Gasdermin D-mediated hepatocyte pyroptosis expands inflammatory responses that aggravate acute liver failure by upregulating monocyte chemotactic protein 1/CC chemokine receptor-2 to recruit macrophages
2019-12-13HongLiXueKeZhaoYiJuChengQuanZhangJunWuShuangLuWeiZhangYangLiuMingYuZhouYaWangJingYangMingLiangCheng
Hong Li, Xue-Ke Zhao, Yi-Ju Cheng, Quan Zhang, Jun Wu, Shuang Lu, Wei Zhang, Yang Liu, Ming-Yu Zhou,Ya Wang, Jing Yang, Ming-Liang Cheng
Abstract BACKGROUND Massive hepatocyte death is the core event in acute liver failure (ALF).Gasdermin D (GSDMD)-mediated pyroptosis is a type of highly inflammatory cell death. However, the role of hepatocyte pyroptosis and its mechanisms of expanding inflammatory responses in ALF are unclear.AIM To investigate the role and mechanisms of GSDMD-mediated hepatocyte pyroptosis through in vitro and in vivo experiments.METHODS The expression of pyroptosis pathway-associated proteins in liver tissues from ALF patients and a hepatocyte injury model was examined by Western blot.GSDMD short hairpin RNA (shRNA) was used to investigate the effects of downregulation of GSDMD on monocyte chemotactic protein 1 (MCP1) and its receptor CC chemokine receptor-2 (CCR2) in vitro. For in vivo experiments, we used GSDMD knockout mice to investigate the role and mechanism of GSDMD in a D-galactose/lipopolysaccharide (D-Galn/LPS)-induced ALF mouse model.RESULTS The levels of pyroptosis pathway-associated proteins in liver tissue from ALF patients and a hepatocyte injury model increased significantly. The level of GSDMD-N protein increased most obviously (P < 0.001). In vitro, downregulation of GSDMD by shRNA decreased the cell inhibition rate and the levels of MCP1/CCR2 proteins (P < 0.01). In vivo, GSDMD knockout dramatically eliminated inflammatory damage in the liver and improved the survival of DGaln/LPS-induced ALF mice (P < 0.001). Unlike the mechanism of immune cell pyroptosis that involves releasing interleukin (IL)-1β and IL-18, GSDMDmediated hepatocyte pyroptosis recruited macrophages via MCP1/CCR2 to aggravate hepatocyte death. However, this pathological process was inhibited after knocking down GSDMD.
Key words: Gasdermin D; Hepatocyte; Pyroptosis; Acute liver failure; Monocyte chemotactic protein 1/CC chemokine receptor-2
INTRODUCTION
Multiple factors such as drugs, alcohol, and hepadnaviruses can lead to large-scale hepatocyte death in a short period of time, which may progress into acute liver failure(ALF) with a mortality rate of up to 80%[1]. The only known therapy, liver transplantation, often cannot be performed in a timely fashion due to the shortage of donors. As of now, there is no effective intervention target or specific drug therapy available for ALF[2].
Massive hepatocyte death is the core event of ALF. The manner of death and number of dead hepatocytes determine the intensity of the expanding inflammatory responses[3]. Necrosis has classically been considered to be the major mechanism of hepatocyte death during the pathogenesis of ALF. However, knockout of the receptorinteracting protein serine-threonine kinases-3 gene or the mixed lineage kinase domain-like pseudokinase gene in mice did not significantly improve the survival rate of mice subjected to ALF[4].
Pyroptosis is a novel programmed cell death mechanism that can induce strong inflammatory reactions and mainly only occurs in immune cells such as phagocytic cells, macrophages, and monocytes[5]. Endogenous and exogenous danger signals can activate caspase 1 or caspases 4 and 5 (human)/caspase 11 (mouse), which cleave gasdermin D (GSDMD) to produce its active N-terminal region [cleaved N-terminal fragment of GSDMD (GSDMD-N)] and an inactive C-terminal fragment. GSDMD-N inserts into the cell membrane and forms a large number of sieve-like pores with a diameter of approximately 10-20 nm through the membrane, resulting in cell death and releasing inflammatory factors such as interleukin (IL)-1β and IL-18[6-8]. Pyroptosis plays an important role in the process of defending against pathogenic microbial infections by the body, but excessive uncontrolled pyroptosis can lead to severe tissue damage[9]. Studies in recent years have found that there is a pyroptosis mechanism in parenchymal cells such as myocardial cells, renal tubular cells, and neuronal cells[10].However, the mechanisms of hepatocyte pyroptosis in ALF are unclear.
The triggering and progression of ALF are closely related to uncontrolled inflammatory responses[11]. Monocyte chemotactic proteins 1, 2, and 3 (MCP1, MCP2,and MCP3) are expressed in many cell lineages. By binding to their common receptor CC chemokine receptor-2 (CCR2), MCPs play a role in amplifying inflammatory responses that aggravate tissue injury by strongly recruiting the directional migration of immune cells[12]. Studies have found that among these, only MCP1 is expressed in hepatocytes and released to extracellular sites in response to hepatocyte injury. MCP1 thus plays a role as an important inflammatory response amplifier in ALF[13,14].However, the functions of MCP1 in hepatocyte pyroptosis have not been fully elucidated.
Our study showed that the expression of GSDMD-N was upregulated significantly in D-galactose/lipopolysaccharide (D-Galn/LPS)-treated AML12 hepatocytes as well as in liver tissue from ALF patients and a D-Galn/LPS-induced mouse ALF model.Reduction of hepatocyte pyroptosis by knockdown of GSDMD significantly inhibited MCP1/CCR2 expression, reduced liver injury, and increased the survival rate of mice.Our results suggested that finding effective intervention targets or drugs to inhibit GSDMD may be a potential approach to the effective treatment of ALF.
