Engineered human pluripotent stem cell-derived natural killer cells: the next frontier for cancer immunotherapy
2019-11-02HuangZhuDanKaufman
Huang Zhu, Dan S.Kaufman*
Division of Regenerative Medicine, Department of Medicine, University of California San Diego, San Diego, CA, USA
Abstract
Keywords: Adoptive NK cell therapy, Cancer immunotherapy, CAR-NK cells, Chimeric antigen receptor, Human stem cells,Natural killer cells
1.INTRODUCTION
Adoptive cell therapy has emerged as novel and promising treatment option for relapsed and refractory malignancies.Chimeric antigen receptor(CAR)-T cell therapies have produced striking responses with overall remission rates of up to 90% in clinical trials.U.S.Food and Drug Administration has approved the first gene modified CAR T-cell therapy tisagenlecleucel(Kymriah) for treatment of relapsed B-cell acute lymphoblastic leukemia (ALL) in children and young adults and the second product axicabtagene ciloleucel(Yescarta)for treatment of adults with certain types of non-Hodgkin lymphoma.1,2Despite this exciting progress with CAR-T cell therapies,more work is needed to overcome some potential limitations of this approach.3CAR-T cell therapy requires collection and genetic modification of a patient's own lymphocytes-a process that requires highly specialized steps that are time consuming and very expensive.4-6In addition,toxicities from CAR-T cells include cytokine release syndrome (CRS) and neurotoxicity can lead to severe morbidity or mortality,requiring treatment at highly specialized clinical centers.7,8
Clinical trials for over a decade demonstrate allogeneic primary NK cells from peripheral blood (PB-NK cells) and umbilical cord blood(UCB-NK cells)have been proven to be safe and effective without significant toxicity such as CRS,neurotoxicity or graft-versus-host disease(GVHD).9-13However,PB-NK cells and UCB-NK cells used for these trials are heterogeneous cell products and vary from each donor, limiting their potential of developing a standardized,cellular immunotherapy product.14,15With progress to generate NK cells from human pluripotent stem cells in the last decade, NK cells from both human embryonic stem cells hESCs (hESC-NK) and human induced pluripotent stem cells (iPSC-NK) are becoming promising candidates to develop standardized adoptive NK cell therapy.Importantly,these cells are homogenous, feasible to genetically modify at a clonal level and easy to be expanded to clinical-scale.16-19This review will highlight recent advances of obtaining and expanding hESC/iPSC-derived NK cells and novel genetic engineering strategies that are being applied to improve their antitumor activity (Fig.1).
2.NK CELL BIOLOGY
NK cells are large granular lymphocytes with an intrinsic ability to kill infected and transformed cells without HLA restriction and without prior antigen sensitization.20,21In humans, they are typically characterized as CD56+CD3-lymphocytes and can be broadly categorized in two subpopulations based on level of CD56 and CD16 expression:CD56brightCD16-cells and CD56dimCD16+cells.22,23The majority of PB-NK cells are CD56dimCD16+which are highly cytotoxic against target cells.In contrast,about 2%to 10%PBNK cells are CD56brightCD16-which have lower cytotoxic activity while displaying a high capacity to produce immunoregulatory cytokines.22,23
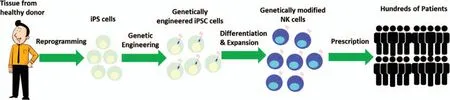
Figure 1.Development of standardized NK cells products using iPSC platform.Human iPSCs are reprogrammed from healthy donor tissue and can be genetically engineered to improve antitumor activities.Several strategies are being exploring to improve NK cells antitumor functions including equipping NK cells with CAR to increase cytotoxicity and target recognition,hnCD16 to enhance ADCC,mbIL15 to increase in vivo persistence and activation.A single iPSC line can be engineered with multiple components.Genetically optimized iPSCs can be stored as master cell bank to provide consistency and an essentially unlimited starting cell population to produce NK cells.iPSC-NK cells can be cryopreserved and ready to use to treating hundreds of patients,potentially with multiple doses per patient.iPSC-NK cells can be used in combination with other therapies such as anti-cancer antibodies to enable targeting of multiple tumor antigens to prevent relapse and better enable long-term remissions and cures.
