Activation of p38/HSP27 pathway counters melatonin-induced inhibitory effect on proliferation of human gastric cancer cells
2019-11-19ChenchenZhuHaonanJiangWenjieDengShuoZhaoKaiquanLiYutingWangQinjunWeiJunDu
Chenchen Zhu, Haonan Jiang, Wenjie Deng, Shuo Zhao, Kaiquan Li, Yuting Wang,Qinjun Wei, Jun Du,✉
1School of Basic Medical Sciences, 2Department of Physiology, 3The Fourth School of Clinical Medicine, 4The Laboratory Center for Basic Medical Sciences, School of Basic Medical Sciences, Nanjing Medical University,Nanjing, Jiangsu 211166, China.
Abstract
Keywords: melatonin, p38, HSP27, gastric cancer, proliferation
Introduction
Heat shock protein 27 (HSP27), a member of the small heat shock proteins, is highly conserved with its function closely correlated with maintaining cell homeostasis in response to harmful stress[1]. The strong protective effect of HSP27 is mainly due to its vital function as molecular chaperone to regulate protein folding, transport, translocation and assembly,thus allowing the cell to survive in different sublethal conditions[2]. Furthermore, an increasing number of studies have shown that HSP27 is essential for cell proliferation regulation[3-5]. Interestingly, recent studies have indicated that HSP27 is overexpressed in multiple types of cancer and plays an essential role in tumor progression and drug resistance[6-11].
Melatonin is an endogenous hormone involved in the synchronization of circadian rhythms of human body. It also displays a potent effect on avoiding deleterious action of diabetic renal injury[12]. In addition, melatonin supplementation enhanced immunity in aged individuals[13]. Recent studies have showed that melatonin reverses morphine tolerance by inhibiting HSP27 expression[14], implying a possible connection between melatonin and HSP27. Intriguingly, disrupting melatonin secretion is associated with the risk of gastrointestinal cancer in human[15]and melatonin treatment has shown some promise in gastric cancer therapy[16-17]. However, whether and how HSP27 is related to the anti-tumor effects of melatonin has not been reported.
In this study, using gastric cancer cell lines BGC-823 and MGC-803, we focused on the regulatory role of HSP27 in the proliferation inhibitory effect induced by melatonin, and tried to gain further insights into the underlying mechanism, which would provide a new theoretical basis for gastric cancer treatment.
Materials and methods
Cell culture
Human gastric cancer cell lines BGC-823 and MGC-803 were obtained from the Cell Biology Institute of Chinese Academy of Sciences (Shanghai, China). BGC-823 and MGC-803 cells were cultured in Dulbecco's modified Eagle's medium (DMEM, high glucose) (Hyclone, Thermo Scientific, Waltham, MA,USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (Hyclone, USA) and antibiotics (100 U/mL streptomycin and 100 μg/mL penicillin) (Invitrogen,USA) in a humidified incubator at 37 °C with 5%CO2. Cells were grown on plastic dishes for transfection and protein extraction.
Treatment and transfection
Melatonin (Sigma, St Louis, MO, USA) was dissolved in dimethyl sulphoxide (DMSO) and cells were treated with melatonin for the indicated time and dose. To determine the effects of inhibitor SB203580(Beyotime, Shanghai, China) on cell growth inhibition, cells were treated with the inhibitor for 30 minutes prior to melatonin treatment.
The sequences of small interfering RNA (siRNA)for HSP27 was 5′-UGAGAGACUGCCGCCAAGU AA-3′, the sequence of control siRNA was 5′-UUCUC CGAACGUGUCACGUTT-3′ (GenePharma Co., Shanghai, China). Cells were transfected with control siRNA or HSP27 siRNA with Lipofectamine 2000,according to the manufacturer's instruction.
MTT assay
Cell proliferation was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide(MTT) assay as described previously[18]. Briefly, cells were seeded at a density of 5×103cells per well into the 96-well plate (Corning, NY, USA) and treated with melatonin for the indicated time and dose. After culture, cells were washed, added with MTT, and the plate was incubated in the dark for 2-4 hours,followed by measurement at 490 nm using a microplate absorbance reader (Elx800, Bio-Tek,Winooski, VT, USA).
