Figure 5 Western blot analyses of Activine and Midkine in patients with pancreatic adenocarcinoma and controls.
ARTICLE HIGHLIGHTS
Research background
Pancreatic cancer has a high mortality rate,due to late diagnosis.Cachexia and perineural invasion have an increased incidence in pancreatic cancer,leading to decreased quality of life.Combating pain and cachexia through optimal treatment options can lead to increased quality of life and improved survival.
Research motivation
We wanted a better understanding of the involvement of cachexia and pain in pancreatic cancer and their relationship with different clinico-pathological factors,constituting a basis for discovering new therapeutic targets.
Research objectives
Defining the profile of cachexia in pancreatic cancer; establishing the degree of perineural invasion in pancreatic cancer; Highlighting the interrelationship between cachexia and perineural invasion in pancreatic cancer.
Research methods
We conducted a prospective study in 114 patients with pancreatic cancer and 125 healthy people as controls.Blood samples were used and pancreatic tissue were collected through EUS-FNA or surgery.The method of determining the biomarkers of cachexia (Activin) and perineural invasion (Midkine) in plasma was western blot,respectively immunohistochemistry in pancreatic tissue.
Research results
The analysis of the data showed an Activin (ACV) and Midkine (MK) proteins overexpression in plasma of patients with pancreatic cancer vs control,results that were correlated with the expression of proteins in the pancreatic cancer tissue.MK was also significantly correlated with advanced T stage,metastasis,diabetes and perineural invasion.ACV was significantly correlated with survival.
Research conclusions
MK can be considered a biomarker for perineural invasion,and ACV a prognostic factor for patients with pancreatic cancer.
Research perspectives
This research would open new perspectives in choosing the treatment that involves activin antagonists in order to prolong survival in patients with pancreatic cancer.
ACKNOWLEDGEMENTS
We thank Dr.Cristian Coman,Institute of Biology Research,Cluj-Napoca and Associate Prof.Manuela Banciu,PhD,Faculty of Biology and Geology,University of Babes-Bolyai,Cluj-Napoca for their support.This study was partially funded by the“Iuliu Haţieganu” University of Medicine and Pharmacy,Cluj-Napoca,through the Doctoral Research Project-2015 (No.7690/36/15.04.2016).The financial support allocated from the grant was used for the acquisition of biomarkers and laboratory supplies.The sponsor had no involvement in the study design,collection,analysis and interpretation of data,writing of the manuscript or decision to submit the manuscript for publication.
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Observation or resection of pancreatic intraductal papillary mucinous neoplasm: An ongoing tug of war
- Improved method for inducing chronic atrophic gastritis in mice
- Protein expression trends of DNMT1 in gastrointestinal diseases: From benign to precancerous lesions to cancer
- Asian Americans have better outcomes of non-metastatic gastric cancer compared to other United States racial groups: A secondary analysis from a randomized study
- Gastric partitioning for the treatment of malignant gastric outlet obstruction
- Difference in failure patterns of pT3-4N0-3M0 esophageal cancer treated by surgery vs surgery plus radiotherapy
