Assessment of groundwater quality with special emphasis on nitrate contamination in parts of Gautam Budh Nagar district,Uttar Pradesh,India
2019-10-31MeenuAgarwalMeenakshiSinghJakirHussain
Meenu Agarwal·Meenakshi Singh·Jakir Hussain
Abstract Progressive developments in industrial and agricultural activities are causing a critical stress on groundwater quality in developing countries.The objective of this paper is to assess and evaluate the contamination level of groundwater caused by leachate in 11 villages of the Gautam Budh Nagar district in Uttar Pradesh,India.We systematically sampled 22 groundwater samples and 13 leachate samples to ascertain the source of pollution on groundwater quality. The standard analytical methods given by the American Public Health Association(APHA)(Standard methods for examination of water and wastewater, 23rd edn. APHA, AWWA, WPCF, Washington,2017)were used for quantitative estimation of hydrochemical parameters of collected samples.The results of the analysis of groundwater samples indicate that pH values range from 7.31 to 8.97.The mean magnesium concentration in groundwater samples is 58.93±21.44 mg/L.Out of the groundwater samples taken,approximately 41%and 73%of samples analysis results have been found beyond the acceptable limit with respect to the parameters of turbidity and total dissolved solids, respectively,according to the Bureau of Indian Standards(Indian standard specification for drinking water (IS:10500). BIS,Manak Bhawan,New Delhi,2012)for drinking water.Around 95.4%of groundwater samples and 92.3%of leachate samples have high nitrate concentrations above the standard limit of BIS(45 mg/L),respectively.The Piper plot shows that 50%of the samples belong to the Ca2+—Mg2+—HCO3-type.Ternary and Durov's diagrams indicate that the mean concentrations of ions are in the order of Na+>Mg2+>Ca2+>K+ (for cations) and HCO3->NO3->Cl->SO42->CO32->F- (for anions) in groundwater of the study area.The spatial variation of the hydrochemical parameters shows that groundwater is heavily contaminated with respect to nitrate.Analytical results indicate that the groundwater of villages Achheja,Bisrakh road,Dujana,Badalpur and Sadopur is not suitable for drinking.
Keywords Leachate·Hydrochemical parameters·Piper plot·Ternary and Durov's diagram·Spatial variation
1 Introduction
Water is one of the vital elements necessary for the sustainable development of life on earth.Groundwater is an important and crucial part of the hydrological cycle.In India,85%of drinking water and 60%of irrigational water requirements are fulfilled by groundwater(Kumar 2017).In recent times,the contamination of groundwater resources by different point and non-point sources has been assumed as a critical issue.The exponentially increasing rate of urbanization and industrialization brought unacceptable changes in the quality of groundwater(Nowak et al.2012;Nagamani et al.2015).Out of the various pollutants entering into the water aquifers,nitrate is causing the most major environmental issue throughout the world(Wu and Sun 2016;Rahmati et al.2015;Asadi et al.2017) and is considered the most severe groundwater pollutant in agricultural areas.According to the Singh and Singh(2004)report,in a few countries of South-East Asia,Africa and Latin America, groundwater is effectively contaminated with the nitrate due to dry areas.
Nitrate is naturally present in water aquifers in low concentration.However,due to domestic sewage,unplanned agricultural activities and increased use of fertilizers,nitrate concentration in groundwater have been on the rise.Recently various studies in China(Su et al.2013;Li et al.2014;Zhang et al.2018),Africa(Elisante and Muzuka 2017;Daw et al.2018),Italy(Lasagna et al.2016;Ducci 2018),Iran(Rezaei et al.2017)and in many other different parts of world(Ahada and Suthar 2018;Izydorczyk et al.2018)have been carried out to assess the extent of nitrate pollution in groundwater due to agricultural activities.Extensive use of nitrogen-rich fertilizers enhances the leaching of nitrogen and further increases the nitrate level in groundwater(Meisinger and Delgado 2002;Rahmati et al.2015).Most of the time,the nitrate requirement of the crop is much less than the quantity of fertilizers added to it;therefore as a result of this excess,nitrate accumulates in the soil(Schepers et al.1991).The abundance and distribution pattern of high nitrate concentration in groundwater in India was studied by several researchers.These studies reflect the anthropogenic and geogenic source of nitrate pollution. Rao (2006) studied the vertical and lateral leaching of nitrate in groundwater of the Srikakulam district of Andhra Pradesh and the nitrate concentration in samples was detected up to 450 mg/LL.The lateral and vertical movement of nitrate is governed by the geological pattern of clay and sand in that particular area.Studies conducted in the arid or semi-arid area in the Thar Desert of India showed that the largest amount of NO3—N(7.10—82.0 mg/L)in groundwater samples is due to the impact of agricultural activities(Suthar et al.2009).From the analytical results made by the Central Ground Water Bureau(CGWB 2010),the occurrence of nitrate concentrations beyond the standard limits according to BIS(45 mg/L)in groundwater samples obtained from various districts of the states in India are shown in Fig.1.The results of the study concluded that nitrate contamination in groundwater is in a very critical stage in states such as Andhra Pradesh,Haryana,Karnataka,Madhya Pradesh,Maharashtra,Orissa,Punjab,Rajasthan and Uttar Pradesh(Table S1)(in the supplementary material).It is documented by several studies made in North India that the rural areas with a large number of livestocks showed more nitrate pollution than urban areas(Singh and Sekhon 1976).
