基于多面体低聚倍半硅氧烷体系的一种独特发光现象
2019-11-08张庆瑞宋明星邓瑞平张洪杰
张庆瑞 宋明星 邓瑞平*, 周 亮 张洪杰*,
(1中国科学院长春应用化学研究所,长春 130022)(2吉林师范大学,四平 136000)
0 Introduction
Since polyhedral oligomeric silsesquioxane(POSS)materials were first pioneered by Scott in 1946,the application of POSS in academic and industrial fields has attracted widespread interest because of their well-defined nanostructure[1-5].Generally,POSS owns a unique cubic cage-shaped nanostructure with the general formula (R-SiO1.5)8[6-7], which contains a polyhedron silicon-oxygen cube skeleton with intermittent siloxane linkages and tunable organic groups at the silicon atoms[8-9],where the R unit is functional organic or aliphatic groups,such as alkyl,aryl or any of their derivatives[10-11]. The average POSS core diameter falls in the range of approximately 0.45~0.53 nm[12].POSShave distinct chemical properties,such as facile chemical modification[13],good solubility[14],high temperature and oxidation resistance properties[15-17].When POSS moieties are incorporated into the compounds,the generated nanocomposites can result in novel interesting physical properties such as increases in glass transition temperature[18-19],thermal stability[20-21],mechanical strength[22-23],and oxidation resistance.
Recently,POSS-based luminescent materials have attracted a great deal of attention due to their good solubility in organic solvents[24-25].Incorporation of POSS moieties into luminescent materials should not only enhance the fluorescence quantum yields of the aggregate state,but also improve the mechanical properties[26-27]. For example,Zhao used 7-allyl-8-hydroxyquinoline (Hq-allyl)as the ancillary ligand to synthesize a new iridium(Ⅲ)coumarin complex,Ir(L)2(q-allyl),and carbazole moieties covalently attached to the POSS,gained good thermal stability and photoluminescence performance[28].At present,most of the POSS-based luminescent materials were chromophores or other groups.In addition,a small amount of POSS-containing luminescent materials were lack of common fluorescent units.For instance,Mohamed synthesized the unusual fluorescent POSS-containing polymers lacking any common fluorescent units:a poly(maleimide isobutyl POSS)homopolymer and poly(styrene-alt-maleimide isobutyl POSS)and poly(4-acetoxystyrene-alt-maleimide isobutyl POSS)alternating copolymers[29-30].However,much less attention has been paid to the luminescence of POSSitself,because there is no conjugated group in their molecule structures.
In this study,we found a unique luminescence phenomenon in the POSS system,that the POSS compounds without any chromophore showed obvious luminescence after heat treatment in their THF solutions.To verify this luminescence behavior,two different POSS monomers,i.e.,the AIPOSS with amino groups and the OIPOSS with all inert groups were investigated.In both of the cases the unique luminescence phenomena were found.For further study of the luminescence mechanism,the chemical structures of the POSScompounds were characterized by means of1H,29Si NMR spectroscopy,and FTIR spectroscopy.The results showed that the POSS compounds contained nearly intact in structures.The valence state of silicon was also determined by means of XPS,which verified that the Si atom remained the valence state before/after the treatment.We considered several possible origination of the luminescence of the POSS,and deduced that this unusual luminescence is most likely caused by the adsorption effect of the solvent in the POSScages.
1 Experiments
1.1 Materials
Aminopropyl isobutyl POSS and octal isobutyl POSS were purchased from Hybrid Plastics Company,USA.THF was purchased from Alfa-Aesar.All chemical reagents were used as received without further purification.
1.2 Characterization
1H,29Si NMR spectra were obtained on an AvanceⅢ400 MHz.FTIR spectra were obtained on a Nicolet-6700 spectrometer.The XPSwas conducted with PHI 5000 Versa Probe(ULVAC-PHI,Japan).Fluorescence spectra were collected at room temperature on a Hitachi F-4500 fluorescence spectrophotometer.
1.3 Preparation of F-AIPOSS
Aminopropyl isobutyl POSS(0.3 g)was dissolved in THF (20 mL),and then heated under reflux at 66℃for 10 h in the nitrogen atmosphere.The solution changes from colorless and transparent into yellow.Then,the solvent was distilled off under reduced pressure,yielding a deep orange oil-like liquid.The resulted sample was denoted as F-AIPOSS.For further FTIR,NMR and XPS characterization,the resulted sample was dried in the vacuum overnight.
1.4 Preparation of F-OIPOSS
The same procedures as above were carried out,yielding a deep orange oil-like liquid.The resulted sample was denoted as F-OIPOSS.For further FTIR,NMR and XPS characterization,the resulted sample was dried in the vacuum overnight.
