MicroRNA-126 and epidermal growth factor-like domain 7 predict recurrence in patients with colon cancer treated with neoadjuvant chemotherapy
2019-11-05TorbenFrstrupHansenAntingLiuCarlsenJuliaTanasTanassiOleLarsenFlemmingBrandtrensenLarsHenrikJensenAndersJakobsen
Torben Frøstrup Hansen,Anting Liu Carlsen,Julia Tanas Tanassi,Ole Larsen,Flemming Brandt Sørensen,5,Lars Henrik Jensen,Anders Jakobsen
1Danish Colorectal Cancer Center South,Vejle Hospital,Institute of Regional Health Research,University of Southern Denmark,Vejle 7100,Denmark.
2Department of Autoimmunology and Biomarkers,Statens Serum Institut,Copenhagen 2300,Denmark.
3Department of Congenital Disorders,Statens Serum Institut,Copenhagen 2300,Denmark.
4Department of Oncology,Herlev Hospital,Herlev 2730,Denmark.
5University Institute of Pathology,Aarhus University Hospital,and department of Clinical Medicine,University of Aarhus,Aarhus 8200,Denmark.
Abstract
Aim:Neoadjuvant chemotherapy may represent a shift in the treatment of locally advanced colon cancer.The angiogenic couple has-microRNA-126 (miRNA-126) and epidermal growth factor-like domain 7 (EGFL7) are transcribed from the same gene and regulates all aspects of angiogenesis and may influence the ability of tumor cells to disseminate.The aim was to analyze the relationship between miRNA-126 and EGFL7 and disease recurrence in patients with locally advanced colon cancer treated with neoadjuvant chemotherapy.
Methods: This study included 71 patients from a phase II study all planned for three cycles of capecitabine and oxaliplatin before surgery.Blood was sampled at baseline and right before and after the operation.Circulating miRNA-126 was analysed by RT-qPCR and a quantitative immunoassay was used for the analyses of EGFL7.
Results:The rates of 5-year disease-free survival (DFS) and overall survival (OS) were 80% and 85%,respectively.The level of circulating miRNA-126 before the operation predicts recurrence,P = 0.035.In patients with values below and above the median the recurrence rate was 31% and 4%,respectively.Similar results applied to EGFL7.A combined estimate identified a subgroup of patients (25 of 71) with no recurrence and a 5-year DFS and OS rate of 100%,respectively.
Conclusion:MicroRNA-126 and EGFL7 are predictors for disease recurrence in patients with locally advanced colon cancer treated with neoadjuvant chemotherapy and may assist in selection of adjuvant chemotherapy.
Keywords: Chemotherapy,colon cancer,epidermal growth factor-like domain 7,microRNA-126
INTRODUCTION
Patients operated for high risk stage II and III colon cancer are candidates for offering adjuvant chemotherapy.Only a minor fraction of the patients benefit from this treatment modality,but nearly all suffer from the side effects,especially when treated with combination chemotherapy[1].Consequently,neoadjuvant chemotherapy is currently under investigation in patients with locally advanced colon cancer as a new treatment approach.This strategy ensures early systemic treatment of minimal disease,while simultaneously providing information about tumor responsiveness[2].The initial phase II experience indicates that a substantial proportion of these patients may convert to a low-risk status at the time of surgery,eliminating the need for adjuvant chemotherapy[3].
Angiogenesis is a key process of tumor growth and the subsequent dissemination of tumor cells,and numerous pro- and anti-angiogenic factors are involved in its regulation[4,5].We have previously focused on microRNA-126-3p (miRNA-126) and epidermal growth factor-like domain 7 (EGFL7),which are transcribed from the same gene.The cross-talk regulation between miRNA126 and EGFL7 is extremely complex and studies indicate that epigenetic modifications may regulate the expression of both parameters although other studies argue for an independent regulation[6-9].This unique couple is involved in several parts of the angiogenic process including endothelial cell (EC) proliferation,migration and formation of angiogenic sprouts[10-13].Overall,miRNA-126 is especially involved in the EC/blood vessel related changes while EGFL7 provides a tight connection to the extracellular matrix.A dual approach to both miRNA-126 and EGFL7 will thus encompass the entire sprouting process characteristic for the activated endothelium.A prognostic impact of miRNA-126,and to a certain degree EGFL7,has been demonstrated both in localized as well as metastatic colorectal cancer[14-17],but their clinical importance in the neo-adjuvant setting is still unknown.