BIS gives the standard limit of 45 mg/L(as NO3-)for safe drinking water. WHO sets the standard value of 50 mg/L as nitrate ion(equivalent to 11.3 mg/L as nitratenitrogen)in drinking water.The standard concentration of nitrate in drinking water as recommended in several countries is given in Table 1.Epidemiological studies have shown the absence of negative health effects(methaemoglobinaemia and thyroid effects)with a nitrate concentration below 50 mg/L in drinking water(Pfnader et al.1993).
The main objective of the current research is to determine the suitability of groundwater for drinking purpose and to analyze the impact of leachate on groundwater quality.
2 Study area
2.1 Locations,climate and land uses
The present study has been carried out in the Gautam Budh Nagar district of Uttar Pradesh in India.The total geographical area of the district is 1442 km2.The study area is located between 28°56′to 28°63′N latitude and 77°47′to 77°54′E longitude.The overall population of this district(as on Census 2011)is 1,648,115.Out of this rural population is 40.9%and urban population is 59.1%.The district Gautam Budh Nagar has three tehsils and four blocks:Bisrakh,Dadri,Dankaur and Jewar.The climate of this area is sub-humid.This part of the country comes under the Yamuna Sub-basin.The major river of this area is the Yamuna and its tributary rivers:Hindon river and Bhuriya Nadi.Hindon originates from Pur Ka Taanda (district Saharanpur)and joins river Yamuna in village Momnathal outside of Delhi.The catchment area of the Hindon River is 7083 km2and it is a monsoon-based river.The average monthly temperature ranges from 32.85°C in the month of June to 14.2°C in the month of January.The average annual wind speed in the district is 6.7 km h-1.The annual rainfall of the district is 700.6 mm.The maximum rainfall occurs from June to September.The month of August gets a maximum rainfall up to 205.8 mm.Relative humidity is also very high during the monsoon season.A major part of the land in the district is used for agriculture(67.93%)and 1.4%covered by forest(Joshi 2009).A large number of small and big industries are present in the Chhapraula Industrial Area of Bisrakh Block.Dense industrial setup in an unplanned manner is responsible for the groundwater pollution of this area(Kumar et al.2017).
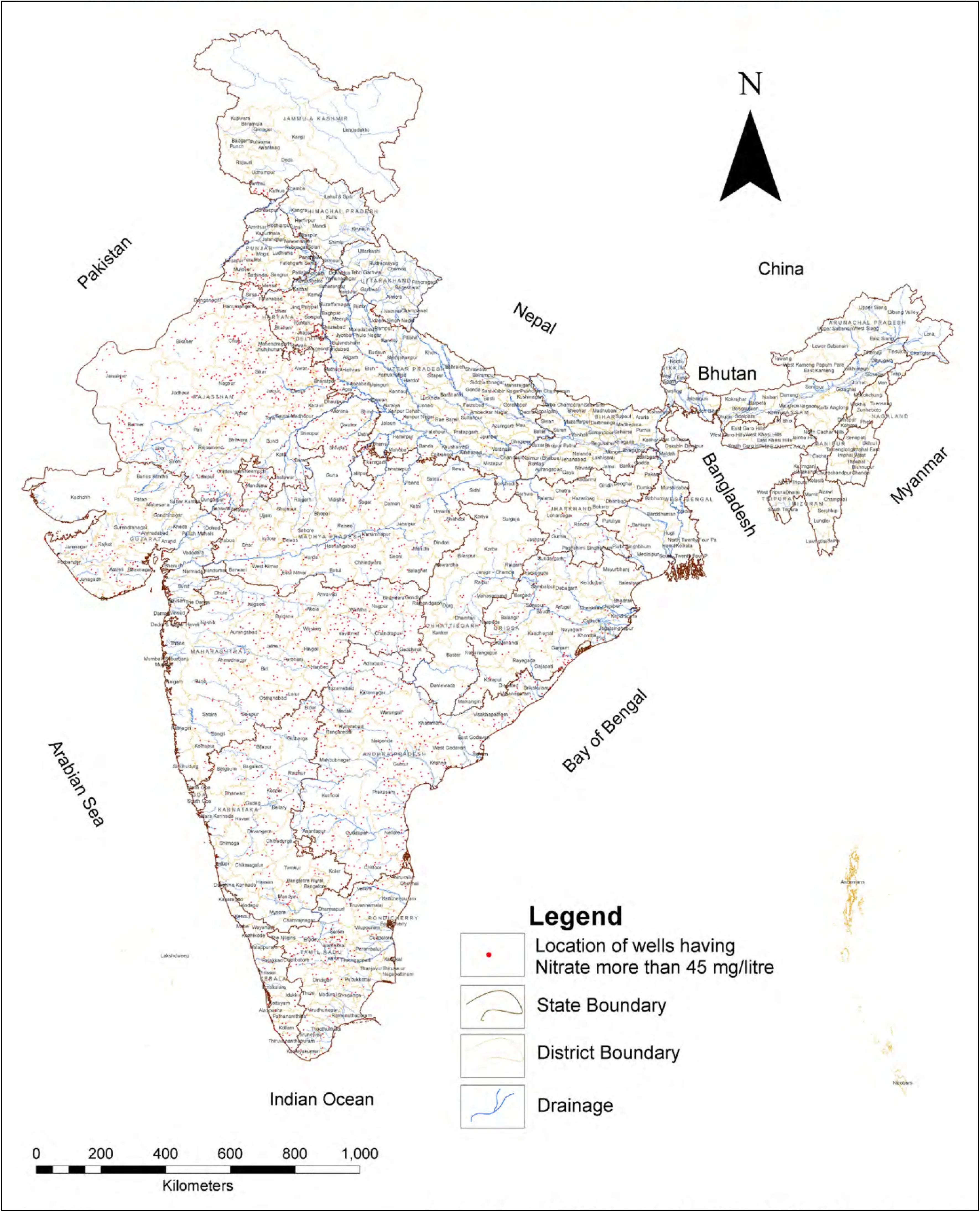
Fig.1 Nitrate in ground water >45 mg/L(Source:CGWB,Ministry of WR,RD&GR)
2.2 Geology
The Gautam Budh Nagar district comes under the Indo-Gangetic alluvial plain of Northwest India.It is a flat land with no hilly areas.The area is divided into floodplain,upland and land adjacent to Petawata.The soil type of the study area is fine loam and sandy loam(bhur,matir and dumat).Bhur is pure sand and Matir is pure clay.Dumat is a mixture of sand and clay,which is a fertile soil.The alluvium is deposited by sand,silt,and clay in gravels of different sizes and is loose or unconsolidated in nature.Most of the part of the district(upland plain)has alluvial soil and is highly fertile(Chaudhary et al.2012).
