Management of atherosclerotic plaque in left internal mammary artery graft five years after angiographic patency: A case report
2019-11-28SavvyNandalOmNarayanPeterBarlisFrancisPonnuthurai
Savvy Nandal,Om Narayan,Peter Barlis,Francis A Ponnuthurai
Savvy Nandal,Om Narayan,Peter Barlis,Francis A Ponnuthurai,Department of Cardiology,The Northern Hospital,Epping,Victoria 3076,Australia
Abstract
Key words: Left internal mammary artery graft; Atherosclerosis; Thrombus; Case report
INTRODUCTION
The left internal mammary artery (LIMA) has demonstrated excellent long term patency rates when used as a bypass conduit[1,2].When vessel occlusion does occur,it is typically associated with the presence of competitive flow from the native circulation leading to atresia,issues relating to surgical technique resulting in distal anastomotic failure or rarely due to dissection (either spontaneous or iatrogenic).The rapid development of de-novo atherosclerosis in a previously non-diseased LIMA,subsequently leading to an acute coronary syndrome (ACS) is rarely encountered.We present the case of a 67-year-old man presenting with an acute myocardial infarction due to the rapid progression of atherosclerotic plaque in the mid shaft of the IMA,culminating in plaque rupture and thromboembolism.
CASE PRESENTATION
Chief complaints
A 67-year-old man presented to the emergency department of our hospital complaining of worsening central chest pain for the duration of 3 h.
History of past illness
The patient had past history of coronary artery bypass graft (8 years ago) LIMA-diagonal to Left Anterior Descending (LAD) artery,free right internal mammary artery (RIMA)-ramus intermedius skip to OM and a left radial artery anastomosed to the PDA and ischaemic cardiomyopathy with moderate segmental systolic dysfunction.He also had history of treated hypertension,dyslipidaemia,rheumatoid arthritis and partial nephrectomy for clear cell carcinoma.His regular medications included aspirin 100 mg daily,atorvastatin 80 mg daily (low density lipoprotein cholesterol 1.7 mmol/L),frusemide 40 mg daily,metoprolol 50 mg twice daily and ramipril 5 mg daily.
Physical and laboratory examinations
The patient’s cardiovascular examination revealed his heart sounds were dual with no added murmurs and his chest was clear.Electrocardiogram showed anterolateral ST changes of ischaemia (Figure1) and troponin elevation to 44 ug/L (reference range <0.05 ug/L).Full blood picture was normal and biochemistry revealed glomerular filtration rate of 63 mL/min/1.73 m2(reference range > 90).C-reactive protein was mildly raised at 6.7 mg/L (reference range < 3.0).
