Facile Solvothermal Fabrication of MoS2 Quantum Dots for Highly Fluorescence Detection of Dopamine
2019-11-06WUJingyanHEDaweiWANGYongshengHUYinFUChenZHAOXuanZHANGLuLIXue
WU Jing-yan, HE Da-wei, WANG Yong-sheng, HU Yin, FU Chen, ZHAO Xuan, ZHANG Lu, LI Xue
(School of Science, Beijing Jiaotong University, Beijing 100044, China)
Abstract: Molybdenum disulfide quantum dots (MoS2 QDs), as a typical representative of transition metal dichalcogenides(TMDs), had attracted much attention due to the unique optical properties and huge potential applications. In this paper, MoS2 QDs were fabricated through a facile solvothermal method from MoS2 powder crystals. Under certain excitation conditions, MoS2 QDs had strong fluorescence properties and were used as fluorescence probes to detect dopamine (DA). The results showed that the average size of the as-prepared MoS2 QDs was about 3 nm, and the fluorescence quantum efficiency was as high as 57.55%. When the concentration of DA changed from 0.02 to 1 000 μmol/L, the fluorescence of the sensing system was quenched with a linear dependence and the limit of detection was 0.32 μmol/L, with extremely high sensitivity. MoS2 QDs sensing system has strong stability and high selectivity for dopamine detection in different pH values and the presence of interferences. MoS2 QDs had potential applications in catalytic reactions, optical imaging, display devices and fluorescence sensing due to the high purity, small size and high fluorescence intensity.
Key words: molybdenum disulfide quantum dots; solvothermal method; fluorescence; dopamine detection
1 Introduction
The discovery of graphene in 2004 set off a worldwide research boom in new materials based on graphene[1]. As a new kind of nanomaterials, graphene nanomaterials had a special layered structure, which gave them many unique electrical and optical properties[2-3]. Transition metal dichalcogenides (TMDs), which were similar to graphene structures, were also of great interest. For instance, TMDs possess great specific surface areas and excellent semiconducting electronic properties, which render them being applied to sensors, catalysis, transistors, photodetectors and energy storage,etc[4-7]. MoS2is made up of Mo atoms sandwiched between two layers of sulfur atoms (S-Mo-S layers) by means of the weak Van der Waals forces existing between layers, which is one kind of the representative 2D TMDs structures. MoS2is easy to form the structure of quantum dot with size less than 10 nm[8-12]. Different from the MoS2bulk material with indirect band gap, MoS2QDs have the characteristic of direct band gap because of the small size and quantum confined effect, which gives rise to the high fluorescence quantum efficiency of MoS2QDs[13-16].The MoS2QDs have properties of low toxicity, biocompatibility, and high fluorescence intensity, which show the potential applications of bio-imaging, catalytic reactions, optical imaging, display devices and fluorescence sensing[17-22].
Up to now, several techniques have been studied for the synthesis of MoS2QDs, such as mechanical exfoliation, electrochemical synthesis and electro-Fenton processing,etc[23-24]. However, most of the synthesis methods have deficiencies including high cost, low quality, uncontrollable preparation, harsh conditions or time-consuming processes[25-29]. By contrast, solvothermal manufacture is facile, economical and controllable, which is a commonly used “top-down” method[30].
3, 4-dihydroxyphenylethylamine (DA) is one of the crucial catecholamine neurotransmitters, which distributes in the central nervous system[31-34]. DA plays pivotal roles in the function of the hormonal, renal and cardiovascular systems[35-37]. Abnormal concentration of DA causes some incurable diseases such as Parkinson and Huntington’s diseases[38-42]. In addition, change of DA contents in the brain leads to the decline in neurocognitive functions such as memory, attention and so on[43-44]. Therefore, DA has been given enormous attention by chemists and neuroscientists in bio-analytical and bio-medical research. In such context, there is a strong demand to establish highly selective and sensitive means for the direct detection of DA.
In this work, MoS2QDs were manufacturedviaa solvothermal treatment of the bulk crystals, and were used as the fluorescent probe. Under the given excitation, MoS2QDs engendered high fluorescence in the absence of DA. When DA was added, DA snapped to the surface of MoS2QDs and formed thin (poly-dopamine) through the self-polymerization effect. The surface of MoS2QDs was altered by the poly-dopamine, which resulted in the fluorescence quenching of MoS2QDs because of the fluorescence resonance energy transfer (FRET) effect. Within the certain range, the concentration of DA has a linear relation with the fluorescence intensity. Through systematic spectroscopic studies, the MoS2QDs were proven to be highly sensitive and selective probe for the detection of DA.