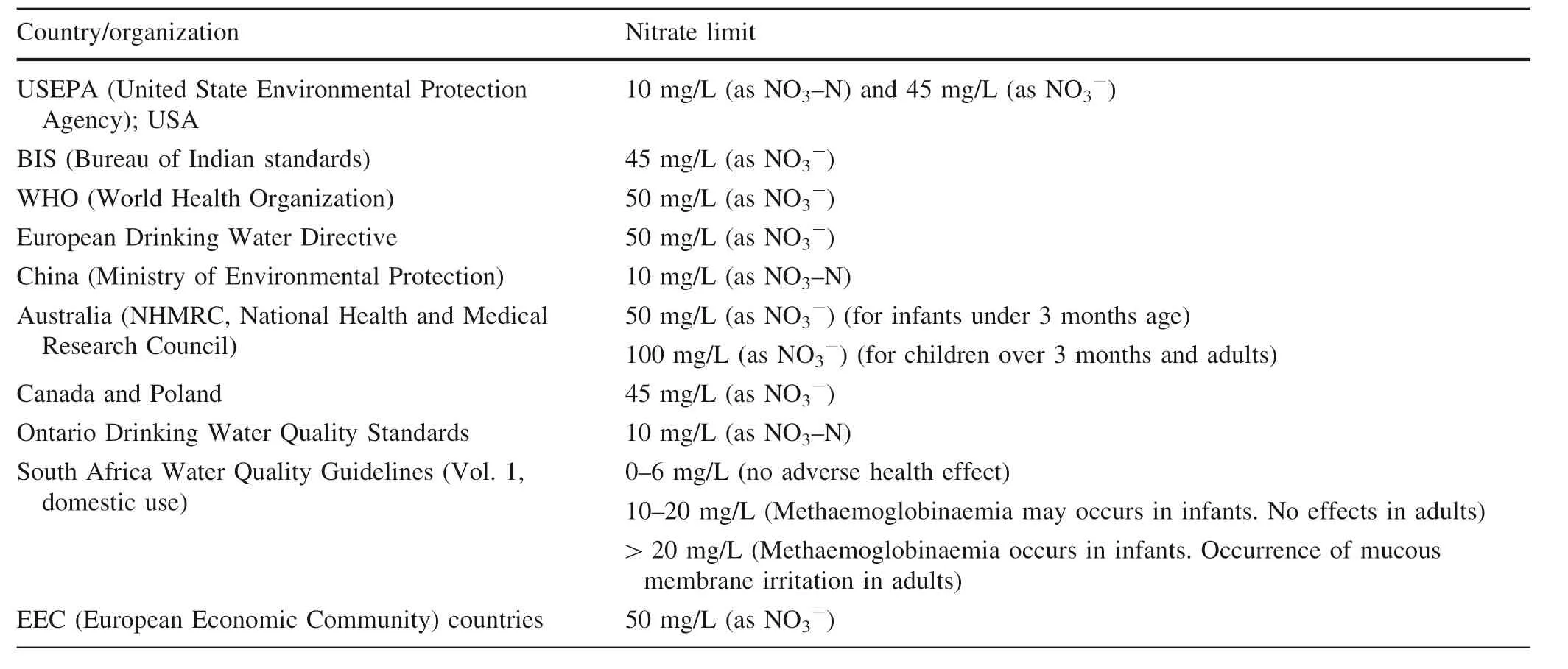
Table 1 Standard nitrate limit for drinking water in different countries
3 Materials and methods

Fig.2 Study area location
Groundwater and leachate samples were collected in June 2017.A total of 35 samples(groundwater S1—S22 and leachate L1—L13)from different villages namely Achheja,Badalpur, Badhpura, Bisrakh road, Bishnuli, Dhoom Manikpur, Dairy Maccha, Duryai, Dujana, Khera Dharampura,Sadopur and Talabpur were collected by a simple randomization method from different groundwater resources like hand-pumps, tube-wells, bore-wells and drains(Fig.2).The standing water was left to run out from the source before collecting the groundwater samples.Polyethylene bottles of 1-litre capacity were used to collect the water samples.The standard analytical methods given by the APHA,23rd editions(2017)were followed for the collection,preservation and analysis of samples.Standard methods of analysis of various parameters of water quality were employed to determine pH (APHA 23rd edn.2017-4500H+B),turbidity(APHA 23rd edn.2017—2130B)and electrical conductivity (APHA 23rd edn.2017—2510B).Total dissolved solids(TDS)was determined through gravimetric analysis(dried at 180°C).Total hardness(calcium and magnesium hardness)waere analysed by EDTA titrimetric method (APHA 23rd edn.2017—2340C). Total alkalinity and chloride (Cl-) was analysed through acid—base titration and argentometric titration respectively.The potentiometric method was used to determine the concentration of fluoride(F-),nitrate(NO3-)and ammonia(NH3).For fluoride analysis,solidstate combination electrode(9609 BNWP)and for nitrate analysis,liquid membrane electrode(9307 BNWP)was used.Sodium(Na+)and potassium(K+)concentration were determined by flame emission photometry by using the SYSTRONICS-128 flame photometer(APHA 3500 Na-B and 3500 K-D).Sulfate was analysed by a turbidimetric method using a spectrophotometer(Varian BIO-100).Certified reference materials were used to make standard solutions for the analysis.