Imaging examinations
A computed tomography aortogram was performed to exclude other differentials for his presentation that revealed no aortic dissection,ulceration or aneurysm apart from scattered small areas of calcified arteriosclerotic plaque.No large central pulmonary embolus was seen either.
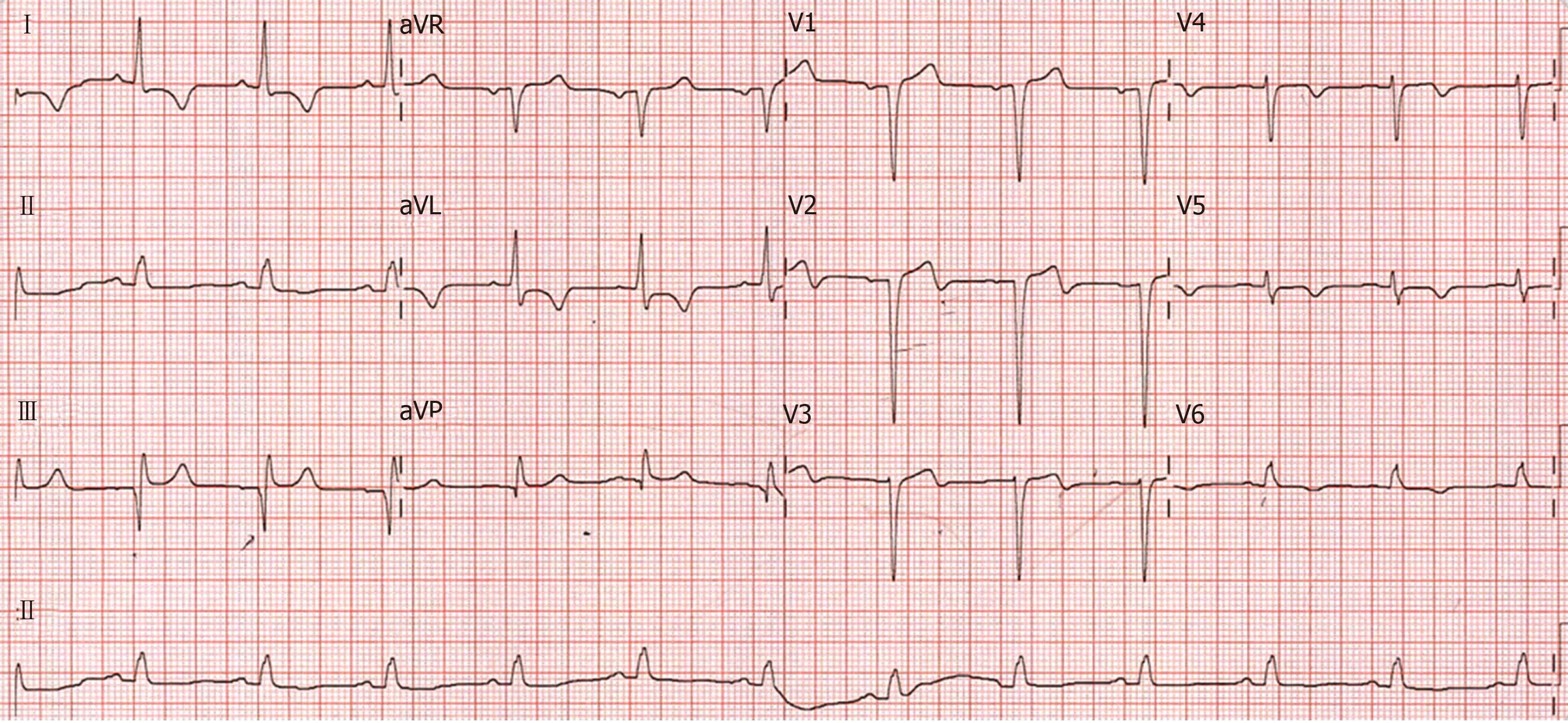
Figure1 12-lead electrocardiograph at presentation.
Further diagnostic workup
Emergent angiography was organised for this patient due to his clinical picture and raised cardiac markers.Notably,he had undergone angiography 5 years ago to investigate episodic chest pain and imaging of the LIMA at the time did not demonstrate any evidence for atherosclerotic plaque formation (Figure2A).Angiography demonstrated a severe diffuse stenosis in the proximal to mid segment of the LIMA,with embolization of a moderate sized thrombus to the distal skip segment and anastomosis with the first diagonal branch (“coronary saddle thrombus”).The LIMA stenosis was characterised by overlying haziness,consistent with acute plaque rupture,associated with residual luminal thrombus (Figure2B).
FINAL DIAGNOSIS
Acute plaque rupture in the LIMA with residual luminal thrombus and distal embolization of the thrombus.
TREATMENT
The patient was managed with intensive antithrombotic therapy initially to reduce the thrombus burden with eptifibatide (10.5 mg/h for 48 h),enoxaparin (1 mg/kg at 80 mg twice daily for 72 h),ticagrelor 90 mg twice daily and aspirin until repeat angiography after 72 h.
At repeat angiography,the thrombus burden was substantially reduced at the distal anastomosis with the diagonal branch and skip graft to LAD.Similarly,the lesion within the proximal to mid LIMA demonstrated marked resolution with the demonstration of multiple plaque cavities and a reduction in overlying thrombus burden (Figure2C).
This lesion was consequently predilated with 2.5 mm × 15 mm balloon.A 2.75 mm× 33 mm everolimus-eluting stent was implanted with post dilatation to a final diameter of 3.0 mm (Figure2D).
OUTCOME AND FOLLOW UP
The patient made a good clinical recovery and was discharged after 6 d.On followup,he remained well with no further episodes of angina.
DISCUSSION
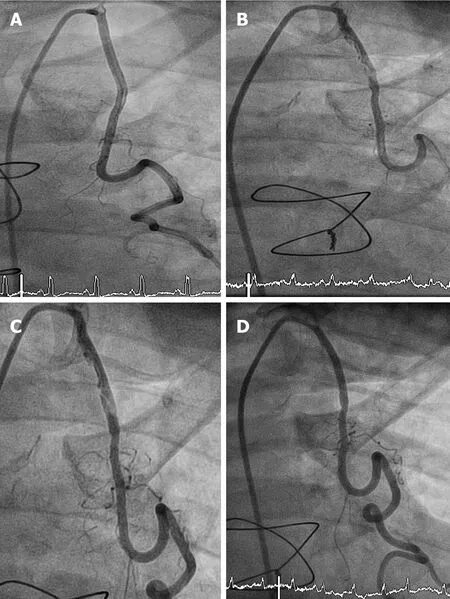
Figure2 Result of angiography.A: LIMA graft Angiogram performed 5 years prior to the current presentation demonstrates no evidence of atherosclerotic plaque formation; B: Proximal LIMA graft lesion; C: Residual atheroma of the LIMA graft post anti-thrombotic therapy; D: Post stent insertion.