2 Experiments
2.1 Reagents and Instrumentation
Molybdenum disulfide(MoS2, 99%), 1-methyl-2-pyrrolidinone (NMP), and dopamine hydrochloride (DA, 99%) were purchased from Alfa Aesar and other chemicals were bought from the commercial supplier. All the chemicals in this work were applied directly without further purification. Transmission electron microscopy (TEM) images were obtained from a JEM-1400 operating at 120 kV. The UV-vis spectra were achieved by a UV-3101 scanning spectrophotometer (Shimadzu, Japan) with a standard 10 mm path length quartz cuvette at room temperature. Fluorescence spectra of MoS2QDs were obtained by a luminescence spectrometer (Fluorolog-3) using a standard 10 mm path length quartz cuvette. The X-ray photoelectron spectroscopy (XPS) measurements were recorded on PHI Quantera (PHI, Japan), the binding energy was calibrated with C1s of 284.8 eV. Fourier transform infrared (FT-IR) spectroscopy was carried on Thermo Fisher Nicolet 6700.
2.2 Solvothermal Fabrication of MoS2
The MoS2QDs were produced from MoS2bulk crystals using the combination of ultrasonication and solvothermal treatments. In a typical procedure, 50 mg MoS2powder was dispersed to 30 mL NMP (1-Methyl-2-pyrrolidinone) in a 50 mL reagent bottle and ultrasonicated for 3 h (150 W). After that, the mixture was transferred into a 100 mL Teflon-lined stainless steel autoclave at 150 ℃ for 3 h. After cooling naturally to room temperature, the solution was centrifuged for 30 min at 8 000 r/min. Then the supernatant containing MoS2QDs was transferred to another test tube and filtrated by the 0.22 μm microporous membrane to remove large MoS2sheets. Finally, to calculate the concentration of MoS2QDs, the suspension was evaporated, weighed and re-dissolved in 50 mL deionized water.
2.3 Procedures for Detection of DA
The 1 000 μmol/L DA solution was prepared by dissolving 0.189 5 g dopamine hydrochloride in 1 000 mL deionized water at a 1 000 mL beaker. Then, the solution of DA was further diluted to various concentrations. After that, the concentrations of DA solution (2 mL) were mixed with MoS2QDs solution (140 μg/mL, 2 mL) together and incubated in oven for 3 h at 60 ℃. After cooling to room temperature, the fluorescence measurements of mixtures were taken. The fluorescent intensity was recorded by fluorescence spectrometer at the excitation wavelength of 365 nm and the slits for the excitation and emission were set to 5 nm.
3 Results and Discussion
3.1 Characterization of MoS2 QDs
The photoluminescence emission spectra (PL) of the MoS2QDs dispersions were obtained under different excitation wavelengths at room temperature. As shown in Fig.1(a), the fluorescence peaks moved from 455 nm to 431 nm with the excitation wavelength ranged from 280 nm to 330 nm. Meanwhile the peak shifted from 435 nm to 490 nm with the excitation wavelength changed from 340 nm to 400 nm, illustrating its excitation-dependent fluorescent property. 3D PL emission spectra (Fig.1(b)) presented the trend of the PL intensity at various excitation wavelength and the maximum emission was observed at around 450 nm under the excitation wavelength of 360 nm. The fluorescence quantum yield of MoS2QDs is 57.55% using quinine sulphate as the reference at the excitation wavelength of 360 nm. The quantum yield of MoS2QDs was calculated according to:
Fig.1 (a) PL emission spectra of the MoS2QDs obtained with excitations ranging from 280 to 400 nm with 10 nm increment. (b) 3D PL spectra of MoS2QDs and the high resolution XPS survey spectra of the Mo 3d state(c) and S 2p state(d).
(1)
whereYis the quantum yield,Fis the calculated integral area of emission intensity, andAis UV-vis absorbance at the excitation wavelength. The subscript “x” denotes MoS2QDs, the subscript “s” refers to quinine sulphate.
The X-ray photoelectron spectroscopy(XPS) measurements were used to measure the chemical valence states and the element composition of the MoS2QDs. As shown in Fig.1(c), two characteristic peaks appear at 232.6 eV and 235 eV corresponded to the Mo 3d5/2and Mo 3d3/2peaks of Mo4+in the MoS2QDs. Meanwhile, the S 2p peaks at around 169 eV and 170 eV are homologized with the S 2p3/2and S 2p1/2for S2-(Fig.1(d)). The atomic ratio (Mo∶S) was close to 1∶2, confirming the charge states of Mo4+and S2-in the MoS2QDs.
3.2 Detection Principle for DA
After being added to the solution, DA attached onto the surface of MoS2QDs and formed poly-dopamineviathe self-polymerization effect. Poly-dopamine changed the surface property of MoS2QDs. Under certain excitation, energy transfers from MoS2QDs to poly-dopamineviathe FRET, resulting in the fluorescence quenching of MoS2QDs, as presented in Fig.2.