The aim of this study was to analyze the ability of miRNA-126 and EGFL7 to predict disease recurrence in patients with locally advanced colon cancer treated with neoadjuvant chemotherapy.
METHODS
This study follows the guidelines presented in REMARK[18].
Study population
This study is based on a phase II trial of neoadjuvant chemotherapy in patients with locally advanced colon cancer[3].The present study population is identical to that of the original trial,consisting of 71 patients that completed neoadjuvant chemotherapy which was followed by surgery between August 2010 and September 2013.In brief,all patients were diagnosed with locally advanced,but resectable,colon cancer as determined by CT scans.The treatment of patients with documented wild-typeKRAS,BRAF,andPIK3CAwas supplemented with anti-EGFR treatment,panitumumab,while patients with any mutations in these genes,or unknown mutational status,were treated with neoadjuvant chemotherapy only.The selection criteria included; age ≥ 18 years,a performance status no higher than two and staged with the tumour T-category of T3with extramural tumour invasion > 5 mm,or T4,based on the diagnostic CT scans,using 64-channel multidetector equipment with a section thickness of 3 mm.Inclusion and exclusion criteria and followup have previously been specified[3].Patients were initially followed for up to three years post-operatively,according to the protocol.It was possible to extend this period to five years in relation to the present study including follow-up data on survival and histopathological verified recurrence of all the enrolled patients.All data recordings were performed as defined with good clinical practice.The study,and the present translational research,was approved by the Regional Committee on Health Research Ethics for Southern Denmark (S-20100014) and the Danish Data Protection Agency.Written informed consent was obtained,prior to inclusion,from all enrolled patients (ClinicalTrials.gov NCT01108107).Blood for the translational research was sampled at the same time with routine,treatment related blood sampling.
Treatment
The neoadjuvant treatment was capecitabine 1000 mg/m2orally two times daily on days 1-14 (28 doses) of a 21-day cycle combined with oxaliplatin 130 mg/m2as a 2-h intravenous infusion on the first day of each cycle.Panitumumab was added in a dose of 9 mg/kg on the first day of each cycle,for patients with wildtype mutational tumour status.Resection of the tumour took place three weeks after the last neoadjuvant chemotherapy.Patients that fulfilled the Danish Colorectal Cancer Group criteria for adjuvant treatment (presence of lymph node metastases,a pT4 tumour,acute surgery due to obstruction of the bowel,neuronal invasion,vascular invasion,high histopathological malignancy grade,and the removal of less than 12 lymph nodes) was offered additional five cycles of the same treatment but without panitumumab.The patients that did not fulfil these criteria were not treated any further.The patients were followed for three years as previously specified[3].
Sampling
Sampling of peripheral blood was carried out before initiation of the neoadjuvant treatment (baseline),after completion of the neoadjuvant treatment,i.e.one or two days before the operation (operation),and at the first follow-up visit approximately four weeks after the operation.Sample availability varied between 75% and 87% [Supplementary Figure 1].Venous blood (whole blood) was drawn from the antecubital area.Samples for serum (EGFL7) analyses were collected in 6 ml dry glasses,left for minimum 30 min for a clot to form,spun down for 10 min at 2500g,followed by the transfer of serum to Greiner tubes (SIGMAALDRICH,USA) and finally frozen at -80oC.Blood intended for plasma (miRNA-126) analyses were collected in 6 mL EDTA-containing tubes,spun down for 10 min at 2500gat room temperature,and plasma was transferred and stored similar to the serum samples.The median storage time from blood sampling to analysis was 2.6 years.Samples were transported on ice from storage to analysis.
Analysis of circulating microRNA-126
Circulating miRNA-126 (cir-miRNA-126) was analysed by staff unaware of the patient outcome and in line with previous descriptions[19].Briefly,the total RNA purification kit (Norgen Biotek Corp,Ontario,Canada) was used for the purification of RNA from 100 μL of each plasma sample as described in the manufacturer's instructions with small modifications as specified earlier.Reverse transcription was performed,using the TaqMan microRNA Reverse Transcription Kit (Applied Biosystems,Foster city,CA,USA).Reverse transcription was performed on an ABI 2720 Thermal Cycler (Applied Biosystems,Foster city,CA,USA).cDNA samples and 3 TaqMan microRNA assays (hsa-miR-126-3p,cel-miR-54 and cel-miR-238) (Applied Biosystems,Foster city,CA,USA) were applied to six 384-well plates according to instructions.All the miR-assays were performed in triplicate.The real-time PCR was performed in the ViiA7 real-time PCR system (Applied Biosystems,Foster city,CA,USA),applying a standard 384 well protocol.Data processing were performed using the Applied Biosystems' ViiA7 real-time PCR analysis software (v.1.2.3).This approach estimates total amount of stable miRNA-126 in a given sample.However,it is not possible to determine if the miRNA originates from microvesicles or from miRNA-protein complexes.