The precision of analytical results was confirmed by comparing the concentration of the total cations(Ca2+,Mg2+,Na+,and K+)and the total anions(,,F-and Cl-)for each water sample.The quality of results was verified by using ionic balance equation.

The calculated error percentage(E)for each analysed water sample was±5%.
Contour maps(to describe the spatial variation),ternary and Durov's diagram of collected samples were plotted by using software Surfer 11.The data generated from the analysis of groundwater samples were plotted on a Piper trilinear diagram using AqQA Rockware Software to assess the hydrochemical facies of the groundwater of the study area.
4 Results and discussion
4.1 Groundwater chemistry
The analysis results obtained for all groundwater samples and leachate samples are given in Tables 2 and 3,respectively.The statistical summary of different hydrochemical parameters for groundwater and leachates samples presenting the mean,minimum,maximum,median and standard deviation values are given in Tables 4 and 5 respectively.The pH values of the analysed groundwater samples range from 7.31 to 8.97 with a mean value of 7.88,indicating slightly alkaline nature of the groundwater.The prescribed standard limit of pH in drinking water(BIS:10500 2012;WHO 2004)is 6.5—8.5.Earlier,Singh and Tripathi(2016)reported pH ranges between 7.1 and 7.9 in the groundwater samples collected from NCR(National Capital Region)area.The pH value of the leachate samples varies from 5.87 to 9.31.One sample(L3)shows a pH value(5.87)less than standard range and samples L8,L9 and L10 have pH values higher than the standard limit,which signifies the anthropogenic sources.The sample S10 and L10,which were collected near a fabric dyeing unit,have higher pH values.This may be due to the release of chemicals containing(like caustic soda,soda ash,sodium silicate etc.)effluent water from various stages of the dyeing process(desizing,kiering,bleaching,mercerizing etc.)into the surrounding water bodies(Ghaly et al.2014).The water becomes turbid due to the occurrence of suspended impurities in it such as sand,silt,organic and inorganic substances.The turbidity value ranges from 0 to 48 NTU for groundwater samples and 16—691 NTU for leachate samples.Around 41%of groundwater samples have a turbidity value higher than the permissible value of BIS i.e.1 NTU.Groundwater samples S1,S2,S10 and S14 have very high values of turbidity—48,43,28 and 14 respectively;indicating the presence of suspended materials in groundwater.These suspended materials in groundwater originate from the percolation of leachate coming from the dumping of domestic,agricultural wastes and effluents from various industries in the study area.The electrical conductivity(EC)of groundwater,in the study area,varies greatly from 538 to 2102 μS/cm.The EC values for leachate samples range from 997 to 4354 μS/cm.A wide variation in the EC values of groundwater reflects that various natural and human activities are affecting the recharge of groundwater.Total dissolved solids(TDS)is the sum of dissolved ionic concentration.Its value in the groundwater samples varies from 350 to 1366 mg/L,with a mean value of 837 mg/L.This is somewhat lesser than the obtained range(507—2949 mg/L)from the report of Singh and Tripathi(2016)for the NCR region.All the samples have TDS values below the BIS permissible limit of 2000 mg/L but 73%of samples have been found above the acceptable limit of 500 mg/L.The entire study area is alluvial plain.Alluvial deposits contain various types of minerals.Dissolution of these minerals into groundwater enhances the TDS of groundwater of this area.In the alluvial plains of the Yellow River in northwest China,TDS ranged from 414 to 1576 mg/L(Li et al.2014).The TDS value of leachate samples ranges from 648 to 2830 mg/L.The ammonia concentration in the groundwater samples ranges from 0.05 to 22.9 mg/L,with a mean value of 4.85±7.40 mg/L.Around 36%of the groundwater samples and 84.6%of the leachate samples have ammonia concentration above the standard limit of 0.5 mg/L(BIS 2012).