MATERIALS AND METHODS
Human serum and liver samples
According to the “Guidelines for Acute Liver Failure” recommended by the European Association for the Study of the Liver in 2017[1], ALF is defined as the occurrence of jaundice and encephalopathy and an international normalized ratio (INR) > 1.5 within fewer than 26 wk after onset. Peripheral serum samples from 30 ALF patients and 30 healthy people were collected from the Affiliated Hospital of Guizhou Medical University. There were seven diseased liver tissue specimens from ALF patients(caused by hepatitis B virus infection) and six healthy liver specimens (from deceased organ donors who died from intracranial bleeding or head injury and were free from chronic diseases) available for study.
Acute liver failure mouse model
Wild-type (WT) male C57BL/6 mice aged 6-8 wk were purchased from the Experimental Animal Center of the Third Military Medical University (Chongqing).Male GSDMD gene knockout (GSDMD-/-) mice (C57BL/6J strain) aged 6-8 wk were a generous gift from Shao Feng’s Laboratory, Beijing Institute of Life Sciences, China.All animals received humane care according to established standards and were maintained in an air-conditioned animal room at 25 °C with free access to water and food. The wild-type mice and GSDMD-/- mice were randomly divided into a normal control group (n = 5) and a D-Galn/LPS group (n = 15). Mice in the D-Galn/LPS group were intraperitoneally injected with D-Galn 300 mg/kg + LPS 10 µg/kg once[15,16], while mice in the normal control group were intraperitoneally injected with an equal amount of phosphate buffer saline (PBS). After 6 h, all mice were sacrificed.Blood was collected from the eyeball vein and centrifuged at 3000 rpm/min for 15 min, and the serum was separated and stored at -80 °C. Liver tissues were harvested by portal vein perfusion. Some of the specimens were fixed using paraformaldehyde for 48 h, and then pathological examination was performed. The remaining tissues were quickly placed at -80 °C.
Culture and treatment of hepatocytes
The mouse liver cell line AML12 was purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences, and was cultured in DMEM-F12 culture medium containing 10% foetal bovine serum, 1% insulin-transferrin-selenium, and 40 ng/mL dexamethasone (Gibco, United States). The cells were cultured in an incubator containing 5% CO2at a constant temperature of 37 °C. The cells were treated with DGaln (15 mmol/L)/LPS (100 µg/mL) for 0, 6, 12, and 24 h to establish hepatocyte injury models at dynamic time periods.
GSDMD RNA interference and transfection in AML12
GSDMD short hairpin RNA (shRNA) and negative control (control shRNA) vectors were purchased from Sangon Biotech and were transfected into the AML12 cells following the manufacturer’s instructions with Lipofectamine 3000 (Invitrogen,United States). The culture medium was replaced with fresh complete medium after 6 h for continuous culture in an incubator containing 5% CO2at the constant temperature of 37 °C for 48 h. Further experiments were performed if the transfection rate was greater than 50%.
Biochemical and coagulation function analysis
Alanine aminotransferase (ALT) and blood ammonia in human and mouse serum samples and the supernatant of cell culture medium were detected using an automatic biochemical analyser (Siemens Advia 1650; Siemens, Bensheim, Germany). The INR of prothrombin was tested using a SysmexCA-7000 coagulation detector.
Western blot analysis
Antibodies against the following proteins were used for Western blot analysis:caspase 1 and caspase 4 (human), caspase 11 (mouse), GSDMD, MCP1, CCR2 (1:1000,Abcam), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:10000, Abcam).The secondary antibody was horseradish peroxidase-conjugated IgG (1:8000, Abcam).For the blots, 30-50 µg of total protein was added into each well and the proteins were separated by electrophoresis on a 10% sodium dodecylsulphate polyacrylamide gel electrophoresis precast gel (Invitrogen, CA, United States). The proteins were then transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, United States). The protein bands were developed with an enhanced luminescent developer(Millipore) and photographed using a Chemi Doc MP System (Bio-Rad, Hercules,United States). The grey value was measured using ImageJ software.
Cell counting kit-8 assay
A total of 2 × 104AML12 cells were seeded in 96-well plates and cultured for 24 h.Then, they were treated for 0, 6, 12, and 24 h, followed by incubation with 10 μL of cell counting kit-8 assay solution in each well for 2 h. The absorbance was measured with a microplate reader at 450 nm. According to the instructions of the kit, cell inhibition rate was calculated as [(control - experimental)/(control - blank)] × 100%.
Cellular immunofluorescence
Cells were fixed with 4% paraformaldehyde and cellular immunofluorescence was performed according to previously described protocols. The cells were incubated with an anti-GSDMD antibody (1:200) at 4 °C overnight. Then, the cells were incubated with an Alexa Fluor 488 fluorescence labelled goat anti-rabbit IgG secondary antibody(1:1000) for 2 h, followed by nuclear staining with 4’,6-diamidino-2-phenylindole(DAPI). The cells were visually observed and photographed using an Olympus FV1000 (Olympus, Tokyo, Japan).
Detection of inflammatory cytokines
Inflammatory cytokines, including IL-1β, IL-18, tumor necrosis factor-alpha (TNFα),and IFN-γ, in human serum samples were detected with the Bioplex multi-cytokine assay kit according to the kit instructions using a BD FACS CantoII flow cytometer(BD Biosciences, NJ, United States). Calculations were conducted using LEGENDplex8.0 software. MCP1, IL-1β, IL-18, TNFα, and IFN-γ in mouse serum were detected based on the kit instructions provided for the enzyme-linked immunosorbent assay (ELISA) kits (Cusabio Biotech Co., Ltd., China).
Haematoxylin and eosin staining and immunohistochemical staining
Haematoxylin and eosin (H and E) staining was performed based on the routine procedure. Pathological changes of the liver were observed with an A12.1503 microscope [Opto-Edu (Beijing) Co., Ltd., Beijing, China]. The degree of inflammation of the liver in mice was evaluated independently by three senior physicians at the Department of Pathology based on the Ishaki score system.
For immunohistochemical staining, F4/80 rat anti-mouse monoclonal antibody(Abcam, diluted to 10 µg/mL) was incubated with the tissue at 4 °C overnight. On the next day, the tissue was incubated with a biotinylated goat anti-rat IgG (1:2000,Abcam) at room temperature for 30 min, then diaminobenzidine staining was performed. Photographs were taken with an Olympus BX41 microscope. The percentage of the average optical density area of five random fields (200×) was calculated using Image J.