Immunoblotting analysis
Cells were washed with phosphate buffer saline(PBS) and lysed for collecting proteins on ice for 20 minutes in radioimmunoprecipitation assay buffer(RIPA buffer) containing 1% protease inhibitor cocktail. The protein concentration was determined by BCA kit. The cellular proteins were then separated by SDS-PAGE and transferred to nitrocellulose membranes. After being blocked with 5% nonfat milk for 1 hour, the membrane was incubated with primary antibody overnight at 4 °C. Then the membrane was washed and incubated with horseradish peroxidaseconjugated secondary antibodies for 1 hour at room temperature. The following antibodies were used:rabbit anti-p38 antibody, rabbit anti-p-p38 antibody,mouse anti-HSP27 antibody, rabbit anti-p-HSP27 antibody, rabbit anti-CDK4 antibody and rabbit anticyclin D1 antibody (Cell Signaling, Danvers, MA,USA), rabbit anti-GAPDH antibody (Santa Cruz,Santa Cruz, CA, USA), mouse anti-β-actin antibody(Sigma, USA). Protein bands were visualized with enhanced chemiluminescence (ECL) reagent(Millipore, Billerica, MA, USA). Digital images of immunoblots were obtained with a Chemidoc XRS and analyzed using the image analysis program Quantity One (Bio-Rad, Hercules, CA, USA).
Flow cytometry analysis
Cell apoptosis was measured with an Annexin VFITC apoptosis detection kit (Beyotime). In brief,cells were seeded in a 25 cm2dish and incubated with different doses of melatonin for the indicated time.The cells were then collected, resuspended in 100 μL of 1× binding buffer containing 2.5 μL of FITCconjugated annexin V and 1 μL of propidium iodide(PI) (100 μg/mL) and incubated for another 15 minutes in darkness. Subsequently, the stained cells were analyzed using flow cytometry.
EdU assay
5-ethynyl-2′-deoxyuridine (EdU) staining was used to measure cell proliferation according to the manufacturer's instruction (Keygen, Nanjing, China).In brief, cells were cultured on coverslips until reaching 70% confluence, then EdU was added to the culture media for 2 hours. After labeling, cells were washed three times with PBS followed by formaldehyde fixation. Then, the cells were incubated with glycine and washed with PBS containing 0.5%Triton X-100. After counterstaining with 4′,6-diamidino-2-phenylindole (DAPI), cells were mounted and imaged by fluorescence microscopy.
Statistical analysis
Data were analyzed by Image J and statistical analyses were carried out using the SPSS software version 15.0 (SPSS Inc., USA). Results were defined as mean±S.E.M. Student'sttest was used to analyze the differences between two groups. ANOVA followed bypost hocBonferroni test was used to for multiple comparisons. Statistical significance was considered whenP<0.05.
Results
Melatonin inhibits proliferation of gastric cancer cells

Fig. 1 Melatonin suppressed proliferation of gastric cancer cells. A: Cell proliferation was evaluated by MTT assays after melatonin treatment for the indicated doses and time. Values were determined by three independent experiments. *P<0.05, **P<0.01. B: BGC-823 cells were stimulated with 1 mmol/L melatonin for 48 hours, and cell cycle was measured by flow cytometry analysis. C: The expression of cyclin D1 and CDK4 were determined by immunobloting analysis. β-Actin was used as a loading control. *P<0.05, **P<0.01. D: Melatonininduced apoptosis was measured by flow cytometry analysis.
Gastric cancer BGC-823 cells were treated with different doses of melatonin and cell proliferation was measured by MTT assay. As shown inFig. 1A, BGC-823 cell growth rate decreased in a dose- and timedependent manner. Statistical analysis showed that melatonin at 1 mmol/L for 24 hours or 48 hours significantly suppressed cell proliferation compared with other groups. To explore whether the proliferation inhibitory effect of melatonin was caused by cell cycle arrest, BGC-823 cells were treated with 1 mmol/L melatonin for 48 hours and stained with PI for flow cytometry analysis. As shown inFig. 1B, the S phase cell population was decreased significantly,while the cells accumulation in G1 phase was increased after melatonin incubation. CDK4-cyclin D1 complex is a major integrator of cell proliferation,which is required for progression through G1 phase and entry into S phase. We then assessed the expression of those cell cycle-related proteins by immunoblotting analysis. As shown inFig. 1C, after incubation with 1 mmol/L melatonin for 48 hours,cyclin D1 and CDK4 expressions were decreased significantly. The above results showed that melatonin suppressed gastric cancer cell proliferationin vitro.