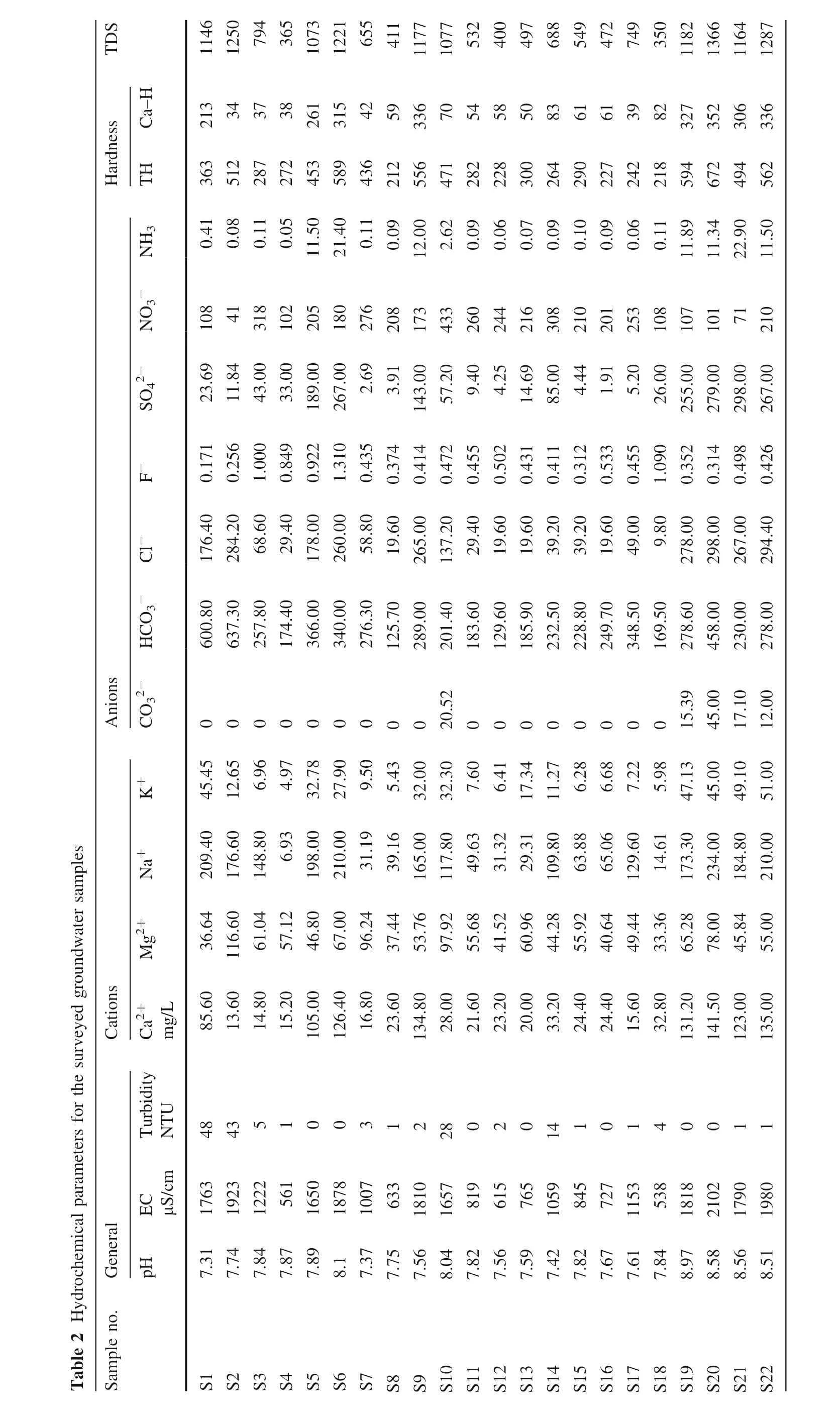

The hardness of water is due to the dissolved Ca2+,Mg2+,,and Cl-ions.Consumption of hard water has no ill effect on health,it only creates a nuisance;however prolonged use of hard water can cause urolithiasis.The total hardness(TH)of water(as CaCO3)ranges from 212 to 672 mg/L,with a mean value of 388 mg/L in the groundwater samples.All the samples show TH above the acceptable limit of BIS i.e.200 mg/L.One sample S20 has a high value of TH(672 mg/L),which is higher than the permissible limit of BIS(600 mg/L).Samples having TH less than 75 are considered as soft water,75—150 moderately hard,150—300 hard and greater than 300 very hard.Accordingly,50%of samples have hard water and the remaining 50%of samples have very hard water.The TH of leachate samples lies between 243 and 903 mg/L.69%leachate samples have high TH above the standard limit of WHO(1993)i.e.500 mg/L.A very high value of TH in groundwater samples S5,S6,S7 and nearby collected leachate sample L5 in the village Achheja indicate the common source of pollution in this area.Similarly leachate samples L8 and L9,which were collected near the source of groundwater samples S9 and S10,also have high hardness values.Several small industries are running in villages Achheja,Dujana,Badalpur and Sadopur and the effluent of these industries are either dumped directly in ground aquifers or allowed to flow in drainage.Leaching of this water leads to the contamination of ground aquifers.The calcium hardness(as CaCO3)values range from 34 to 352 mg/L in the groundwater samples.Leachate samples have calcium hardness value from 63 to 565 mg/L.