We used a two-step normalization.First we spiked-in cel-miRNAs (cel-miRNA-54 and cel-miRNA-238) for the technical normalization,then further normalization with whole set data of miRNA-126.The Cq values were then normalized as described earlier and transformed according to the 2-ΔΔCq method[20].This means that the presented estimates for cir-miRNA-126 are relative values without a dimension.
Analysis of circulating EGFL7
We used a sandwich enzyme-linked immunosorbent assay (Cloud-Clone Corp,SEL643Hu,Houston,TX,USA) to quantify circulating EGFL7 (cir-EGFL7) in the serum samples,according to the manufacturer's protocol.In short,100 μL of standard or sample was added to each well of a 96-well strip plate that was precoated with an antibody specific to EGFL7.Then incubation for 2 h at 37oC,aspiration,and addition of detection reagent A,incubation for an additional hour at 37oC,aspiration,and three times washing.The described step was repeated for detection reagent B,using 30 min of incubation and five washes.Substrate solution was added and incubated for 15 minutes at 37oC followed by the addition of a stopping solution and finally absorbance reading at 450 nm.The concentrations of EGFL7 were assessed through comparisons with the standard curve followed by multiplication with the initial dilution factor (100 fold).If samples had concentrations above the standard curve they were diluted further (and multiplied accordingly).
Samples were analysed in duplicate and we used the average for comparison with the clinical data.Inhouse analytical coefficients of variation on three levels were < 10% for Intra-Assay and < 12% for Inter-Assay.Protein concentrations are expressed in ng/mL.
Statistics
We report median values followed by a 95% confidence interval.The Wilcoxon rank sum test was used comparing median values.Fisher's Exact test was used to evaluate differences between proportions.Disease free survival (DFS) was defined as the time from the operation to the first documented tumour recurrence or death of any cause.Occurrence of other malignancies led to censoring of the DFS data in six cases.Data were censored from the day of diagnoses.Accordingly,overall survival (OS) was calculated from the date of the operation to death of any cause.Adjustment for multiple comparisons was not carried out.All statistics were performed using the NCSS statistical software (NCSS Statistical Software,Kaysville,UT 84037,USA,version 2007).Pvalues < 0.05 were considered significant.All tests were two-sided.
RESULTS
Patient characteristics
The patient characteristics are summarized in Table 1.At the time of data analysis,i.e.,at a median followup of 4.0 years,disease recurrence had occurred in 14 patients.
MicroRNA-126
Low-level miRNA-126 levels was related to a higher pN category (baseline,P= 0.036),the absence of tumor fixation,perineural invasion,microsatellite stable status,and high pT category (preoperatively,P< 0.050),and a higher pT category (at follow-up,P= 0.012,Table 2).A relationship between wild typeKRAS,BRAF,andPIK3CAand age (P< 0.05) was also seen [Supplementary Table 1].
Overall,the median miRNA-126 decreased significantly during neoadjuvant chemotherapy followed by an increase postoperatively [Figure 1].
Epidermal growth factor-like domain 7
With the exception of performance status,no relationships between EGFL7 and clinical or pathoanatomical characteristics were detected [Supplementary Tables 2 and 3].Similar to that of miRNA-126,the median EGFL7 tended to decrease during neoadjuvant chemotherapy followed by a significant increase postoperatively [Figure 1].

Table 1.Clinical and pato-anatomical characteristics,n = 71
Recurrence and prognosis
The distributions of miRNA-126 and EGFL7 values,according to recurrence is shown in Figure 2.Patients with disease recurrence were characterized by a significantly lower levels of miRNA-126 at all sampling points (P< 0.05),compared to patients without disease recurrence.Also,high levels of EGFL7 tended to be associated with disease recurrence.