In addition, we examined cell apoptosis rate in response to 1 mmol/L melatonin treatment, and BGC-823 cells showed enhanced cell apoptosis rate after incubation with melatonin for 48 hours. However, the number of apoptotic cells stimulated by melatonin was limited and below 3% of total cells (Fig. 1D).Therefore, the marked increase of cell growth inhibitory rate by melatonin most likely reflected its inhibition on gastric cancer cell proliferation rather than cell apoptotic process.
HSP27 prevents melatonin-induced inhibition of cyclin D1
We then determined whether melatonin was capable of regulating HSP27 activation. We treated cells with different doses of melatonin (0.01-1.00 mmol/L) for 48 hours and found significant induction of phosphorylated HSP27 in BGC-823 cells. In contrast,the levels of total HSP27 were constant at all the time points (Fig. 2A). To determine the involvement of HSP27 in melatonin-stimulated inhibition of cell proliferation, specific siRNA for HSP27 were applied(Fig. 2B). After the depletion of HSP27, BGC-823 and MGC-803 cells were treated with 1 mmol/L melatonin. As shown inFig. 2C, the results showed that the expression of cyclin D1, a marker of cell proliferation, was greatly decreased in those cells.These results indicate that HSP27 phosphorylation prevents the downregulation of cyclin D1 expression induced by melatonin.
p38 activation is required for melatonin-induced HSP27 activation
As shown inFig. 3A, 1 mmol/L melatonin significantly induced p38 phosphorylation in BGC-823 cells. To confirm the involvement of p38 phosphorylation in melatonin-induced proliferation inhibition, we analyzed the effects of p38 inhibitor,SB203580, on melatonin-mediated cyclin D1 expression. As shown inFig. 3B, the combination of SB203580 and melatonin significantly suppressed cyclin D1 protein level, suggesting that like HSP27,melatonin-induced p38 activation was also resistant to melatonin-mediated inhibition of cyclin D1 expression.Meanwhile, pretreatment with SB203580 remarkably suppressed melatonin-induced HSP27 activation(Fig. 3C). These results indicate that p38 may be an important upstream activator for HSP27 in gastric cancer cells.
Activation of p38/HSP27 signaling pathway against the inhibitory effect of melatonin on gastric cancer cell proliferation
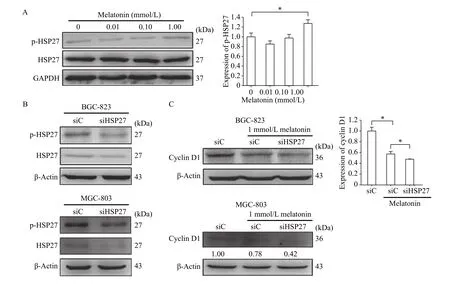
Fig. 2 HSP27 participated in the inhibitory effect of melatonin on cyclin D1 expression. A: After incubated with melatonin for the indicated doses for 48 hours, total proteins were extracted from BGC-823 gastric cancer cells for immunoblotting analysis to evaluate phosphorylation of HSP27 and total HSP27. GAPDH was used as a loading control. Experiments were repeated at least three times. *P<0.05.B: BGC-823 and MGC-803 gastric cancer cells were transfected with HSP27 siRNA and control siRNA and the knockdown efficiency was checked. C: After incubated with 1 mmol/L melatonin for 48 hours, the expression of cyclin D1 in HSP27-depletion cells was detected. β-Actin was used as a loading control. The immunoblotting analysis was processed in three independent experiments. *P<0.05.

Fig. 3 p38/HSP27 pathway activation countered the inhibitory effect of melatonin on cyclin D1. A: After incubated with melatonin for the indicated doses for 48 hours, total proteins were extracted from cells for immunoblotting analysis to evaluate phosphorylation of p38 and total p38. GAPDH was used as a loading control. B: After pre-treated with 20 μmol/L SB203580 for 30 minutes, cells were stimulated with 1 mmol/L melatonin for 48 hours and the expression of cyclin D1 was determined by immunobloting analysis. β-Actin was used as a loading control. C: After pretreatment with SB203580 and then incubation with 1 mmol/L melatonin for 48 hours, the expression of p-HSP27 and HSP27 level were analysed. GAPDH was used as a loading control. Experiments were repeated at least three times. *P<0.05, **P<0.01.