4.2 Cation chemistry


Major cations in water samples are alkali metals i.e.sodium and potassium and alkaline earth metals i.e.calcium and magnesium.These metals are present naturally in aquifers in significant quantities.The presence of a considerable amount of these metal ions is due to the geological conditions of the study area.The residence time of groundwater among the rocks is very high due to the gentle slope of the study area.It leads to a greater degree of dissolution of mineral-rich rocks.But the occurrence of very high values of cation concentration signifies the anthropogenic source for these metals.The observed concentration of calcium and magnesium ions in groundwater samples varies from 13.60 to 141.50 mg/L and 33.36 to 116.6 mg/L,respectively.36%of the samples have calcium concentrations above the acceptable limit of BIS(75 mg/L)but below the permissible limit of BIS(200 mg/L).The Mg2+concentrations in all the groundwater samples are above the acceptable limit of BIS i.e.30 mg/L.A range of 55 to 269 mg/L for Ca2+and 18 to 137 mg/L for Mg2+was reported in the groundwater of the Noida region of the Gautam Budh Nagar district in pre-monsoon season(Singh et al.2011).The concentration of Ca2+and Mg2+in leachate samples ranges from 25.2 to 226.9 and 36.24 to 123 mg/L respectively. All the leachate samples have magnesium content above the acceptable concentration of BIS(30 mg/L).From the alkalies,the concentration of sodium and potassium ions in groundwater samples ranges from 6.93 to 234.0 and 4.97 to 51.0 mg/L,respectively.The concentration of sodium in 18%of samples is above the standard value of WHO for Na+(200 mg/L).The concentration of sodium and potassium in leachate samples lies between 88.82 to 720.8 and 9 to 138.1 mg/L,respectively.
4.3 Anion chemistry
In groundwater samples,the concentration of bicarbonate varies from 125.7 to 637.3 mg/L.Only five samples of groundwater show the occurrence of carbonate ranges from 12.0 to 45.0 mg/L.The bicarbonate concentration in leachate samples lies in between 224.4 and 762.9 mg/L.Chloride concentration in the groundwater samples varies from 9.8 to 298.0 mg/L.32%of the groundwater samples exceed the acceptable limit of 250 mg/L of chloride in drinking water but all the samples are below the permissible limit of chloride 1000 mg/L(BIS).Leachate samples have chloride concentration from 68.60 to 450.0 mg/L.Sulfate concentration in groundwater samples ranges from 1.91 to 298.0 mg/L.Groundwater samples S6,S19,S20,S21 and S22 have sulfate concentrations higher than the acceptable limit of BIS i.e.200 mg/L but all the samples are well below the standard limit of 400 mg/L as per BIS and WHO.A range of 11 to 837 mg/L of sulfate was reported by Singh et al. (2011) in parts of Noida metropolitan city.Presence of excess of sulfate in drinking water imparts an unpleasant taste to it and can cause gastrointestinal disorders.Sulfate-rich water can corrode the metal pipeline of the water distribution system.Sulfate concentration in leachate samples lies in between 3.11 and 883.9 mg/L.All the samples except one(L10)lie below the standard limit of sulfate.Fluoride value in groundwater samples varies from 0.171 to 1.310 mg/L.All the samples are within the permissible limit of fluoride 1.5 mg/L(BIS)and 91%of the samples show fluoride values below the desirable limit of 1.0 mg/L in drinking water.Fluoride concentration in leachate samples ranges from 0.40 to 1.31 mg/L. The nitrate concentration in groundwater samples ranges from 41 to 433 mg/L,with a mean of 196.96 mg/L.95%of groundwater samples and 92%of leachate samples have nitrate concentration above the standard limit of 45 mg/L(BIS).A study made by Singh et al.(2013)in Bathinda district of Punjab showed that 33%in pre-monsoon(nitrate concentration up to 83 mg/L)and 45%in post-monsoon(nitrate concentration up to 90 mg/L)of groundwater samples were not suitable for drinking.Ahada and Suthar(2018)reported a range of 38.45 to 198.05 mg/L of nitrate concentration in Malwa district of Punjab.On the basis of Cl concentration and NO3/Cl ratio,they suggested the multiple anthropogenic sources of nitrate to groundwater.The major origin of nitrate in groundwater is anthropological activities.The study area is densely populated and as a result of poor sanitation conditions,a large proportion of nitrogenous wastes from human and animal excreta mix in the leachates.The open dumping of animal excreta is very likely to be seen in this area.Due to wind and rainwater,this waste goes into the soil layers and finally mixes with leachate.In North America,the proportions of waste entering into groundwater are approximately 40%from animals,30% from crop residues and 20—25% from municipal wastes out of the total waste(Power and Schepers 1989).The application of nitrate-rich fertilizers(urea,diammonium phosphate,calcium ammonium nitrate etc.)enhances the percentage of nitrate in leachate.The natural origin of nitrate in groundwater is the nitrification of ammonia(from fertilizers)by microbes.The percolation of this nitrate-rich water over a period of time,increases the concentration of nitrate in groundwater.The soil of Talabpur,Duryai and Dujana villages is quite rich in organic matter due to extensive agricultural practices in this area.Dudley(1990)has suggested that slow leaching of nitrate derived from organic matter in soil can lead to a steady rise in the nitrate concentration of groundwater over a long period.In these villages,farming is traditional and nitrogen fertilizers such as urea and ammonium sulphate are used in small amounts on a regular basis.Many farmers also use manure to increase crop production.Samples collected from these villages show the high nitrate concentration in groundwater.To reduce the leaching of soil nitrate,Hosseini et al.(2018)suggested a drip irrigation system in place of irrigation through tube-wells or bore-wells.Consumption of nitrate-rich water over a long period of time causes toxicity in humans.The high concentration of nitrate in drinking water affects the functioning of the cardiovascular system and central nervous system in adults.But a low concentration of nitrate in drinking water affects the oxygen carrying ability of blood and causes methemoglobinemia or blue baby syndrome in infants.Nitrate itself is not toxic to the human body.A dose of 9 mg/day of sodium/ammonium nitrate can safely be given to a person to cure phosphatic kidney disorders;but in human body nitrate is reduced into nitrite causing an adverse effect. Nitrite combines with the haemoglobin,resulting in the formation of methemoglobin, which reduces the oxygen-carrying capacity of blood. Freshwater invertebrates are also affected by nitrogen present in the aquatic environment.A maximum limit of 2 mg/L NO3—N was recommended by Camargo et al.(2005)for freshwater invertebrates and 20 mg/L NO3—N for marine creatures.Nitrate toxicity in aquatic animals increases with the increase of exposure time and an increase of nitrate concentration in water.Increase in body size of aquatic animals and an increase in salinity of water lower the toxic effects of nitrate(Camargo and Ward 1995).