In order to assess the relationship between the analyzed parameters and disease recurrence,patients were divided into two groups using the median values as cut-off.Based on these values,the recurrence rates differed significantly at the time of operation both for the miRNA-126 levels (31%vs.4%,P= 0.035) and the EGFL7 levels (37%vs.4%,P= 0.017),respectively [Table 3].Using combined estimates [Table 3] led to the identification of a group at very high risk (50%) of recurrence and a no-risk (0%) group.

Figure 1.Changes in circulating microRNA-126 (cir-miRNA-126) and epidermal growth factor-like domain 7 (cir-EGFL7) during treatment.Median values are illustrated with horizontal lines marking the respective upper and lower limits of the 95% confidence intervals (CI).P-values refer to differences between the individual time points (baseline,operation,and follow-up) based on the Wilcoxon Signed-Rank Test for differences at the medians

Figure 2.Dot plots illustrating the results from microRNA-126 (miRNA-126) A-C,and epidermal growth factor-like domain 7 (EGFL7) D-F analyses according to recurrence status,at baseline,operation,and follow-up,respectively.Horizontal bars represent medians

Table 3.MicroRNA-126 and EGFL7 as individual and combined estimates in relation to disease recurrence
For the entire cohort the rate of 5-year DFS and OS was 80% and 85%,respectively.The relationship between disease recurrence and miRNA-126 and EGFL7,at the time of operation,translated into a significant benefit as to DFS [Figure 3].There were no significant relationships with OS,P= 0.149 andP= 0.126,respectively.
DISCUSSION
Neoadjuvant chemotherapy represents a new and promising treatment approach for patients with localized advanced colon cancer.While our initial phase II data are currently being validated in an ongoing phase III trial (EudraCT no:2013-002363-26),the present translational study,with a median follow-up of four years,indicates a very promising outcome for this patient category in general.The data suggest a relationship between miRNA-126,EGFL7,and disease,which expands the possibility for treatment selection.
The present results demonstrated a significant relationship between low cir-miRNA-126 and high cir-EGFL7 levels and the clinically relevant endpoint of disease recurrence.This was especially pronounced in the “before operation” samples,which precedes the decision about offering adjuvant chemotherapy.This motivated us to generate a combined estimate focusing specifically on the presumed low- and highrisk combinations (miRNA-126 high + EGFL7 low,and miRNA-126 low + EGFL7 high).Twenty-five patients were identified as low risk,based on the combined miRNA-126/EGFL7 estimate from the “before operation” or the “follow-up” samples.None of these patients experienced disease recurrence,but 10 were still high-risk and thus received adjuvant chemotherapy.This underlines the predictive potential of these two biomarkers in the clinical setting.Although there are no directly comparable studies in the neoadjuvant setting,several preclinical studies have demonstrated relationships between low miRNA-126 expression and increased angiogenesis,tumor growth,migration,and the metastatic potential,supporting the present results[6,21-23].The same pattern was seen with high cir-EGFL7.This is in agreement with our previous study analyzing EGFL7 expression in 126 patients with stage II-IV colon cancer[17].It showed a significantly higher EGFL7 expression in the primary tumor of recurred patients than of those who remained recurrence free after the operation.The correlation with prognosis is well in line with previous studies in locally advanced cancer as concerns miRNA-126[16,24],and with disseminated disease with respect to EGFL7[15].A meaningful multiple Cox Regression analysis was not feasible with only 14 events in the DFS analyses.