2 Results and discussion
The chemical structures of AIPOSS and OIPOSS are depicted in Scheme 1.Generally,both AIPOSS and OIPOSS are regarded as non-luminous materials,because there is no conjugated group in their molecule structures.Interestingly,we found that the treated AIPOSS(denoted as F-AIPOSS)showed bright blue fluorescence after stirred and refluxed in THF solution.To study the unusual luminescence phenomenon,another POSS compound OIPOSS with only inert groups was investigated also.The treated OIPOSS(denoted as F-OIPOSS)showed bright blue emission also.Fig.1 shows the photographs of the F-AIPOSS and F-OIPOSS and their untreated counterparts under 365 nm UV illumination.It could be observed that both the F-AIPOSS and F-OIPOSS showed a strong blue fluorescence.
The luminescence properties of the POSS samples in THF solution and in solid state were investigated.Fig.2 presents the PL spectra of FAIPOSS,AIPOSS,F-OIPOSS,and OIPOSS in THF solution (1 mmol·L-1).And Fig.3 exhibited the PL spectra of F-AIPOSSand F-OIPOSSin solid state.
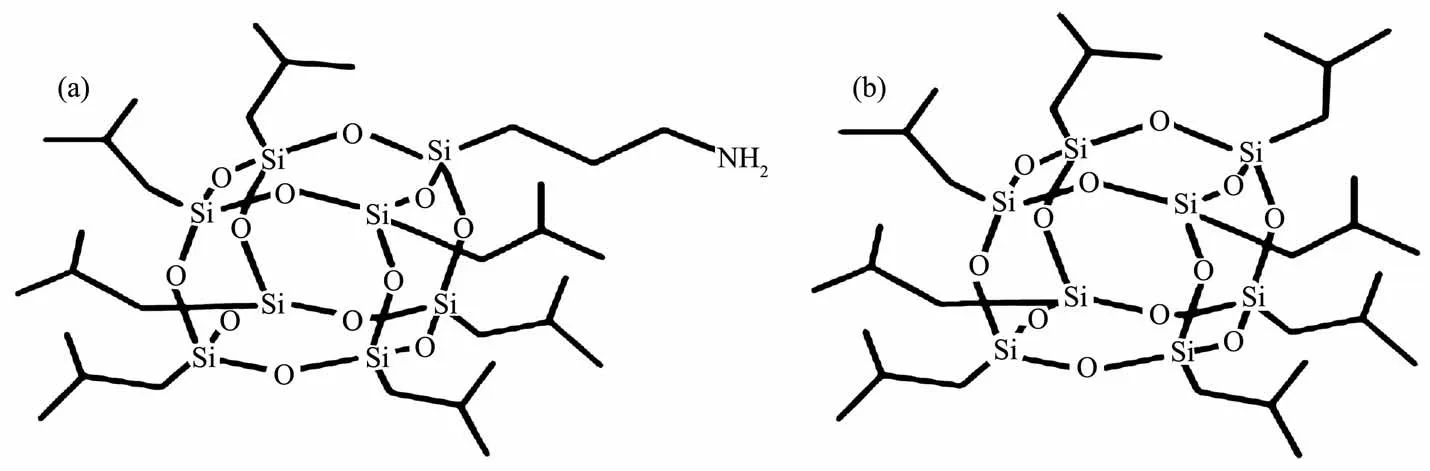
Scheme 1 Chemical structures of:(a)aminopropyl isobutyl POSS(AIPOSS);(b)octal isobutyl POSS(OIPOSS)

Fig.1 A1:F-AIPOSS/THF solution;A2:AIPOSS/THF solution;B3:F-OIPOSS/THF solution;B4:OIPOSS/THF solution;C1 and C3:Photographs of F-AIPOSSand F-OIPOSSin the solid state under 365 nm UV illumination,respectively;D:Photographs of A1,A2,B3,and B4 under 365 nm UV illumination,respectively

Fig.2 PL spectra of F-AIPOSS,AIPOSS,F-OIPOSS,and OIPOSSsolutions in THF(1 mmol·L-1)under different excitation wavelength
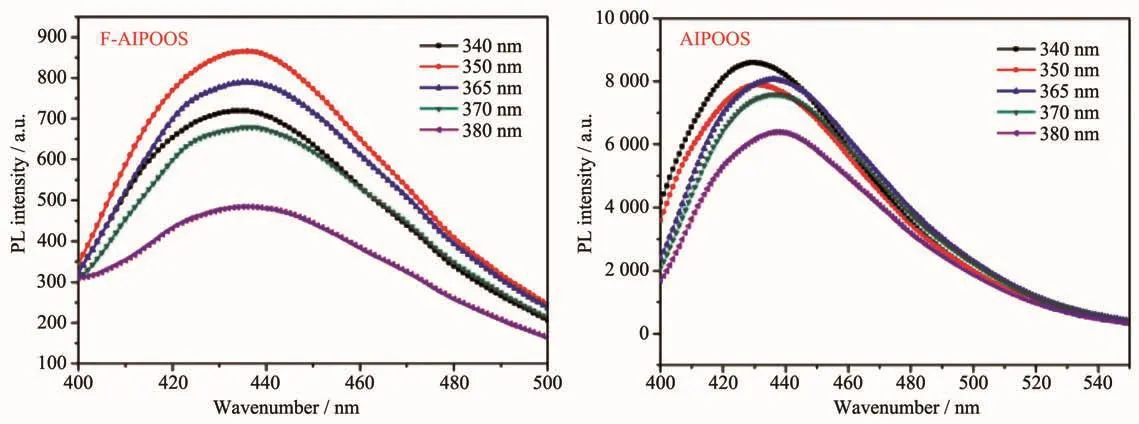
Fig.3 PL spectra of F-AIPOSSand F-OIPOSSunder different excitation wavelength in the solid state
The F-AIPOSS and F-OIPOSS featured strong emission peaks at about 430 nm either in THF solutions or solid states.Contrastively,the AIPOSS and OIPOSS THF solutions showed similar luminescence as well,but their emission intensities were rather low,and no luminescence at all could be detected in their solid states.In addition,it was noted that the F-AIPOSS and F-OIPOSS solutions exhibited maximum emission intensity under excitation wavelength of 380 and 370 nm,respectively,which was a little red shift compared with that of the AIPOSSand OIPOSSsolutions(360 nm)and the solid F-AIPOSSand F-OIPOSSsamples(350 and 340 nm,respectively).The corresponding data were listed in Table 1.