NK cells can distinguish healthy cells from infected or tumor targets by the balance between inhibitory and activating signals from cell surface receptors20,24Inhibitory signals from receptors such as killer immunoglobulin-like receptors (KIRs) and CD94-NKG2A recognize class I major histocompatibility complex(MHC) molecules which are constitutively present on normal cells to inhibit NK cell cytotoxic activity,thus prevent the killing of healthy cells.25,26NK cells can recognize and kill infected or tumor cells as these cells often lose or down regulate MHC-class I molecules, leading to the removal of inhibitory signals.27In addition, virus infected or malignant cells often up-regulate ligands which bind to NK cell activating receptors,triggering the activation of NK cells.28These activating receptors include NKG2D, KIR (activating forms), natural cytotoxicity receptors(NCRs), and CD16.29
Once activated, NK cells release preformed cytolytic granules containing granzyme and perforin to lysis tumor cells.30NK cell degranulation also occur through antigen-dependent cellular cytotoxicity(ADCC)which is a key effector mechanism mediated by CD16.31,32Also, engaged NK cells can trigger tumor cell apoptosis through death-receptor pathways by inducing death ligands such as FAS ligand and TRAIL.33In addition, NK cells secrete proinflammatory cytokines such as interferon (IFN)-γ which also exert antitumor effects.34
3.ADOPTIVE NK CELL THERAPY
Recently, cell-based anti-cancer immunotherapies have made great advances with approval of two autologous CAR-T cell therapies(KymriahTMand YescartaTM)by the FDA in 2017 for treating lymphoma.1,2While CAR-T cells have attracted considerable attention, NK cells have been shown to possess potent anti-acute myeloid leukemia (AML) activity without eliciting serious adverse effects such as CRS and neurotoxicty that frequently occurs from CAR-T cell treatments.9,12Early NK cell trials used IL2 to stimulate autologous NK cells to treat hematological malignancies, metastatic melanoma, renal cell carcinoma,and advanced digestive cancer.35,36While these trials demonstrated safety with no toxic side effects,the clinical benefits were typically limited as NK cell antitumor activity was likely inhibited by self HLA molecules.35-37In contrast, clinical trials using allogenic PB-NK cells have demonstrated that about 30%of patients with refractory or relapsed AML achieved complete remission.9,12,38,39Besides AML,allogeneic NK cell therapy has been tested for treating solid tumors,40such as ovarian, breast cancer,10lung cancers,41renal cell carcinoma, colorectal, and hepatocellular.42These studies indicate NK cells can play a therapeutic role in the treatment of solid tumors.This has led to increased interest on NK cell-based adoptive immunotherapy and explosion of NK cell-based clinical trials to treat a variety of both hematological malignancies and solid tumors.14,37,43-45
4.SOURCES OF NK CELLS USED IN CLINICAL TRIALS
To enable expanded therapeutic use of NK cells,a strategy to efficiently produce a large number of cells suitable for clinical trials is required.To date,a variety of cell sources have been used in adoptive NK cell clinical trials including PB-NK cells (both autologous and allogeneic cells), UCB-NK cells, umbilical cord blood CD34+ cell-derived NK cells (UCB-34+-NK) and the NK cell line NK-9211,12,46,47(summarized in Table 1).Although trials using these cells have demonstrated favorable safety profile,the efficacy can be modest and each cell source is confined by limitations.14,48The major limitation of using PB-NK and UCBNK is that efficacy can be very much donor-dependent and not derived from a single renewable source.This makes it difficult to generate a standardized product and challenging to develop multi-dose treatment schema.15,49Unlike autologous CAR-T cells which were reported to persist and remain functional years beyond transplantation,50,51allogeneic NK cells typically survive only a few weeks in the adoptive transfer setting.9,10So a multidose treatment schema becomes necessary for adoptive NK cell therapy to maximize efficacy.43In addition,primary NK cells are in general challenging to genetically engineer due to highly variable efficiency and viability following genetic modification.52Again providing another challenge to develop consistent and reproducible genetically engineered NK cell therapies.