The cover effect of poly-dopamine was confirmed by the TEM. Fig.3(a) described that the diameters of pure MoS2QDs are mostly distributed in the range of 1-5 nm, with the average diameter of ~3 nm in size (Fig.3(b)). The diameter of MoS2QDs obviously increases after adding DA(Fig.3(c)). The average value increases to ~20 nm (Fig.3(d)).

Fig.3 TEM image(a) and size distribution histogram(b) of MoS2QDs. TEM image(c) and size distribution histogram(d) of MoS2QDs with 1 000 μmol/L DA.


Fig.4 (a) UV-vis absorption spectra of DA, MoS2QDs and MoS2QDs-DA. The inset: MoS2QDs solution exposed under natural light(left) and 365 nm UV light (middle), and MoS2QDs-DA exposed under 365 nm UV light(right). (b) FT-IR spectra of MoS2QDs and MoS2QDs-DA.
3.3 Analytical Performance of The Sensor
The detection of DA was studied in order to explore the capability of the MoS2QDs sensing system. As depicted in Fig.5(a), when the concentration of DA increases from 0 μmol/L to 1 000 μmol/L, the fluorescence intensity of MoS2QDs solution at 455 nm gradually reduces under the excitation wavelength of 365 nm. The emission intensity of MoS2QDs solution cuts down about 41.8% of its original value when the concentration of DA reaches 1 000 μmol/L.
The connection between the concentration of DA and (F0-F)/F0(the degree of fluorescence quenching) is depicted in Fig.5(b). The inset of Fig. 5(b) indicates that there is a linearity between DA concentration and (F0-F)/F0ranging from 0 to 1.0 μmol/L and the correlation coefficient is 0.972. The detection limit is 0.32 μmol/L based on 3σ/k(σis the standard deviation of the corrected blank signals of the MoS2QDs andkis the slope of the fitting line).
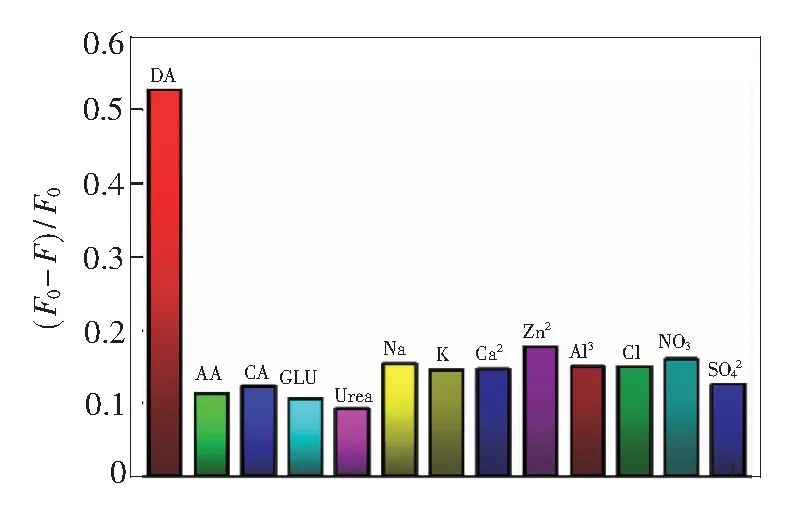
The effect of pH on the efficiency of the sensing system is further investigated. As presented in Fig. 5(c), the fluorescence of MoS2QDs with and without DA was measured at different pH values ranging from 2 to 13. The fluorescence intensity of MoS2QDs and MoS2QDs-DA (MoS2QDs with 100 μmol/L DA) has no obvious change when the pH value varies. Fig. 5(d) reveals that the fluorescence intensity of MoS2QDs and MoS2QDs-DA is independent on the pH, which ensures the MoS2QDs as a stable sensing platform in the complicated environment.

Fig.5 (a) Emission spectra of MoS2QDs in the presence of DA with different concentrations at a range from 0 μmol/L to 1 000 μmol/L. (b) (F0-F)/F0plot of MoS2QDs with different concentrations DA. (c) PL emission spectra of MoS2QDs with 1 000 μmol/L DA under different pH values. (d) Line chart about the relationship between pH values and fluorescence intensity.

4 Conclusion
MoS2QDs were fabricated through a facile solvothermal method from bulk MoS2crystals. The MoS2QDs possess strong fluorescence and the fluorescence quantum efficiency was as high as 57.55%. The as-prepared MoS2QDs can be significantly quenched by DA through FRET effect which were further studied as a sensing probe to detect DA. The fluorescence of sensing system is quenched with a linear dependence ranging from 0 to 1.0 μmol/L and the limit of detection is 0.32 μmol/L. In addition, the system is stable in different pH environment and exhibits high selectivity for the DA detection. All the above results indicate that the sensing system based on MoS2QDs is a superior sensor platform, and will contribute to various applications in the bio-analytical and bio-medical researches.