We describe a case of ACS secondary to atherosclerotic plaque rupture complicated by distal embolization in a LIMA conduit 8 years after surgery and 5 years after angiography revealing a patent graft.Most LIMA occlusions occur in the early postoperative period and are associated with surgical complications such as dissection,hematoma,spasm,or anastomotic stenosis.LIMA-LAD grafts are associated with excellent long-term patency and improved outcomes compared to the saphenous vein grafts.The 10-year patency rate of LIMA grafts is approximately 90%if the graft is patent 1 wk after the procedure[1,2].Myocardial infarction caused bydenovoatherothrombotic disease within the LIMA in the late postoperative period is rare.Our patient’s clinical history of dyslipidaemia,hypertension and rheumatoid arthritis increases rate of atherosclerosis progression.Atherosclerotic lesions are also more prone to rupture in patients with rheumatoid arthritis[3].
Atherothrombotic graft occlusion within the IMA itself is rarely described[4,5].Several unique structural and physiological characteristics protect the LIMA from atherogenesis,which include fewer fenestrations in the endothelial layer,lower intercellular junction permeability,enhanced endothelial expression of antithrombotic molecules such as heparin sulfate and tissue plasminogen activator,and higher endothelial nitric oxide production[6].
The most common late complication of LIMA-LAD graft has been dissection,either spontaneous or post intervention,even this is rarely reported in the literature[7].Spontaneous coronary artery dissection (SCAD) type III was also considered in our case given the appearance of a long lesion with haziness and linear stenosis.However,this was considered less likely due to the appearance of the lesion with multiple plaque cavities and the overlying thrombus.
At the time of angiography,the patient was clinically stable and pain free therefore we elected to defer a percutaneous strategy at the index procedure and use an initial antithrombotic strategy for the following reasons of large thrombus burden,risk of further distal embolization and challenges with protecting both the LAD and diagonal territories.The reduction in thrombus burden would also potentially reduce lumen compression if SCAD were the underlying pathology.
Whilst thrombectomy was considered for acute management,we were dissuaded by the significant tortuosity of the LIMA (limiting deliverability),concerns regarding the passage of a thrombectomy device into the diagonal vessel could lead to thrombus dislodgment and embolization into the LAD (or vice versa).Whilst SCAD was considered less likely,there remained a risk that wiring the vessel could inadvertently lead to sub-intimal wire passage with consequent distal propagation of the dissection.
At repeat angiography,the thrombus burden was substantially reduced suggesting that this strategy may have mitigated the risk of distal embolization and periprocedural infarction.Similarly,whilst further imaging with Optical Coherence Tomography (OCT) or Intravascular ultrasonography (IVUS) was considered,we felt the angiographic appearances of the lesion to be sufficiently characteristic of an atherosclerotic process rather than SCAD.In particular,OCT imaging would necessitate pressurized contrast delivery to achieve clearing of the blood pool,hence raising the risk of either propagation of an underlying dissection or hydraulic dissection of the LIMA ostium that such evaluation would be rendered redundant.In addition,there is risk of further extension of SCAD with OCT due to pressurised contrast injection.
The available medical literature comparing the incidence of atheromatous plaque formation with that of SCAD within LIMA conduits is scant,perhaps due to the relative rarity of such events.However,prompt recognition and appropriate management is clearly critical,given the life-threatening nature of such occlusions and the important technical considerations needed to achieve successful reperfusion.Relevant considerations include attention to guiding catheter and coronary wire length (guide catheter shortening and the use of longer length coronary guide wires may be required).The risk of vessel occlusion due to guide wired induced straightening of a tortuous LIMA is a further consideration.This case highlights the rapid development of atherosclerotic disease in a graft 5 years after documented patency and the importance for consideration of expectant thrombus management in a patient with atheromatous plaque rupture of the LIMA graft,to our knowledge; this is the first case in literature describing this strategy.
CONCLUSION
Whilst use of IMA conduits is associated with excellent long-term patency,late atherogenesis complicated by plaque rupture is rarely encountered and the natural history of atheromatous plaque rupture in the LIMA is unknown.Prompt recognition,together with judicious use of antithrombotic and anti-platelet therapy may facilitate optimal percutaneous reperfusion.Confirmatory imaging with IVUS or OCT may provide useful additional lesion definition and help to distinguish between dissection and atheromatous aetiologies.