Statistical analysis
Data were analysed using Student’s t-test (Sigma Plot, SPSS Inc., IL, United States) for differences between two groups and are expressed as the mean ± square error. For comparisons between multiple groups, three-way analysis of variance was performed,followed by t-tests with Bonferroni correction using SAS 9.3 (SAS Institute Inc., Cary,NC, United States). In addition, a log-rank test was used for survival analysis. All experiments were repeated at least three times. Differences were considered statistically significant at P < 0.05 (aP < 0.05,bP < 0.01, andeP < 0.001).
RESULTS
Pyroptosis-associated inflammatory cytokines and proteins increase in human ALF
To investigate whether pyroptosis is involved in the pathogenesis of ALF, we first collected serum samples from 30 ALF patients and 30 normal controls. Serum ALT,TBIL, blood ammonia, and the INR of prothrombin were significantly higher in samples from patients with ALF compared to the control samples (Figure 1A).Pyroptosis-associated inflammatory cytokines, including IL-1β, IL-18, TNFα, and IFNγ, increased in the ALF cases (Figure 1B). Pyroptosis-associated protein expression in liver tissues from patients with ALF and normal controls were then detected by Western blot. The expression levels of caspase 1/4, full-length GSDMD (GSDMD-FL),and GSDMD-N in liver tissues increased in ALF cases compared to the controls(Figure 1C), and the expression of GSDMD-N increased significantly. These data suggest that pyroptosis is involved in the pathogenesis of ALF.
Pyroptosis-associated proteins increase in a D-Galn/LPS-induced hepatocyte injury model
Hepatocyte death is the core event of ALF, and in order to investigate whether there is GSDMD-mediated pyroptosis when hepatocyte injury occurs, the liver cell line AML12 was used to establish a dynamic time-axis model of hepatocyte injury at 0, 6,12, and 24 h using D-Galn/LPS. With the prolongation of the intervention, the cell inhibition rate increased gradually, and ALT content in the supernatant increased as well (Figure 2A and B). It was observed under a confocal fluorescence microscope that GSDMD protein was only slightly expressed in normal AML12 cells. However, the expression of GSDMD protein increased in the hepatocyte cytoplasm over time after the intervention. After 24 h of intervention, the GSDMD protein gradually accumulated on the medial side of the hepatocyte membrane, which is in line with the previous observations that cleaved GSDMD-N segments were displaced from the cytoplasm to the cell membrane and then inserted into the medial membrane to form membrane pores, thus leading to cell death (Figure 2C).
Protein expression levels of caspase 1/11, GSDMD-FL, and GSDMD-N were found to increase over time by Western blot (Figure 2D). However, the downstream inflammatory cytokines IL-1β and IL-18 were not detected in the supernatant, but MCP1 content increased with the prolongation of intervention time (Figure 2E). These results suggested that pyroptosis is an important form of death in this D-Galn/LPSinduced in vitro hepatocyte injury model. However, hepatocyte pyroptosis may mediate and expand the inflammatory responses by mechanisms different from the mechanisms used for pyroptosis in immune cells.
GSDMD knockdown by shRNA decreases the expression of MCP1 and CCR2 in a DGaln/LPS-induced AML12 hepatocyte injury model