A ternary graph for cations and anions(Ca2+,Mg2+,Na+,)in groundwater and leachate samples,collected from the study area,was plotted to explain the quality of water(Figs.3,4)(Buccianti and Pawlowsky-Glahn 2005).Most of the groundwater samples lie towards the Na+and Mg2+(for cations)and NO3-(for anions)apex.A similar type of pattern is observed for cations in leachate samples where most of the leachate samples lie towards the Na+and Mg2+apex.Groundwater samples(S1 and S2)and leachate samples L1,L2 and L3 collected from village Badhpura and Dhoom Manikpur shows high concentrations of chloride.The analytical results indicate the leaching of chloride rich water.The water groups are dominated by bicarbonate ion.This is clearly demonstrated by the Durov diagram of groundwater samples in Fig.5(Durov 1948).The descending order of the mean concentration of cations and anions (in mg/L) in groundwater samples are Na+>Mg2+>Ca2+>K+and>respectively.
4.4 Hydrochemical facies
The hydro-geochemical pattern of the area was studied by plotting the concentration of cations and anions(Ca2+,Mg2+,Na+,K+,CO32-,HCO3-,SO42-,and Cl-)in milliequivalents per liter on the Piper trilinear diagram(Fig.6).This plot contains two triangles(cations plot and anions plot).The cations and anion plots are combined in a diamond-shaped plot,which gives the hydrogeochemical facies of the study area.The Piper plot is more useful to explain the composition of water than any other plotting method.Hydrochemical facies are distinct zones that possess cation and anion concentration categories (Piper 1944).The Piper diagram of the groundwater samples explains the association and variation among different kinds of groundwater in the study area.The diamond field of the Piper plot is divided into four sub-fields:(1)Ca2+—Mg2+—Cl-—SO42-(2)Na+—K+—Cl-—SO42-(3)Na+—K+—HCO3-,and(4)Ca2+—Mg2+—HCO3-.Around 50%of water samples come into the Ca2+—Mg2+—HCO3-sub-field,36.4%comes in Ca2+—Mg2+—Cl-—SO42-sub-field and the remaining 13.6%comes under Na+—K+—HCO3-sub-field.The sub-field Ca2+—Mg2+—Cl-—SO42-contains most of those samples,which have high values of TDS and EC.For cation concentrations,the Ca—Mg type(86.4%)and Na—K type(13.6%)of water dominate in the study area.On the basis of anion concentrations,the groundwater found is the HCO3-type (63.6%) (Table 6). For Cl-, SO42-, and HCO3-concentrations,the groundwater sources can be classified into normal chloride (less than 15 meq/L),normal sulfate(less than 6 meq/L),and normal bicarbonate(2—7 meq/L) water types (Soltan 1998). 86.4% of groundwater samples are of normal bicarbonate type,95.5%of the samples is of normal sulfate type and all the samples show the normal chloride type of water in the study area.

Fig.3 Ternary plots showing cation and anion composition in groundwater samples of study area

Fig.4 Ternary plots showing cation and anion composition in leachate samples of study area

Fig.5 Durov's diagram demonstrating the HCO3-type of water
4.5 Spatial distribution pattern of hydroparameters in groundwater
The contour maps for the spatial distribution of selected hydrochemical parameters are shown in Fig.7.Darker points on the map indicate the higher concentration and lighter points show the lower concentration of that particular parameter.The northeast part of the study area is the agricultural area.The distribution pattern of pH in thestudy area clearly shows the presence of alkaline water.A dark zone in the northeast part signifies the high pH of groundwater in that area.Contour mapping of pH clearly indicates that the groundwater of the villages Achheja,Dujana and Badalpur is highly alkaline(Fig.7a).Higher electrical conductivity is also observed in the same part of the study area(Fig.7b).The distribution pattern of nitrate signifies that the groundwater of the northwest part is polluted(Fig.7c).The pH,EC,ammonia,fluoride and sulfate show the same pattern of distribution although their concentrations differ with each other(Fig.7a,b,d,e,f).A similar type of distribution pattern indicates the same source for these parameters in groundwater.The spatial distribution of hydrochemical parameters of groundwater shows greater contamination in the central part of the study area.The heavy industrial setup in the northwest part and the dense population in southwest part may be the cause of this pattern.The source of nitrate in groundwater is probably due to the leaching of agricultural wastes and nitrification of nitrogen excreted by humans and to a lesser extent by animals inside the settlement.Most of the residents of the study area use septic tanks for the disposal of
human excreta.The leakage of nitrogenous wastes via septic systems and sewerage system increased the nitrate concentration in groundwater.The northwest part of the study area can be considered as the principal source of contamination of groundwater.