Accordingly, we examined BGC-823 cell proliferation by MTT assay and the results indicated that inhibitory effect of melatonin on proliferation was associated with p38/HSP27 pathway. The combination of melatonin and SB203580 or HSP27 siRNA significantly downregulated cell proliferation rate,compared with melatonin treatment alone (Fig. 4A).In addition, the pretreatment with the SB203580 or HSP27 siRNA alone had limited influence on melatonin-mediated apoptosis in BGC-823 cells, and the percentage of apoptotic cells in each group was below 3% (data not shown). Similar results were found in MGC-803 cells by EdU staining (Fig. 4B). In summary, these results indicated that p38/HSP27 pathway activation counters the inhibitory effect of melatonin on gastric cancer cell proliferation (Fig. 4C).
Discussion
Since melatonin impairs the proliferation and promotes apoptosis effectively in multiple types of cancer without any toxicity, it is regarded as one of the most important compounds in cancer prevention[19-20]. Gastric cancer was the fourth most common malignancy and the second in mortality of all cancers worldwide[21]. To date, despite the extensive exploration of anti-gastrointestinal cancer actions in melatonin[22], further experimental studies are still needed to improve the efficacy of melatonin treatment in gastric cancer.
In addition to its well-recognized pro-apoptotic characteristic[23-24], in this study, we noticed that melatonin mainly showed anti-proliferation effect on gastric cancer cell line BCG-823 and MGC-803.Melatonin stimulated HSP27 phosphorylation in a dose-dependent manner. Also, depletion of HSP27 resulted in a decrease in cyclin D1 expression in both cell lines. These results indicate that HSP27 is actively involved in maintaining homeostasis of gastric cancer cells against melatonin. Actually, HSP27 can support cell survival under stressed conditions[25]. Suppression of HSP27 could induce long-term dormancy in human breast cancer[8]. Intriguingly, HSP27 interacts with multiple proliferation-related signaling pathways. For example, HSP27 coordinately promotes proliferation and inhibits Fas-induced apoptosis by regulating PEA-15[16]. HSP27 phosphorylation can promote HUVECs proliferation through PI3K/Akt and ERK1/2[26].Collectively, these findings confirm phosphoHSP27 is a potentially resistant molecule in melatonin-induced inhibitory effect on cell proliferation.
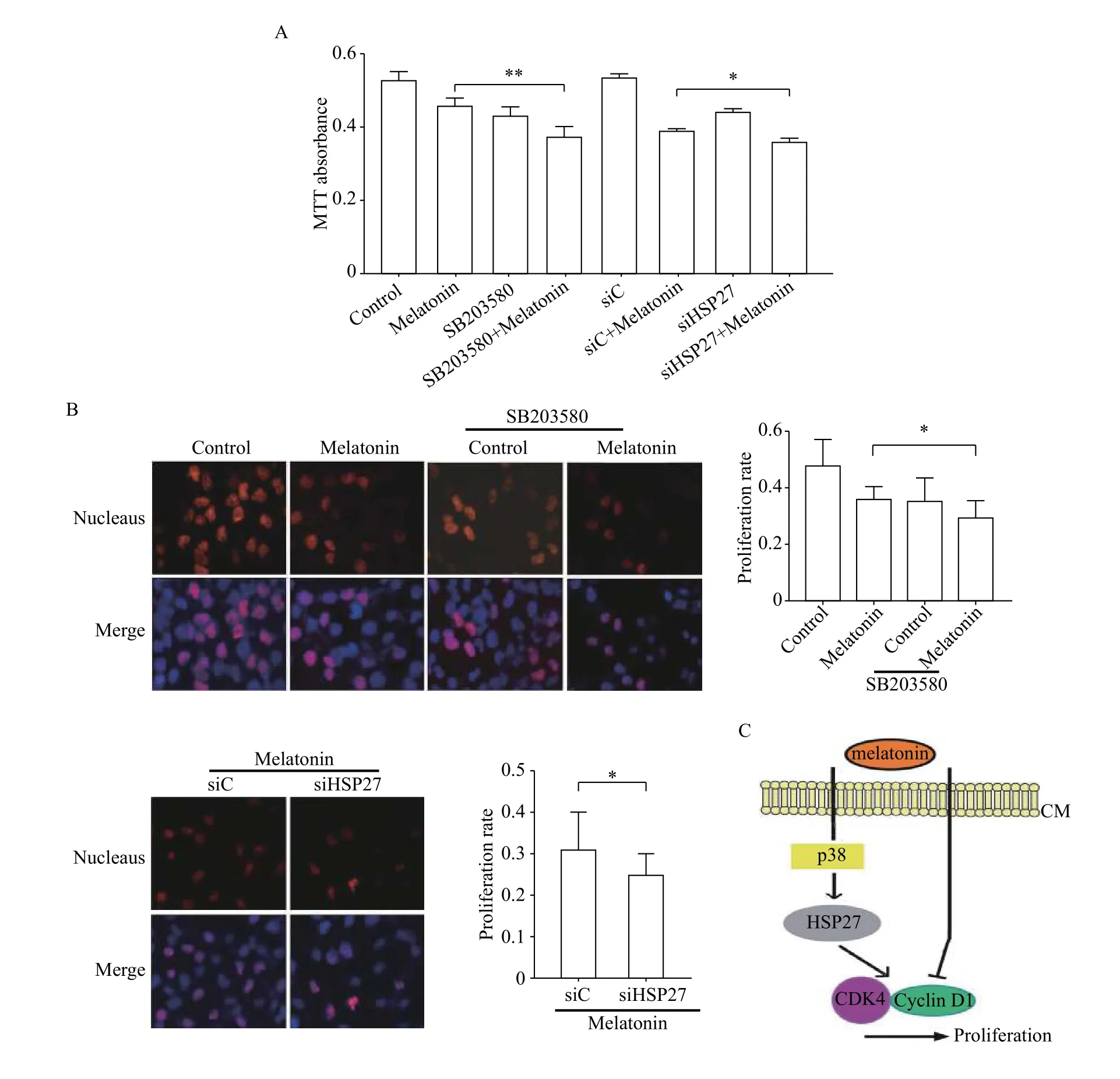
Fig. 4 p38/HSP27 pathway activation countered the inhibitory effect of melatonin on gastric cancer cell proliferation. A, B: After transfected with HSP27 siRNA or incubation with 20 μmol/L SB203580 and then treated with 1 mmol/L melatonin for 48 hours, cell proliferation was measured by MTT assays (BGC-823) (A) and Edu staining (MGC-803) (B). *P<0.05, **P<0.01. C: A model of the mechanism.Melatonin activated p38/HSP27 signaling pathway which countered the inhibitory effect of melatonin on gastric cancer cell proliferation.