It has been confirmed in previous studies that the MCP1/CCR2 signalling pathway has the effect of strongly triggering neutrophil aggregation to mediate immune injury of the liver and is thus involved in the pathogenesis of ALF. The above study results showed that D-Galn/LPS-induced hepatocyte pyroptosis did not release IL-1β and IL-18, but the levels of MCP1 increased significantly in the cell supernatant. To further observe whether the pyroptosis executing protein GSDMD has regulatory effects on MCP1/CCR2, Lipofectamine 3000 was used to transfect shRNA to knock down GSDMD expression in AML12 hepatocytes (Figure 3A). Our results revealed that downregulation of GSDMD expression significantly reduced the protein expression levels of MCP1 and CCR2 in the D-Galn/LPS-induced hepatocyte injury model(Figure 3B). Moreover, the cell inhibition rate decreased (Figure 3C), and the ALT and MCP1 contents in the cell supernatant were also reduced (Figure 3D).
GSDMD knockout dramatically eliminates inflammatory damage in the liver and improves the survival of D-Galn/LPS-induced ALF mice
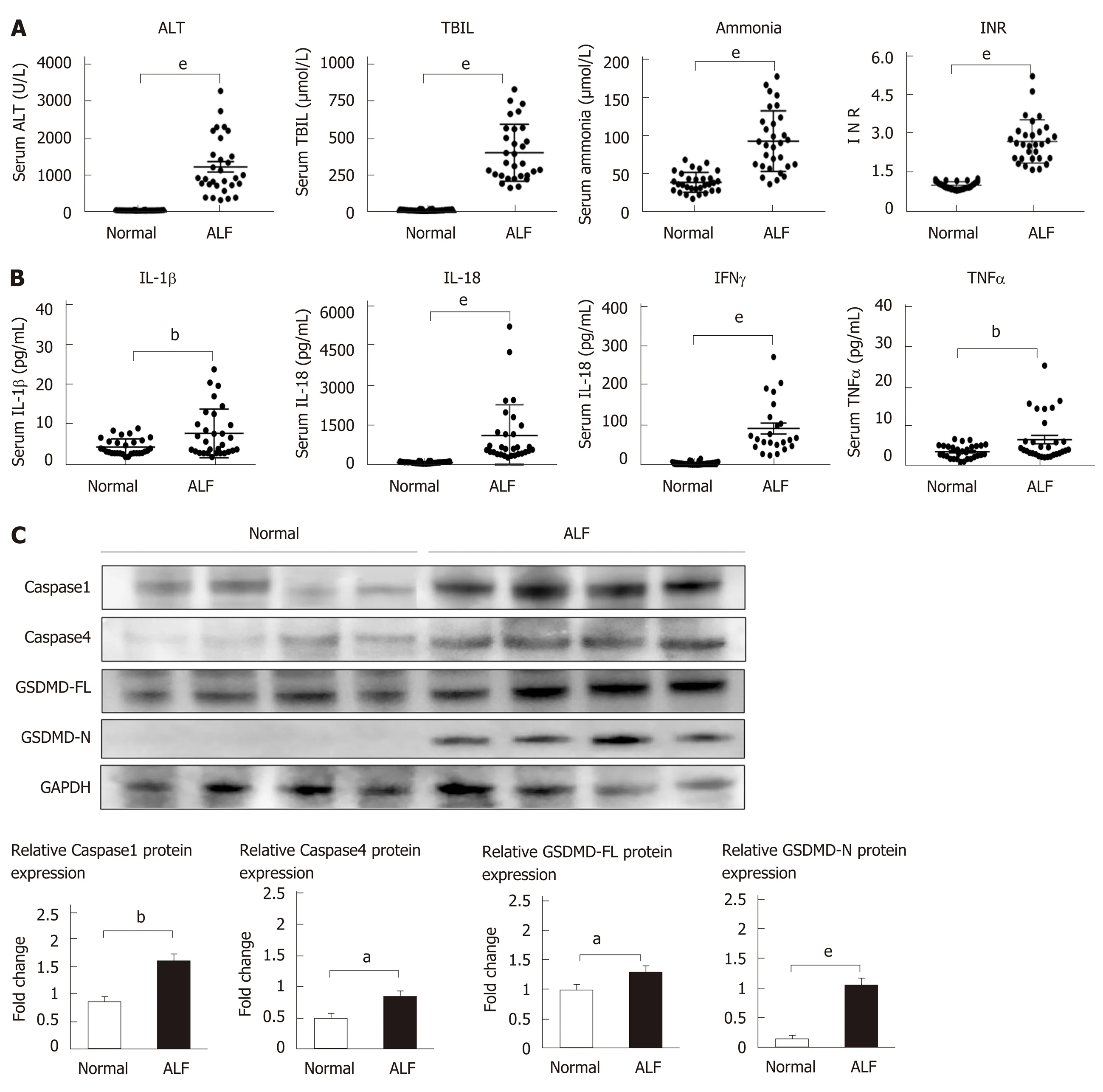
Figure 1 The levels of pyroptosis-associated inflammatory cytokines and proteins increase in human acute liver failure.
In the D-Galn/LPS-induced ALF model in mice, the WT + D-Galn/LPS group started to die at approximately 6 h, and only 1 out of the 15 mice survived to 24 h. In the GSDMD-/- + D-Galn/LPS group, the 24-h survival rate improved tremendously with only 3 out of the 15 mice dying (Figure 4A). Anatomy analysis showed that the liver surface was smooth and fine, the colour was dark pink, the edge was sharp, and the texture was medium for mice in the WT + PBS and GSDMD-/- + PBS groups. For mice in the WT + D-Galn/LPS group, the liver was significantly congested and swollen, the liver volume was enlarged with a dark brown colour, and the membrane was tight. For mice in the GSDMD-/- + D-Galn/LPS group, the liver was slightly congested and swollen (Figure 4B). Compared to the WT + PBS group, ALT, INR, and blood ammonia increased significantly for mice in the WT + D-Galn/LPS group, but the above indicators decreased significantly for mice in the GSDMD-/- + D-Galn/LPS group compared to mice in the WT + D-Galn/LPS group (Figure 4C). H and E staining of the pathological sections of the liver (Figure 4D) showed that the hepatocyte cytoplasm was evenly stained red, the size of the nucleus was normal, the hepatic sinus was clear, and the hepatic cords were neatly organized for the mice in the WT + PBS group and the GSDMD-/- + PBS group. For mice in the WT + DGaln/LPS group, the hepatocytes suffered from massive necrosis, the structure of the hepatic lobule collapsed, a large number of red blood cells were deposited in the hepatic sinus, and there was extensive inflammatory cell infiltration into the portal area and the surrounding area. For GSDMD-/- mice, H and E staining of the liver showed that the hepatocytes were swollen and degenerated, there was focal necrosis,the liver lobular structure existed, and the inflammatory cell infiltration was reduced significantly. The Ishak score showed that the degree of inflammation in the GSDMD-/- + D-Galn/LPS group was reduced significantly compared to the WT + DGaln/LPS group (Figure 4E).