It is interesting that the treatment heating in the THF solution could result in the luminescence of these POSS compounds.As depicted in Scheme 1,there is no chromophore in these POSS compounds,and the used solvent has no luminescence also in the visible region.Naturally,it was speculated that the structures of the AIPOSS and OIPOSS changed after heating in the THF solution.So their structures were characterized by means of1H NMR,29Si NMR and FTIR,respectively.For the characterization,all the samples were dried in vacuum to insure the purity.

Table 1 Excitation wavelength for maximum(λEx,max)and minimum(λEx,min)emission intensity of the POSSsamples
1H NMR spectrum of F-AIPOSSsample in CDCl3(Fig.S1 in Supporting Information)features a signal at 2.68,1.85,1.54,0.93 for the SiCH2CH2C H2NH2methylene,SiCH2CH(C H3)2methine,SiC H2CH2CH2NH2methylene and SiC H2CH(CH3)2methyl groups,respectively; and a signal at 0.61 for both the SiC H2CH2CH2NH2and SiC H2CH(CH3)2methylene groups.For the AIPOSS (Fig.S2 in Supporting Information),there are signals at 2.72,1.86,1.60,0.96 for the SiCH2CH2C H2NH2methylene,SiCH2C H(CH3)2methine,SiCH2C H2CH2NH2methylene and SiCH2C H(CH3)2methyl groups,respectively;and a signal at 0.66 for both the SiC H2CH2CH2NH2and SiC H2CH(CH3)2methylene groups.
1H NMR spectrum of the F-OIPOSS (Fig.S3 in Supporting Information)features signals at 1.86,0.96,0.60 for the SiCH2C H(CH3)2methine,SiCH2CH(C H3)2methyl and SiC H2CH(CH3)2methylene groups,respectively.For the OIPOSS (Fig.S4 in Supporting Information),signals arise at 1.86,0.95,0.60 for the SiCH2C H(CH3)2methine,SiCH2CH(C H3)2methyl and SiCH2CH(CH3)2methylene groups,respectively.
According to the1H NMR results,there is little difference in the POSSsamples before/after heating in THF solution,indicating that they keep nearly unchanged chemical structures before/after the treatment.But very tiny difference could be found after amplifying the1H NMR spectra of the F-AIPOSS/AIPOSS and F-OIPOSS/OIPOSS,that two signals in the treated POSS (F-AIPOSSand F-OIPOSS)arise at about 1.43 and 1.26,respectively,which might be due to the signals from the residual THF (Fig.S5 in Supporting Information).And if the oil-like F-AIPOSS or F-OIPOSSsamples rather than the dried ones were used in the1HNMR characterization,the THF related signals increased obviously.