NK-92,a human NK cell line originally derived from a patient with lymphoma,overcomes the limitations of primary NK cells.53They are well-characterized, homogenous, easy to genetically modify and unlimited source of NK cells,which are all attractiveproperties of developing standardized NK cell therapy products.54So NK-92 cells are engineered with various CARs or CD16 to increase cytotoxicity for both hematologic and solid malignancies.55,56However, for the concern of tumorigenicity,NK-92 cells need to be irradiated prior to administration to human,which limit the antitumor activity of these cells as studies of PB-NK cells clearly show that persistence of the NK cells in patients attribute to treatment outcome.9,54

Table 1 Sources of NK cells being using in clinical trials.
5.NK CELLS DERIVED FROM HUMAN PLURIPOTENT STEM CELLS
As the technical advance of obtaining clinical scale mature NK cells from both hESCs and iPSCs, NK cells derived from human stem cells are becoming the most promising cell population for adoptive NK cell therapy.17,18,57-59There are several advantages of using hESC-NK or iPSC-NK over primary NK cells and NK cell lines.14,60First,hESC-NK/hiPSC-NK cells are uniform, reproducible and unlimited source of NK cells which can be develop multi-dose treatment schema,eliminating the donor-to-donor variability as well as concern of limiting cell numbers when using PB-NK or UCB-NK cells.14,18Second, it is feasible and consistent to genetically engineer hESCs and iPSCs to derive NK cells with improved antitumor activity or in vivo persistence.Diverse genome-editing technologies, such as, lentivirus, transposons, or the CRISPR-Cas9 system can be used to engineer hESCs and iPSCs, followed by differentiating these into NK cells from the genetically modified stem cells.61,62Clinical trials using iPSC-NK cells to treat cancers have now been approved by the US FDA and have commenced.63,64
The methods to generate NK cells from human pluripotent stem cells in vitro has evolved in the last decade.16,17,65Early studies in our lab used murine stromal cell lines(e.g.,S17 or M2-10B4 cells) to obtain CD34+CD45+hematopoietic progenitor cells from undifferentiated hESCs with media containing fetal bovine serum (FBS) followed by sorting and then moved to a second stromal cell line (e.g., AFT024 or EL08-1D2 cells) in media supplemented with SCF,Fms-like tyrosine kinase 3 ligand(FLT3L), IL-3, IL-15, IL-7 to direct differentiation towards CD45+CD56+NK cells.17NK cells generated using this method have demonstrated mature phenotypes and functionalities similar to primary NK cells.16,17They can kill diverse hematologic and solid tumor cell lines in vitro and eliminated leukemia cells(K562 cells) xenografted in immune-deficient mice.17Interestingly,hESC-derived NK cells showed better antitumor activity than UCB-34+-NK cells which were typically phenotypically immature.17,18To produce hESC and iPSC-derived NK cells with more clinically compatible conditions and at a scale suitable for clinical trials, our group adopted a “spin-EB” protocol that eliminated the use of stromal cell lines and FBS.19,66These serum-free,stromal-free conditions produce even more CD34+CD45+hematopoietic progenitor cells than the stroma plus FBS conditions used previously.18The EBs containing hematopoietic progenitor cells can be transferred without sorting to either stromal cells(OP9-DL4)or uncoated plates in serum-free media supplemented with cytokines to effectively produce mature CD45+CD56+NK cells.Indeed,EBs on uncoated plates can form adherent cells to support attachment and differentiation,making the entire process from stem cells to mature NK cells serum-free and stromal-free which are attractive for clinical applications.67Using these conditions,functional NK cells have been effectively produced from multiple different hESC and iPSC lines.59Recently, we further improved this method by adapting the mouse embryonic fibroblast-dependent hESC/hiPSC to feederfree culture conditions and including Rho-associated protein kinase inhibitor (ROCKi) during EB formation.68
To expand hESC-NK and hiPSC-NK cells to a clinical-grade,a K562-based artificial antigen-presenting cells(aAPCs)expressing membrane-bound IL-21 (mbIL21) have been used.69These aAPCs have been used to propagate clinical-grade PB-NK cells for human trials of adoptive immunotherapy.These conditions can routinely produce >109CD45+CD56+NK cells from 106starting undifferentiated hESCs or hiPSCs.18Since clinical protocols of PB-NK cells or UCB-NK cells typically use does of approximately 107NK cells per kg, in vitro differentiation protocol developed by our group plus expansion system can effectively be used for clinical-grade NK cell production.18hESCNK and hiPSC-NK cells express a typical repertoire of activating and inhibitory receptors found on PB-NK or UCB-NK cells and effectively kill both hematological malignancies and solid tumor cells.16,18More importantly, hESC-NK and hiPSC-NK cells are effective against leukemia and ovarian cancer without any evidence of teratomas or other untoward effects in xenograft tumor models in vivo.17,59,61Additional studies also showed hESC-NK and hiPSC-NK cells both were able to inhibit HIV-infection in vitro and in vivo.70