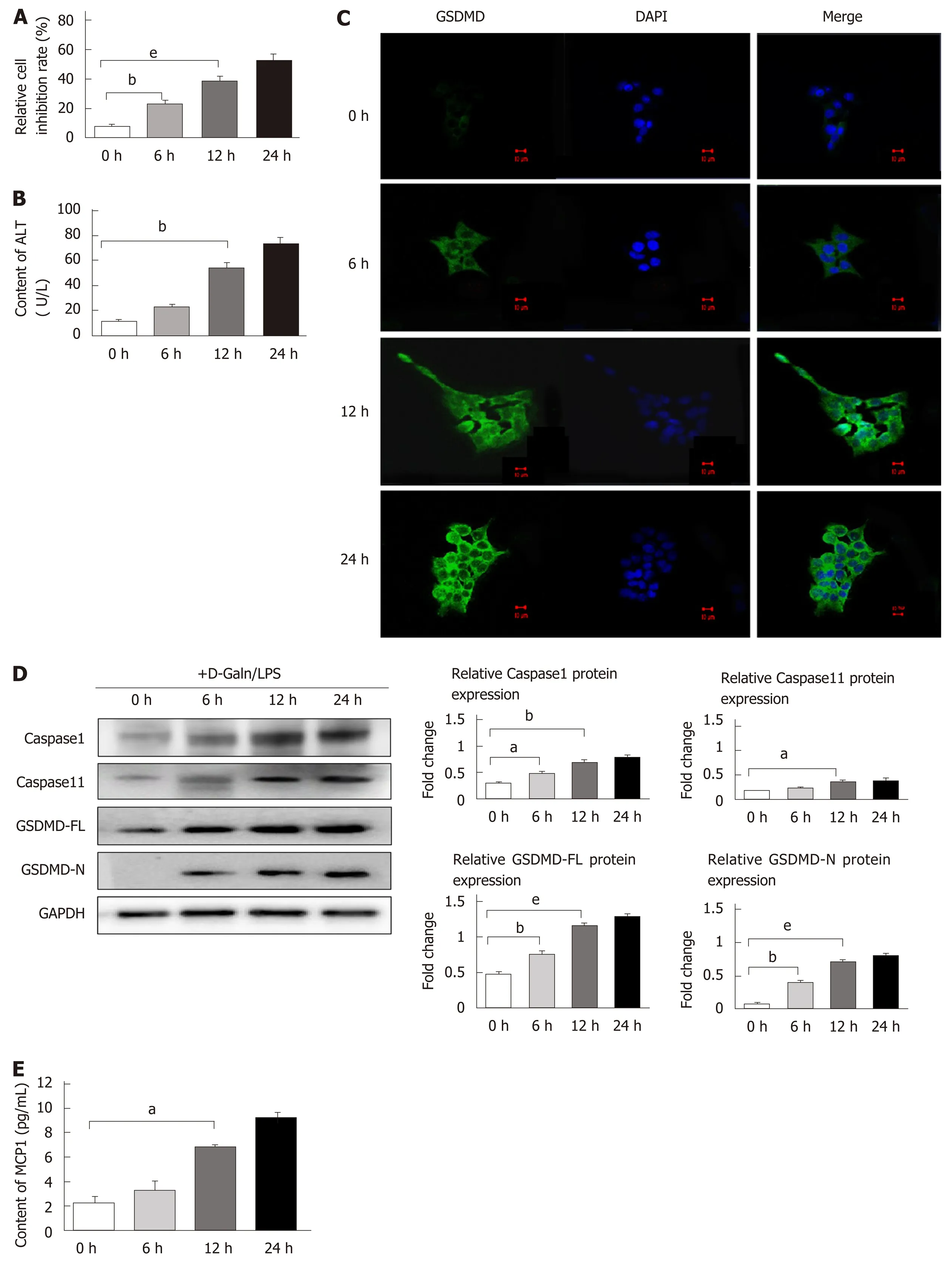
Figure 2 Expression of pyroptosis pathway-associated proteins increases in a D-galactose/lipopolysaccharide-induced AML12 hepatocyte injury model.
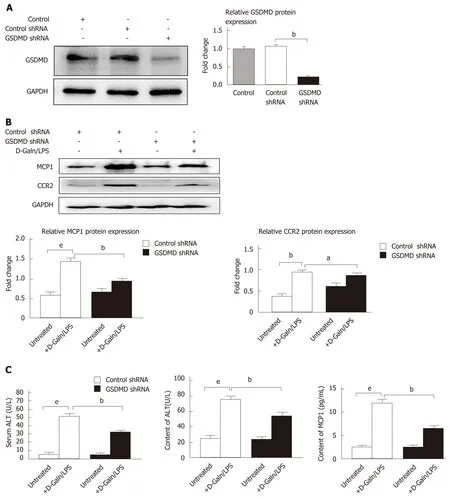
Figure 3 Gasdermin D knockdown by shRNA decreases the expression of monocyte chemotactic protein 1 and CC chemokine receptor-2 in a D-galactose/lipopolysaccharide-induced AML12 hepatocyte injury model.
GSDMD knockout significantly decreases the expression of MCP1/CCR2 and macrophage infiltration in D-Galn/LPS-induced ALF mice
The expression of GSDMD-N in the liver of the WT + D-Galn/LPS group mice increased significantly compared to that in the WT + PBS group mice. The protein expression of MCP1/CCR2 in the GSDMD-/- + D-Galn/LPS group decreased compared to that in the WT + D-Galn/LPS group (Figure 5A). Immunohistochemistry of the liver showed that macrophage-specific F4/80 protein expression in the GSDMD-/- + D-Galn/LPS group was reduced significantly compared to that in the WT + D-Galn/LPS group (Figure 5B). Serum MCP1, IL-1β, IL-18, TNFα, and IFN-γ were decreased in the GSDMD-/- + D-Galn/LPS group compared to the WT + DGaln/LPS group (Figure 5C).
DISCUSSION
The aetiology of ALF varies and the pathogenesis is thus slightly different in different geographic regions, but massive hepatocyte death and the secondary severe inflammatory responses are the same and are more important than the initial trigger[17,18].
GSDMD is a key substrate protein for pyroptosis, which rapidly leads to cell death and attracts a large number of immune cells. Therefore, GSDMD is known to be one of the important switches for inflammatory responses[19]. The role and mechanism of GSDMD-mediated hepatocyte pyroptosis in the pathogenesis of ALF remain unclear.
First, we clarified that the protein expression levels of caspase 1/4, GSDMD-FL,and GSDMD-N increased in the liver and the levels of IL1β, IL-18, TNFα, and INFγ in the serum were elevated in human cases of ALF compared with controls. Second, we established 0, 6, 12, and 24 h dynamic hepatocyte injury models in AML12 cells by treatment with D-Galn/LPS. We found that along with the gradual increase in the cell inhibition rate, the protein expression levels of caspase 1/11, GSDMD-FL, and the cleaved N-terminal fragment increased with the prolongation of the intervention time.However, knocking down GSDMD by shRNA obviously decreased the cell inhibition rate in the D-Galn/LPS AML12 hepatocyte injury model. Third, in in vivo experiments, compared to the WT + D-Galn/LPS group, serum ALT, ammonia, and inflammatory factors including IL1β, IL-18, TNFα, and INFγ were decreased significantly in the GSDMD-/- + D-Galn/LPS group and the INR was shortened.Furthermore, H and E staining of histopathological sections of the liver showed that hepatocyte death was reduced significantly, the hepatic inflammatory response was alleviated significantly, and the survival rate improved dramatically. These results showed that GSDMD-mediated hepatocyte pyroptosis plays a key role in both human ALF and the D-Galn/LPS induced mouse ALF model.