To make sure the structures of the POSSsamples further,their29Si NMR spectra were investigated.For the29Si NMR spectra of F-AIPOSS (Fig.4),two peaks appear,centering at-67.54 (peak a)and-67.82(peak b),corresponding to their-O Si CH2CH2CH2NH2and-O Si CH2CH(CH3)2units,respectively.For that of AIPOSS,two peaks appear at-67.81 (peak a1)and-67.99 (peak b1),corresponding to their O Si CH2CH2CH2NH2and O Si CH2CH(CH3)2units,respe-ctively.For the F-OIPOSS/OIPOSS cases (Fig.5),only one peak appears at-67.84(peak c)or-67.92(peak c1)for FOIPOSS and OIPOSS,respectively,attribu-ting to their O Si CH2CH(CH3)2units.
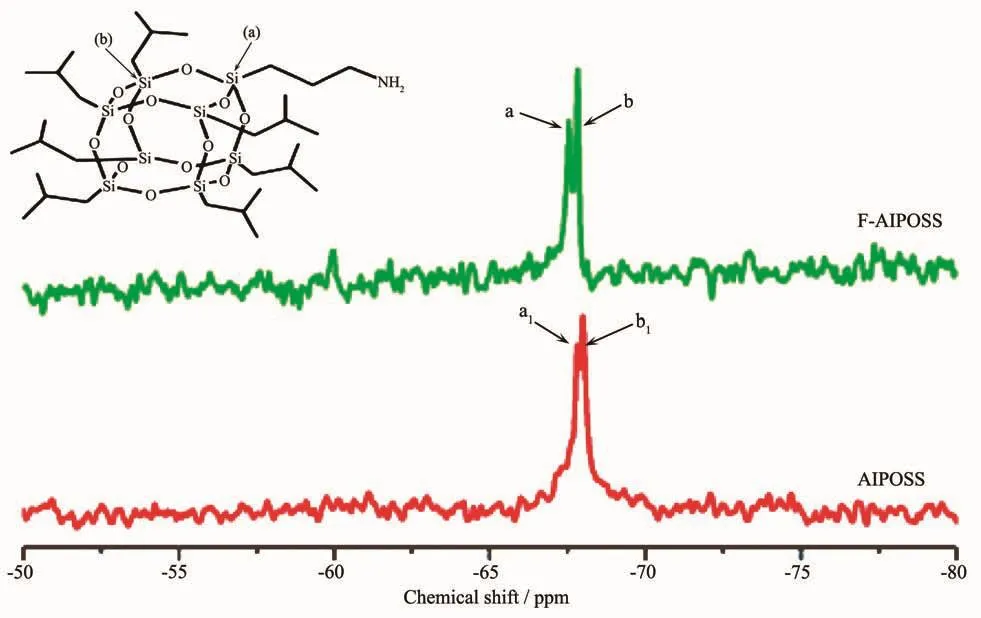
Fig.4 29Si NMR spectra of F-AIPOSSand AIPOSSin CDCl3

Fig.5 29Si NMR spectra of F-OIPOSSand OIPOSSin CDCl3
These29Si NMR spectra results suggest that no cage cleavage occurred in the POSSsamples after the heat treatment in THF,proving that the POSScores of F-AIPOSSand F-OIPOSSremaining intact.
Fig.6 displays the FTIR spectra of F-AIPOSS,AIPOSS,F-OIPOSSand OIPOSSat room temperature.Characteristic absorption bands appear at 2 950~2 870 cm-1and 1 110 cm-1,representing the isobutyl C-H stretching and the Si-O-Si stretching in the POSS structure,respectively.This result indicates that no obvious functional groups appear in the treated POSS samples.
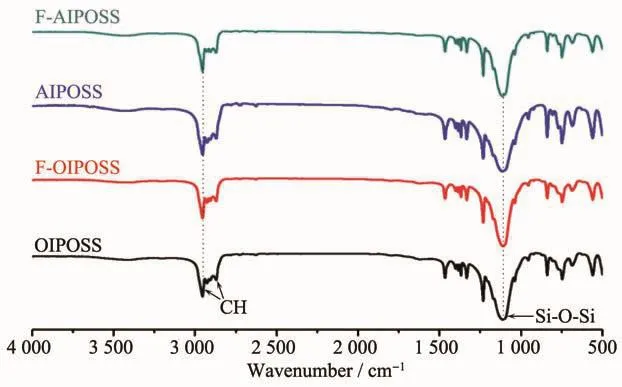
Fig.6 FTIR spectra of F-AIPOSS,AIPOSS,F-OIPOSS and OIPOSS
According the structure characterization results mentioned above,there is no obvious chemical structure change could be found in the treated POSS compounds.It is obscure what the origination of the luminescence is.Because excellent light-emitting Si nanocrystals could be synthesized from hydrogen silsesquioxane[31],the formation of trace silicon nanocrystals is one of our speculations.But the XPS analysis result of F-AIPOSSdid not support this point.As shown in Fig.7,only one peak at 102.0 eV was detected from Si2p region of F-AIPOSS,which is consistent with O-Si-C species,and no signal of Si(0)at 98.9 eV had been detected.Therefore no Si nanocrystal formed in our experiments.