6.GENETIC MODIFICATIONS OF HIPSC-NK CELLS TO ENHANCE ANTITUMOR FUNCTIONS
Despite the increasing interest on using adoptive NK cell therapy for treating cancers, the efficacy in human trials have been limited especially for solid tumors.40One of the major reasons is the adoptive transfer of primary NK cells or irradiated NK-92 cells demonstrate low persistence in vivo that limits their antitumor efficacy.40,71In addition,the tumor microenvironment could cause NK cell exhaustion and dysfunction through several different mechanisms.72,73For instance, many immunosuppressive cytokines and metabolites such as TGF-β,prostaglandin E2(PGE2), indoleamine 2,3-dioxygenase (IDO), and adenosine produced by tumor cells in the tumor microenvironment could suppress the proliferation and function of NK cells.74-76Moreover, the tumor microenvironment is rich in other host immune cells such as myeloid-derived suppressor cells(MDSCs)and regulatory T cells(Treg cells)that can be activated by IL2 to inhibit NK cell function and limit their antitumor activity.38,74Finally, NK cells without genetic modifications have low or no cytotoxicity against some tumor targets such as B cell lymphoma and choriocarcinoma cell,likely due to lack of ligands for NK cell activating receptors and high expression of inhibitory ligands.Thus, improving NK cell in vivo persistence, overcoming the immunosuppressive tumor environment and potentiating antitumor cytotoxicity through genetic modifications are highly desirable for improving NK cell antitumor activity and developing next generation adoptive NK cell therapy products.71However,as noted, NK cells currently used in clinical trials can be hard to consistently genetically modify (primary NK cells) or have to be irradiated (NK-92 cells) before administration to human.In contrast, hESC/iPSC-derived NK cells are homogeneous and readily able to be genetically modified to provide an ideal cell population to develop standardized immunotherapy products(Fig.1).The following section highlights the strategies are being exploring to enhance antitumor functions of hiPSC-NK cells.
7.ENGINEERING HIPSC-NK CELLS WITH CAR TO ENHANCE TUMOR ACTIVITY
CARs on different source of NK cells such as PB-NK,UCB-NK,and NK92 cells have been recently explored to direct antitumor activity.77,78CAR engineering NK-92 cells targeting a serial of antigens including CD19,CD20,ErbB2(HER2),CD38,EGFR,GD2, and so on have shown promising results in preclinical in vivo models.55In addition,UCB-NK cells engineered with CARCD19, IL-15 and inducible caspase-9 suicide (iC9) gene have shown improved persistence and antitumor activity in a Raji lymphoma mouse models.46These preclinical proof of concepts studies have led to clinical testing of CAR-NK cells for the treatment of both hematological malignancies and refractory solid tumors.79However, these trials all utilize CARs designed for T cells and not optimized for NK cell signaling.
Our group engineered iPSC-NK cells with a novel CAR containing the transmembrane domain of NKG2D, the 2B4 co-stimulatory domain,and the CD3ζ signaling domain to mediate strong antigen-specific NK cell signaling.61This NKCAR was specifically designed to enhance NK cell activity targeting mesothelin-expressing tumors.61,80In an ovarian cancer xenograft model, NK-CAR expressing iPSC-NK cells significantly inhibited tumor growth and prolonged survival in comparison with PB-NK cells, unmodified iPSCNK cells, or iPSC-NK cells expressing T CAR (CD28-41BBζ).Interestingly, in the same in vivo model, NK-CAR expressing iPSC-NK cells showed higher persistence in circulation,spleen,and peritoneal fluid compared with PB-NK cells and iPSC-NK cells at 10 days after intraperitoneal injection.Furthermore,comparison of third-generation T-CAR-expressing T cells to CAR-expressing NK cells in an ovarian cancer xenograft model suggested that CAR-expressing NK cells can exert antitumor effects in a safer way compared with T-CAR-expressing primary T cells.61Using this CAR targeting other antigens is underway.81CAR-targeted hiPSC-NK cells in combining with platform of clinical-scale production of iPSC-derived NK cells are enabling clinical trials for treatment of refractory malignancies.