We next examined the potential activators for HSP27 in our system. It was reported that MAPKactivated protein kinase-2 could mediate the incorporation of p38 into the pre-existing complex of HSP27, leading to its phosphorylation[27]. Thus, it is worthwhile to explore whether p38/HSP27 pathway is involved in melatonin-induced proliferation inhibitory effect on gastric cancer cells. The observation here showed that melatonin treatment induced a dosedependent increase in p38 and HSP27 phosphorylations and inhibition of cell proliferation. Blocking p38 with SB203580 impaired upregulation of HSP27 phosphorylation and aggravated cell proliferation arrest by melatonin. In non-small cell lung carcinoma cells, barbaloin inactivated p38/HSP27 pathway by inhibiting p38 nucleus translocation[28]. Similarly,lncRNA BX357664 exhibited its inhibitory role in renal cell carcinoma metastasis by blocking p38/HSP27 pathway[29]. We previously identified that p38/HSP27 pathway could be induced by ATP depletion and phosphorylated HSP27 acted as a regulator of cell cytoskeleton reorgnization[30]. Therefore, it may be reasonable to conclude that, at least in part, p38/HSP27 pathway activation counters the proliferation inhibition induced by melatonin.
In the present study, we revealed the distinguishable anti-proliferation role of melatonin on gastric cancer cells. Furthermore, p38/HSP27 pathway activation exerts protective effect on gastric cancer cells against proliferation inhibition induced by melatonin. Our results suggest that co-treatment of melatonin and p38/HSP27 pathway inhibitor might improve the therapeutic efficacy of melatonin on gastric cancer.
Acknowledgments
This work was supported by the Training Programs of Innovation and Entrepreneurship for Undergraduates by Jiangsu Province (201810312018Z) to Jun Du, Chenchen Zhu, Kaiquan Li, the Training Programs of Innovation and Entrepreneurship for Undergraduates by Nanjing Medical University to Jun Du, Yuting Wang.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Sonic Hedgehog stimulates migration of MCF-7 breast cancer cells through Rac1
- Association of long non-coding RNA HOTAIR and MALAT1 variants with cervical cancer risk in Han Chinese women
- Developmental changes in contraction of gastric smooth muscle cells in rats correlate with their differences in RhoA/ROCK pathway
- Characterization of oral yeasts isolated from healthy individuals attended in different Colombian dental clinics
- Anti-inflammatory effects of natural volatile organic compounds from Pinus koraiensis and Larix kaempferi in mouse model
- Cost effective use of mosquito net mesh in inguinal hernia repair