Pyroptosis promotes and expands inflammatory responses by releasing inflammatory factors IL-1β and IL-18, but we did not detect IL-1β and IL-18 in the culture supernatant of the D-Galn/LPS induced AML12 hepatocyte injury model by ELISA, which is consistent with the experimental results of Kofahi et al who used HCV infection to induce Huh-7.5 human hepatocyte pyroptosis in vitro[20]. In addition,Geng et al[21]also reported that the process of hepatocyte pyroptosis cannot be inhibited by the interfering heat shock-induced ALF model in rats by using the IL-1 receptor antagonist anakinra, suggesting that the mechanisms of hepatocyte pyroptosis in inducing inflammatory responses are different from those of immune cell pyroptosis.
In this study, we found that the content of MCP1 in the supernatant was increased in the AML12 hepatocyte injury model. Downregulation of GSDMD by shRNA can significantly reduce the protein expression levels of MCP1/CCR2 and the amount of MCP1 released to the supernatant.
The previous experiments showed that the hepatic inflammatory response was alleviated and the survival rate improved significantly in the GSDMD-/- + DGaln/LPS group mice. Downregulation of GSDMD inhibited MCP1/CCR2 in vitro,thus it is important to explore whether GSDMD-mediated hepatocyte pyroptosis expands inflammatory responses by upregulating MCP1/CCR2 to recruit macrophages in ALF mice. We found that the expression levels of MCP1, CCR2, and F4/80 in the liver of the WT + D-Galn/LPS group mice were increased, and compared to the WT + D-Galn/LPS group, the expression levels of MCP1, CCR2, and F4/80 in the liver were decreased significantly in the GSDMD-/-+D-Galn/LPS mice. Our study has provided new evidence for the role and mechanisms of hepatocyte pyroptosis in ALF.
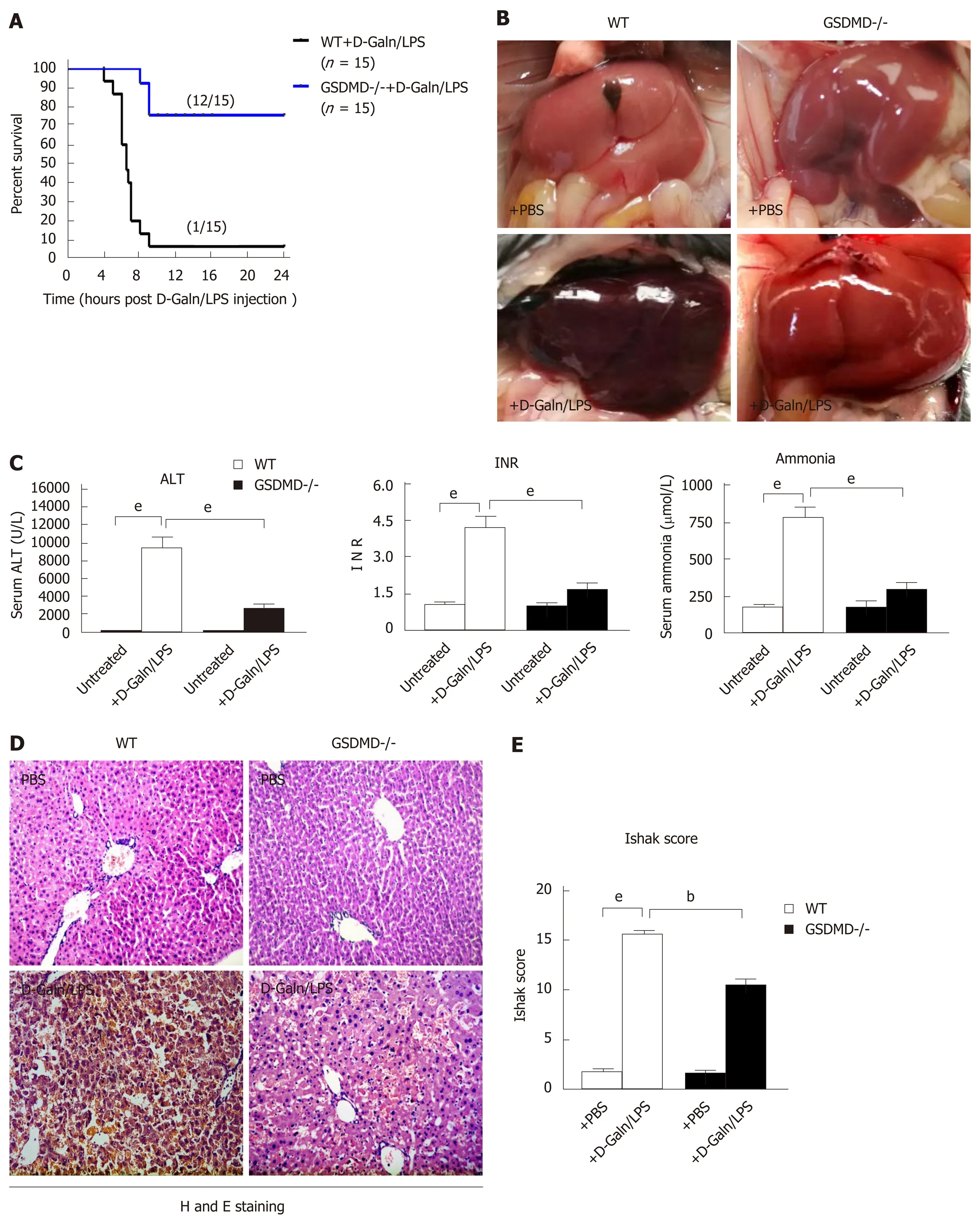
Figure 4 Gasdermin D knockout dramatically eliminates inflammatory damage in the liver and improves survival in D-galactose/lipopolysaccharide-induced acute liver failure mice.