8.ENGINEERING HIPSC-NK CELLS WITH CD16 TO ENHANCE ADCC
Antibody-dependent cell-mediated cytotoxicity(ADCC)is a key effector mechanism used by NK cells enabling the antitumor effect of therapeutic mAbs.82This process is mediated by CD16a,which binds the Fc portion of antibodies.Interestingly,CD16a is cleaved by the metalloprotease ADAM17 that is produced by both tumor cells and activated NK cells.32,83CD16a has two single nucleotide polymorphism(SNP)variants,the high Fc affinity FcγRIIIa158V and low affinity FcγRIIIa158F.Patients with the CD16a high affinity SNP treated with cetuximab, trastuzumab, or rituximab have been shown to have greater objective response and progression free survival than patients with the low affinity SNP.84-86When NK cells are activated, the CD16a molecule is cleaved from the surface of activated NK cells by ADAM17 expressed on the surface of both tumors and NK cells,leading to reduced ADCC capacity during mAb therapy.32Our group and others previously identified the ADAM17 cleavage site of CD16a and created a non-cleavable version of CD16a by mutating the cleavage site.32By engineering hiPSC-NK cells with to express the high affinity CD16a that also contains the ADAM17-resistant point mutation, a high affinity, non-cleavable version of CD16(hnCD16)can be expressed on ip[SC-derived NK cells to prevent activation induced shedding upon stimulation thus exhibit enhanced ADCC against various tumor targets in vitro and in xenograft mouse models.87The beauty of engineering hiPSC-NK cells with hnCD16 is that the modified NK cells could be combined with essentially any readily available antitumor antibodies and universally targeted to diverse tumors without need to engineer them with CARs-specific to each tumor antigen.Notably,it is also feasible to integrate hnCD16 to CAR expressing hiPSC-NK cells to further improve NK cell functions and possibly target multiples antigens simultaneously by combing with antibodies or CARs.
9.ENGINEERING HIPSC-NK CELLS WITH IL15 SIGNALING TO IMPROVE PERSISTENCE AND FUNCTION
Cytokines such as IL2 and IL15 are key stimulators of NK cell activity, including differentiation, proliferation, activation, and survival.88,89IL2 has been widely used to expand NK cells in vitro and prolong NK cell survival after administration to patients.9However, IL2 can cause s adverse effects such as capillary leak syndrome inpatients.90Also,IL2 activatesT-regcellswhich in turn inhibit NK cell function and decrease its anticancer effect.91IL15 has been shown to stimulate NK cell proliferation and IL-15 levels correlated with in vivo expansion of infused NK cells in patients.9In nonhuman primates,IL15 stimulate NK cell expansion without exerting apparent toxicities.92Notably,IL15 does not activate TReg cells.Thus,IL15 may hold more promise compared with IL2 to support NK cell-based therapies.Several IL-15 products are in clinical development including the IL-15 superagonist (ALT 803).63,93ALT-803 was shown to be well tolerated by patients and was able to promote CD8+T and NK cell expansion in vivo without stimulating Treg cells.94In physiological condition,endogenous IL-15 in serum binds to IL-15Rα to form a natural complexwhich istrans-presented tocells expressing the dimericIL-15β(CD122)/γc(CD132)receptor including NK cells.88,95Upon binding to IL-15β and γc subunits,IL15 activates Janus kinase 1(Jak1)and Janus kinase 3(Jak3),which leads to phosphorylation and activation of signal transducer and activator of transcription 3(STAT3)and STAT5.96