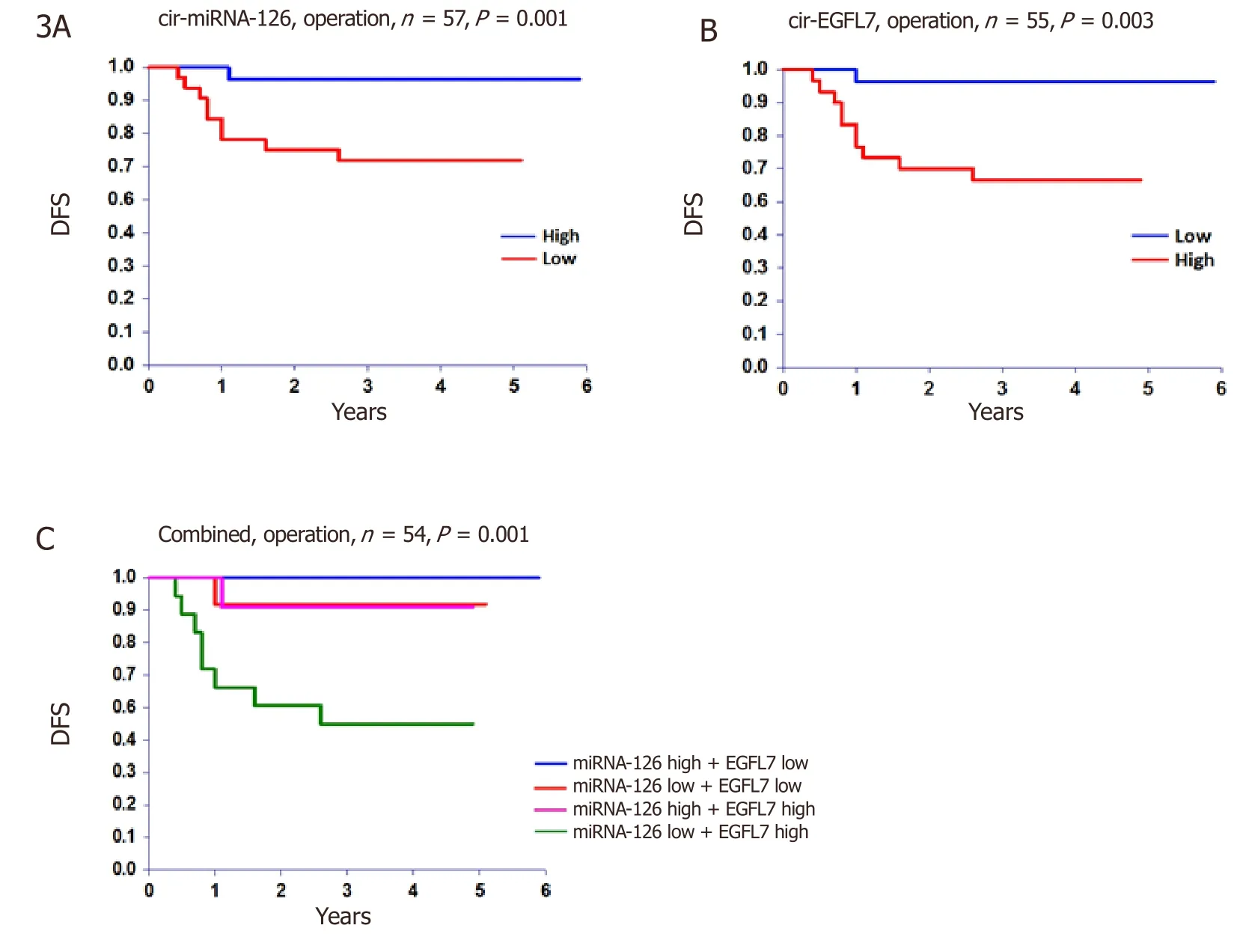
Figure 3.Disease free survival (DFS).The DFS at time of operation according to A,circulating microRNA-126 (cir-miRNA-126),B circulating epidermal growth factor-like domain 7 (cir-EGFL7),and C the combined estimate
The levels of miRNA-126 and EGFL7 seem to decline during treatment and rise again after the operation.This may be explained by a decreased number of immature blood vessels during treatment,which is particularly pronounced in the malignant tumor.The general increase seen in the postoperative samples are most likely also influenced by the postoperative stress response,but it is interesting that the relationship with recurrence status is also recapitulated at this time point.
An opportunity to analyze miRNA-126 expression in the diagnostic biopsies appeared after the generation of these originally planned blood-based results.This was performed byin situhybridization and image guided analyses as previously described[25,26]and the results suggest a similar distribution with a lower expression of miRNA-126 in the patients who eventually experienced disease recurrence supporting the presented results [Supplementary Figure 2].
Post-transcriptional gene regulation is one of the main functions related to miRNAs that often have multiple targets.This is also true for miRNA-126 and the regulation of several of these mRNA targets may all impact on the risk of disease recurrence from local colon cancer.Some of the more well described targets areSPRED1,p85β,andPI3KR2governing vascular integrity[10,27],VEGF-A regulating AKT-pathway signaling[28],CXCR4 and IRS-1 involved in CRC cell proliferation and migration[29,30],and KRAS impacting on the viability of the mutated tumor cells[31].Recent reviews have also highlighted the clinical potential of miRNA-126[32-34].