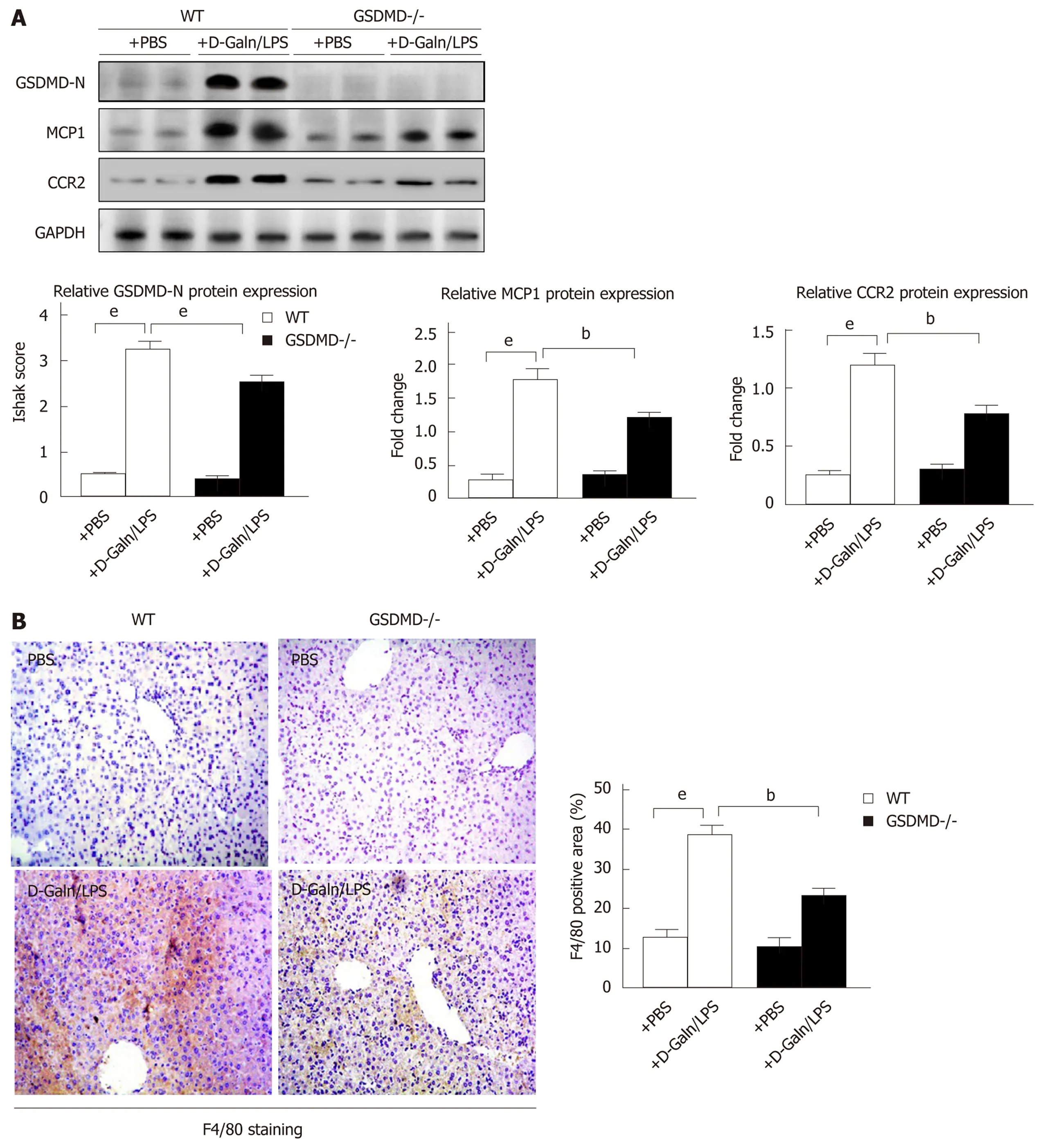
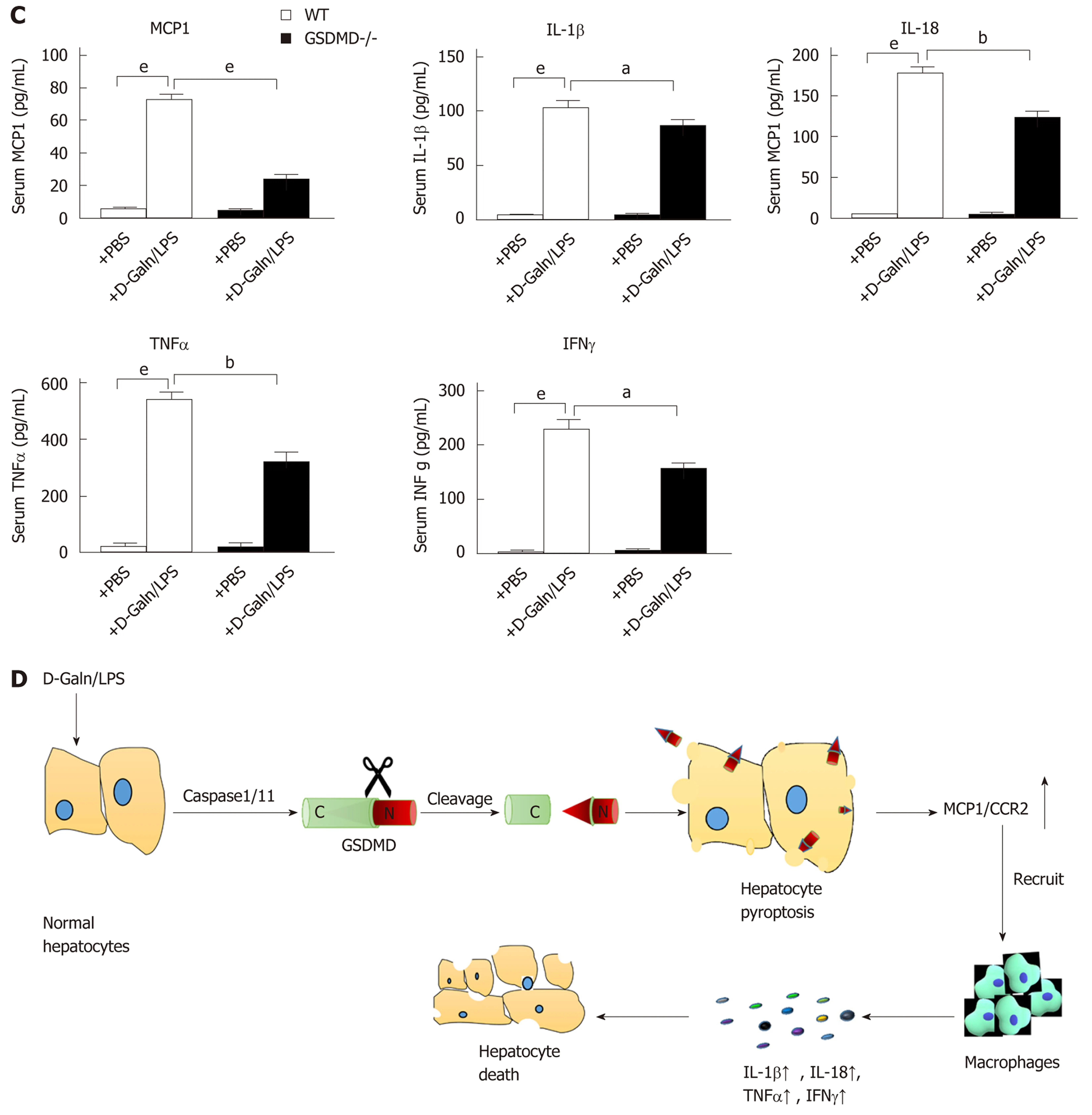
Figure 5 Gasdermin D knockout significantly decreases the expression of monocyte chemotactic protein 1/ CC chemokine receptor-2 and macrophage infiltration in D-galactose/lipopolysaccharide-induced acute liver failure mice.