One group created a membrane-bound IL15 (mbIL15) by linking human IL15 gene to that encoding CD8α and transduced to PB-NK cells.97They showed that human NK cells expressing mbIL15 survival and expansion better in vitro and in vivo without exogenous cytokines than unmodified NK cells or NK cells expressing non-membrane bound IL-15.In addition,mbIL15 NK cells exhibited enhanced cytotoxicity against leukemia, lymphoma and solid tumor cells in vitro,and against leukemia and sarcoma cells in xenograft models.97Recently, another group created a different mbIL15 by fusing IL15 and IL15Rαwith a Ser-Gly linker and co-expressed with second generation CAR in T cells using the Sleeping Beauty (SB) system.mbIL15-CAR T cells exhibited improved persistence and antitumor effects in leukemia xenograft models.98Interestingly, the long-term persistent mbIL15-CAR T cells show memory stem-cell phenotype (CD45RO-negCCR7+CD95+).98We and our collaborators engineered hiPSC-NK cells with NK-CAR against CD19 and mbIL15 and showed significantly increased expansion rate in the absence of cytokines in vitro.81
Another way to potentiate cytokine signaling in NK cells is to modulate negative regulators of cytokine signaling.The suppressor of cytokine (SOCS) proteins (CIS; SOCS1-7) are reported to be important negative regulators of cytokine signaling, and CIS has been shown to be a potent inhibitory checkpoint in NK cell-mediated tumor immunity as deletion of Cish in mouse NK cells made them hypersensitive to IL-15 and enhanced cytotoxicity toward tumors.99,100Deletion of CISH in iPSC-NK cells demonstrated CIS could regulate antitumor activity in human NK cells similar but not identical to that in mouse NK cells.101
10.HIPSC-NK CELLS DERIVED FROM UNIVERSAL,HYPOIMMUNOGENIC HIPSC
A major challenge for adoptive cell therapy using stem cell derived products including iPSC-NK cells is the rejection of allogeneic cells by the recipient's immune system.Creating a universal and hypoimmunogenic hiPSC line which is“invisible”to immune system has recently been proposed by several groups to solve this allogeneic rejection problem.102-106To avoid T cell mediated rejection, HLA class I surface expression has been eliminated by knocking out the accessory chain beta-2-microglobulin(B2M).102,106However,knocking out B2M also disrupt the surface expression of non-classical HLA-E and HLA-G,which are required to prevent killing by NK cells.107,108To prevent NK cell mediated rejection,some groups expressed HLAE or HLA-G in B2M knockout iPSC.103,105In addition,immune checkpoint PD-L1 and macrophage“don't-eat me”signal CD47 were employed to create universal and hypoimmunogenic hiPSCs.104,105NK cells derived from the universal, hypoimmunogenic hiPSC line would presumably be resistant to rejection by the recipient's immune system, thus have longer in vivo persistence.One concern for this strategy is deleting HLA might lead to loss of function in NK cells due to disarming.109However,there is currently no report on studies to generate NK cells from these universal, hypoimmunogenic hiPSC lines.
11.CONCLUDING REMARKS
Recent advances in cell-based adoptive immunotherapy have revolutionized cancer treatments.Clinical trials in adoptive NK cell therapy using allogeneic PB-NK or UCB-NK and NK-92 cells have shown to be safe and yield promising results.9-13However,there are some obstacles of using these cells that limits the widespread use of this promising new therapy and development of off-the-shelf, standardized NK-cell products.14,60NK cells derived from human stem cells circumvent these challenges.They can be readily genetically modified on a clonal level and provide a platform to produce uniform and consistent engineered NK cells with improved activity.61More importantly,hiPSC-NK or hESCNK cells can be expanded into clinical-scalable cell population suitable to provide a standardized cellular immunotherapy product that can be used to treat hundreds or thousands of patients.18NK cells genetically engineered with antigen specific CARs, enhanced CD16 signaling and mbIL15 in combination with antibodies targeting checkpoint inhibitors and tumor antigens hold great promise for the future of adoptive NK cell therapy.
杂志排行
血液科学的其它文章
- Successful ex vivo expansion of mouse hematopoietic stem cells
- The role of cholesterol metabolism in leukemia
- Will immune therapy cure acute myeloid leukemia?
- Hematopoietic stem cell metabolism and stemness
- Epigenetic regulation of hematopoietic stem cell homeostasis
- Macrophages in leukemia microenvironment