The limitations of the current study are associated with the general caveats related to interpreting circulating biomarker data.The analysis of miRNAs is influenced by several technical aspects such as choice of blood fraction,sampling,handling,processing,normalization procedures,and the possible contamination from platelets during processing.Our results being influenced by one or more of these steps cannot be excluded,but the consistency of the presented results is remarkable.The blood sampling at baseline,surgery,and follow-up represents three distinct time points with weeks and months between them.The same significant relationship between disease recurrence and miRNA-126 is demonstrated at all three time points.This would probably not be the case,if data were strongly influenced by pre-analytical and analytical factors.Multiple comparisons,which may be inherent to this hypothesis generating study,could influence some of the correlations with the clinical and patho-anatomic characteristics.Also,blood samples from all patients at all sampling points would have been preferable,but an availability of 75%-87% is acceptable.The study population,comprising all patients enrolled in a well-described clinical phase II trial,is a strength of the study.
Our initial experience with neoadjuvant chemotherapy is that half of the patients converted from high to low-risk status during the three cycles of preoperative chemotherapy actually do surprisingly well (4-year OS rate of 90%).They are spared a substantial amount of adjuvant chemotherapy and the adverse events associated with this treatment.The identification of prognostic/predictive biomarkers in this clinical situation is of importance,as many of the unconverted patients also remain recurrence free,and thus could be spared from the adjuvant chemotherapy if identified.The analyses of miRNA-126 and EGFL7,and especially the combined estimate,may procure such information.It is well known,however,that the crosstalk regulation between miRNA-126 and EGFL7 is extremely complex and the exact interpretation of these data in a neoadjuvant setting is consequently scheduled for further validation in an ongoing phase III trial.
In conclusion,miRNA-126 and EGFL7 are predictive of disease recurrence in patients with locally advanced colon cancer treated with neoadjuvant chemotherapy and may be instrumental in the identification of patients to be spared of adjuvant chemotherapy.
DECLARATIONS
Acknowledgements
We are very thankful for the technical assistance provided by Camilla Davidsen,Lone Frischknecht,Birgit Roed Sørensen,Boye Schnack Nielsen and Margit Søgaard Jakobsen and for the linguistic editing provided by Karin Larsen.Special thoughts and thanks goes to Niels H.H.Heegaard how participated in this study but unfortunately passed away all too soon.This study was supported by The Cancer Foundation,The Danish Council for Independent Research,Direktør Jacob Madsen & Hustru Olga Madsen's Foundation,and the Regional Strategic Council for Research in the Region of Southern Denmark,none of which had any influence on any part of the study.
Authors' contributions
Conceived and designed the experiments:Hansen TF,Sørensen FB,Jakobsen A
Performed statistical analyses of the data and drafted the manuscript:Hansen TF
Performed the miRNA analyses:Carlsen AL
Performed the protein analyses:Tanassi JT
Being responsible for patient inclusion in the trial:Larsen O,Jensen LH,Jakobsen A
Supervised the project:Jakobsen A
Availability of data and material
The datasets supporting the conclusions of this article are included within the article (and its additional files).
Financial support and sponsorship
This work was supported by the Cancer Foundation (no reference),the Danish Council for Independent Research (No.10-093589),Direktør Jacob Madsen & Hustru Olga Madsen's Foundation (No.5297),and the Regional Strategic Council for Research in the Region of Southern Denmark (No.14/32395).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethics approval and consent to participate
The study,and the present translational research,was approved by the Regional Committee on Health Research Ethics for Southern Denmark (S-20100014) and the Danish Data Protection Agency.Written informed consent was obtained from all patients enrolled in the study (ClinicalTrials.gov NCT01108107).This study represents translational research related to a clinical phase II trial (ClinicalTrials.gov NCT01108107).Blood for the translational research was sampled at the same time with routine,treatment related blood sampling.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2019.
杂志排行
Cancer Drug Resistance的其它文章
- Enhanced Kat3A/Catenin transcription:a common mechanism of therapeutic resistance
- Longitudinal monitoring for the emergence of epidermal growth factor C797S resistance mutations in non-small cell lung cancer using blood-based droplet digital PCR
- Regulation of ABCB1 activity by microRNA-200c and microRNA-203a in breast cancer cells:the quest for microRNAs' involvement in cancer drug resistance
- Genetic variations in triple-negative breast cancers undergoing neo-adjuvant chemotherapy
- Use of MRl,metabolomic,and genomic biomarkers to identify mechanisms of chemoresistance in glioma
- The role of exosomal microRNAs; focus on clinical applications in breast cancer