Our study demonstrated that GSDMD-mediated pyroptosis played an important role in the pathogenesis of human ALF and D-Galn/LPS-induced hepatocyte injury in vitro as well as in mouse ALF in vivo. Unlike the mechanism of immune cell pyroptosis expanding inflammatory responses by releasing IL1β and IL-18,hepatocyte pyroptosis recruited immune cells to infiltrate the liver by upregulating MCP1/CCR2, further expanding the cascading inflammatory responses. Inhibition or knockdown of GSDMD can reduce hepatocyte pyroptosis by downregulating MCP1/CCR2, which alleviates the inflammatory cytokine storm, thus improving ALF. Developing drugs that have the effects of targeted inhibition of GSDMD is thus a potential new therapeutic approach to develop ALF therapies. However, the present study has its shortcomings. In the present study, we did not use adenovirus to promote GSDMD gene or protein expression, and thus observing the effects of overexpression of GSDMD on hepatocyte pyroptosis will be the focus of our future work.
ARTICLE HIGHLIGHTS
Research background
Acute liver failure (ALF) seriously endangers human life due to its high mortality. There is currently no specific treatment method or drugs available for treating ALF. Pyroptosis is a highly inflammatory type of programmed cell death. Gasdermin D (GSDMD), as the final executor of pyroptosis, is also known as one of the important control switches in inflammatory responses.However, the actual effects of GSDMD in hepatocyte pyroptosis and ALF are still unclear.
Research motivation
Our findings may provide a research basis for developing inhibitors or drugs with targeted inhibition or knockdown of GSDMD for the treatment of ALF.
Research objectives
To detect GSDMD expression in liver tissues from humans and mice with ALF and in injured hepatocytes and to investigate the possible molecular mechanism of GSDMD-mediated hepatocyte pyroptosis for expanding inflammatory responses.
Research methods
The expression levels of pyroptosis pathway proteins in liver tissues from humans with ALF, the injured AML12 cell line, and liver tissues from Galn/LPS-induced ALF mouse models were detected by using Western blot. In further study of the molecular mechanism, downregulation of GSDMD by shRNA was induced in vitro, and GSDMD knockout mice were used in a Galn/LPSinduced ALF model.
Research results
The expression of the cleaved N-terminal fragment of GSDMD protein (GSDMD-N) was increased significantly in liver tissues from humans and mice with ALF and in an in vitro injured AML12 hepatocyte cell line. The mechanism of inflammation induced by hepatocyte pyroptosis was different from the release of interleukin (IL)-1β and IL-18 by immune cell pyroptosis.Hepatocyte pyroptosis promoted and expanded inflammatory responses by upregulating monocyte chemotactic protein 1 (MCP1)/CC chemokine receptor-2 (CCR2). GSDMD knockout can significantly alleviate D-galactose/lipopolysaccharide (D-Galn/LPS)-induced ALF in mice,reduce serum inflammatory cytokines, and improve the survival rate of the ALF mice. Its effects were associated with a decrease in the expression of the MCP1/CCR2 proteins and a reduction of MCP1 release. However, the effects of downregulating GSDMD in ALF patients are still unclear and should be confirmed in clinical studies.
Research conclusions
GSDMD-mediated hepatocyte pyroptosis plays a key role in ALF, both in humans and DGaln/LPS-induced ALF mice. GSDMD upregulates MCP1/CCR2 to release inflammatory cytokines, which leads to deterioration of the condition in ALF. Inhibition or knockdown of GSDMD can significantly reduce the levels of inflammatory cytokines and alleviate liver injury in ALF.
Research perspectives
The present study clarified the role of hepatocyte pyroptosis in ALF as well as its mechanism of inducing and expanding inflammatory responses by upregulating MCP1/CCR2. This study also demonstrated that targeted GSDMD inhibitors or effective intervention drugs may be a treatment approach to the prevention and treatment of ALF.
杂志排行
World Journal of Gastroenterology的其它文章
- Inositol 1,4,5-trisphosphate receptor in the liver: Expression and function
- Reduced microRNA 375 in colorectal cancer upregulates metadherin-mediated signaling
- Long noncoding RNA NALT1-induced gastric cancer invasion and metastasis via NOTCH signaling pathway
- Cystic duct cancer: Should it be deemed as a type of gallbladder cancer?
- Real life efficacy and safety of direct-acting antiviral therapy for treatment of patients infected with hepatitis C virus genotypes 1, 2 and 3 in northwest China
- Chronic pancreatitis and the heart disease: Still terra incognita?