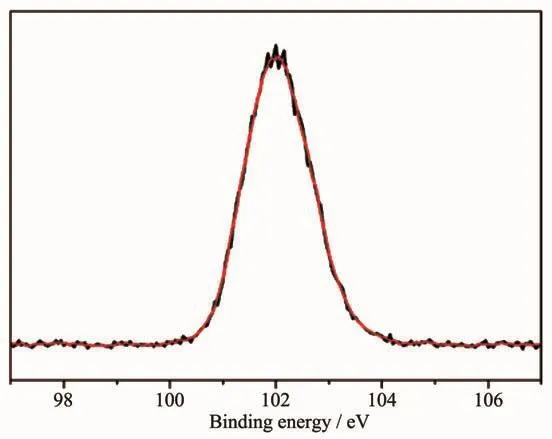
Fig.7 High resolution XPSspectrum of Si2p region of the F-AIPOSS
Another speculation is the formation of the POSS/solvent adduct. Considering the cage-shaped nanostructure of the POSS compounds,it is possible that the solvent molecules enter into the cages of the POSS,forming the POSS/solvent adducts,which modulate the electron structures of the POSS,and consequently leads to the luminescence.In our experiments,the resulted F-AIPOSS and F-OIPOSS are oil-like before dried under vacuum,indicating that there is much solvent in the samples.And these samples could not dried entirely from the solvent,tiny THF related1H NMR signals were still detected in the dried F-AIPOSSand F-OIPOSSsamples as mentioned above.We suppose that the formation of the POSS/solvent adduct determines the emission properties of the corresponding samples.After heating in the THF solution,much solvent enters into the POSS cages,and many POSS/solvent adducts form consequently,giving rise to intensive luminescence from the FAIPOSSand F-OIPOSSTHF solutions(Fig.2).When the F-AIPOSS and F-OIPOSS dried further,some of the solvent molecules escape from the cages,which destroys part of the POSS/solvent adducts,resulting in decreased emission intensity of the solid samples(Fig.3).For the case of untreated AIPOSS and OIPOSS compounds,they have no emission at all in the solid states,because there is no POSS/solvent adduct in the sample.But very weak emission could be detected in their THF solution,it is attributed to a few solvent molecules enter into the POSS cages.Due to the rate of formation of the POSS/solvent adducts is very low at the room temperature,these solutions exhibit weak luminescence only(Fig.2).It is supposed also that the more the POSS/solvent adducts form in the samples,the more the electronic delocalization is resulted to some extent.Therefore,the F-AIPOSS and F-OIPOSS THF solutions exhibited red shift in theirλEx,maxcompared with their solid counterparts and the AIPOSSand OIPOSSsolutions(Table 1).Unfortunately,we have no direct evidences for how the POSS/solvent adducts form.It is still need further investigation for this luminescence behaviour.
3 Conclusions
In summary,an unusual luminescence behavior from the POSScompounds was observed,that the nonluminous POSScompounds showed intensive luminescence after heating and stirring in THF solution.The chemical structures of the corresponding POSSsamples were characterized by using1H NMR,29H NMR,XPS,and FTIR spectroscopy.No obvious structure changes could be detected after the POSS compounds heating in the solvent,except the tiny difference of the1H NMR spectra.It is supposed that this unusual luminescence is most likely ascribed to the formation of the POSS/solvent adducts in the system,which alters the electronic structures of the POSS compounds,and consequently leads to this unusual luminescence behavior.
Acknowledgements:The authors are grateful for financial support from the National Natural Science Foundation of China(Grant No.51502285),National Basic Research Program of China (Grant No.21521092), National Natural Science Foundation for Creative Research Group (Grant No.21701047),the Science and Technology Development of Jilin Province of China (Grant No.20180520191JH),and the Thirteenth Five-Year Program for Science and Technology of the Education Department of Jilin Province(Grant No.JJKH20191024KJ).
Supporting information isavailable at http://www.wjhxxb.cn