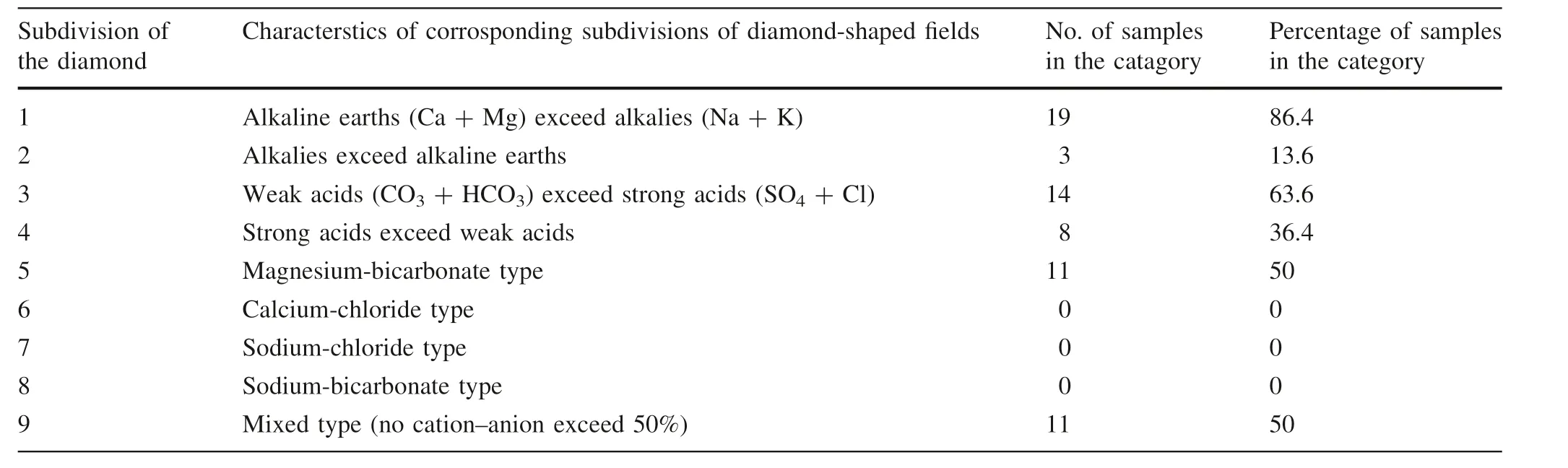
Table 6 Characterization of groundwater samples on the basis of Piper trilinear diagram

Fig.7 Spatial distribution maps of selected hydrochemical parameters in groundwater samples a pH distribution,b electrical conductivity distribution,c nitrate distribution,d ammonia distribution,e fluoride distribution and f sulfate distribution
5 Conclusion
The analytical results of hydrochemical parameters of groundwater of the Gautam Budh Nagar district revealed that the groundwater of this area has been contaminated to a large extent.The pH value of groundwater samples ranges from 7.31 to 8.97 with a mean of 7.88,which shows a slightly alkaline nature of water.Forty-one percent of groundwater samples have a turbidity value higher than the acceptable limit of BIS(1 NTU).Total hardness of all the groundwater samples is above the desirable limit of BIS(200 mg/L).100%of samples exceed the acceptable limit of Mg2+in groundwater samples.Na+and K+concentrations are also high in groundwater samples.18%of groundwater samples are above the standard limit of WHO for Na+.The hydrochemical study of groundwater shows the HCO3-type of water,while the major cations are Ca2+and Mg2+.The Piper plot shows that groundwater of study area is Ca2+—Mg2+—HCO3-type and alkaline earth metal ions exceed alkali metal ions.All the samples show fluoride concentrations within the standard range of 1.5 mg/L.The major findings of the current study are:
· The average nitrate level in the groundwater of the study area(196.96±93.18 mg/L)is much higher than the standard limit of 45 mg/L.
· The groundwater samples collected from villages Dairy Machha,Khera Dharampura,Bisrakh road,Duryai and Bishnuli have nitrate values higher than 250 mg/L.
· The extensive use of fertilizers and disposal of wastes containing high N content are the main sources of nitrate in the groundwater of this area.
· The overall hardness of groundwater of the study area reflects geological origin,but exceptionally high value of hardness in groundwater and leachate samples of village Achheja and Bisrakh road indicate leaching is dominant at these places.
The overall quality of groundwater in the study area is poor.Particularly groundwater of villages Achheja,Bisrakh road,Dujana,Badalpur and Sadopur is heavily contaminated. Results of the study indicate that the groundwater of these villages is unfit for drinking purpose.The spatial variation of various hydrochemical parameters indicates that the monitoring of the impact of the surrounding environment on groundwater quality along the industrial belt is essential for the health of residents in the study area.
Compliance with ethical standards
Conflict of interestOn behalf of all authors,the corresponding author states that there is no conflict of interest.
杂志排行
Acta Geochimica的其它文章
- The Magma Engine and subduction initiation
- Influence of the biological carbon pump effect on the sources and deposition of organic matter in Fuxian Lake,a deep oligotrophic lake in southwest China
- Geochemistry and sediment in the main stream of the Ca River basin,Vietnam:weathering process,solute-discharge relationships,and reservoir impact
- The mixing of multi-source fluids in the Wusihe Zn-Pb ore deposit in Sichuan Province,Southwestern China
- Spatial prediction of landslide susceptibility using GIS-based statistical and machine learning models in Wanzhou County,Three Gorges Reservoir,China
- Fluid properties and sources of Sixiangchang carbonateassociated mercury deposit,southwest China
