A new species of the genus Xenophrys(Anura:Megophryidae)from northern Thailand
2019-10-31Yun-HeWu,ChatmongkonSuwannapoom,NikolayA.PoyarkovJr.等
DEAR EDITOR,Species of Xenophrys are conserved morphologically and live primarily in forests. In Thailand, the genus harbors many cryptic species.Herein we report the collection of specimens from Doi Inthanon,Chiang Mai Province,northern Thailand,which were identified previously as X.minor.Molecular and morphological analyses find that these specimens differ significantly from other known congeners,and therefore we describe a new species.Further,our phylogenetic analyses indicate that X. latidactyla is a junior synonym of X.palpebralespinosa.
The Indo-Burma and Sundaland biodiversity hotspots span Thailand and host a high diversity of amphibian species(Myers et al.,2000).In Thailand,at least 32 of 193 species of amphibians appear to be endemic(Frost,2019).Some recent studies of Megophrys sensu lato, Leptobrachella and Fejervarya have shown that the diversity of amphibians in Thailand is underestimated (Chen et al., 2017, 2018;Suwannapoom et al., 2016). Further, 10 new species of amphibians have been discovered in the past three years alone(Frost,2019;Pawangkhanant et al.,2018;Poyarkov et al.,2018;Suwannapoom et al.,2018).Thus,the extensively rich amphibian diversity in Thailand appears to remain underestimated. Like many other regions, high rates of deforestation owe to increased development (Royal Forest Department,2006)and this drives a high risk of extinction even before the discovery of new species.
Genus Xenophrys Günther, 1864 (family Megophryidae)occurs in the southern and eastern Himalayas,Indochina,and northward to the Qinling and Huangshan mountains of mainland China (Chen et al., 2017). Of the 66 species,Thailand has seven species only:X.aceras,X.lekaguli,X.longipes,X.major,X.minor,X.parva and X.takensis(Chanard, 2003; Mahony, 2011; Nutphund, 2001; Stuart et al.,2006).Xenophrys minor Stejneger was described originally from Kwanghsien (now Guan Xian, Dujiangyan City),55 kilometers northwest of Chengtu (Chengdu), Szechwan(Sichuan),China.Several researchers recorded it for montane areas of northern Thailand(Chan-ard,2003;Chuaynkern&Chuaynkern,2012;Nabhitabhata et al.,2000;Nabhitabhata and Chan-ard,2005).Several recent publications revised the X.minor complex(Li et al.,2014;Mahony et al.,2013;Wang et al., 2012). Further, the type locality occurs in Sichuan,China and a distance of over 1 500 km separates them from the known localities in northern Thailand.Thus,the taxonomic status of Thai populations previously referred to as X.minor requires further investigations.
Our recent fieldwork in Thailand resulted in the collection of specimens of Xenophrys cf."minor"from Chiang Mai Province(Doi Inthanon). Further phylogenetic analyses of mtDNA sequences and morphological examinations showed that this species is distantly related to X.minor from China and can be distinguished from all known congeners both by molecular and morphological characters. Based on an integrative taxonomic approach,we describe this population as a new species of the genus Xenophrys.
Six individuals were collected from Doi Inthanon,Chiang Mai Province, Thailand in August 2017 (Figure 1). After specimens were euthanized using benzocaine, liver tissue was taken and preserved in 95%alcohol.The specimens were fixed with 10%formalin for 24 hours and subsequently transferred to 70%ethanol.All specimens were deposited in the herpetological collection of the Museum of the Kunming Institute of Zoology (KIZ), Chinese Academy of Sciences(CAS)and University of Phayao(AUP).Six preserved adult specimens were examined and measured to the nearest 0.1 mm using digital calipers.Morphological terminology followed Fei et al.(2009)and Poyarkov et al.(2017)(Supplementary Methods).
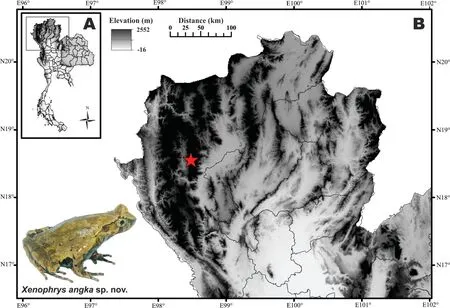
Figure 1 Known distribution of Xenophrys angka sp.nov.from Doi Inthanon,Chiang Mai Province,Thailand
Whole genomic DNA was extracted,and a partial fragment of the mitochondrial 16S rRNA were amplified and sequencing.DNA extraction,primers and PCR cycle protocols are in Supplementary Methods.Matrilineal genealogies were reconstructed to study the phylogenetic relationships among Xenophrys based on the partial mitochondrial 16S rRNA gene.Homologous sequences of related species of Xenophrys,and those of the outgroups Leptobrachella oshanensis, L.ventripunctata, Leptobrachium boringii, Megophrys nasuta,and M. baluensis, were downloaded from GenBank(Supplementary Table S1).Trees were reconstructed using maximum likelihood (ML) and Bayesian inference (BI)(Supplementary Methods).Apart from tree-based methods,we also calculated row pairwise sequence divergence using uncorrected p-distances implemented in MEGA v6.0.6(Tamura et al.,2013).
The results of the ML and BI analyses yielded essentially identical topologies with relatively high nodal support values for most terminal nodes (Figure 2). The tree resolved monophyly of Xenophrys with two major lineages,corresponding to the subgenera Xenophrys sensu stricto and Panophrys(Figure 2).Xenophrys cf."minor"from Chiang Mai Province assigned to Panophrys and phylogenetic relationships within which remained essentially unresolved.The Chiang Mai population of Xenophrys cf."minor"was a strongly supported lineage(BPP=1,BS=100;Figure 2),which different notably from X. minor sensu stricto from China(Figure 2). Genetic distance between the Chiang Mai population and other species of Xenophrys ranged from 4.5%(X. rubrimera, subgenus Panophrys) to 14.4% (X.mangshanensis,subgenus Xenophrys)(Supplementary Table S2).The tree nested X.latidactyla within the radiation of X.palpebralespinosa with high support (BPP=1.0, BS=94)(Figure 2).The genetic divergence between X.latidactyla and X.palpebralespinosa was 0.9%.
The Chiang Mai population of Xenophrys cf."minor"differs in a number of taxonomically important diagnostic characters from other congeners,including X.minor from China.Thus,both mtDNA and morphological analyses clearly indicate that this population represents a separately evolving lineage and an undescribed species,which we describe below.
Taxonomic account
Xenophrys angka sp.nov.
Figures 3-4;Table 1.
Chresonymy:Xenophrys minor(partim)—Chan-ard,2003;Nabhitabhata and Chan-ard,2005;Nabhitabhata et al.,2000;Chuaynkern and Chuaynkern,2012.
Holotype:KIZ040591,an adult female collected from Kiew Mae Pan nature trail in Doi Inthanon,Chiang Mai Province,Thailand(N18.556187°,E98.482229°;elevation 2 190 m a.s.l.), collected by Chatmongkon Suwannapoom, Parinya Pawangkhanant and Nikolay A.Poyarkov on 29,August,2017.
Paratypes:Two males KIZ040595 and AUP-00077, three females KIZ040592,AUP-00076 and AUP-00055;collected at the same locality and same collection information as the holotype.
Etymology:The specific epithet"angka"is given as a noun in apposition and refers to the name of the highest mountain of Thailand,Doi Angka,located in the Doi Inthanon,Chiang Mai Province,Thailand,where the new species occurs.
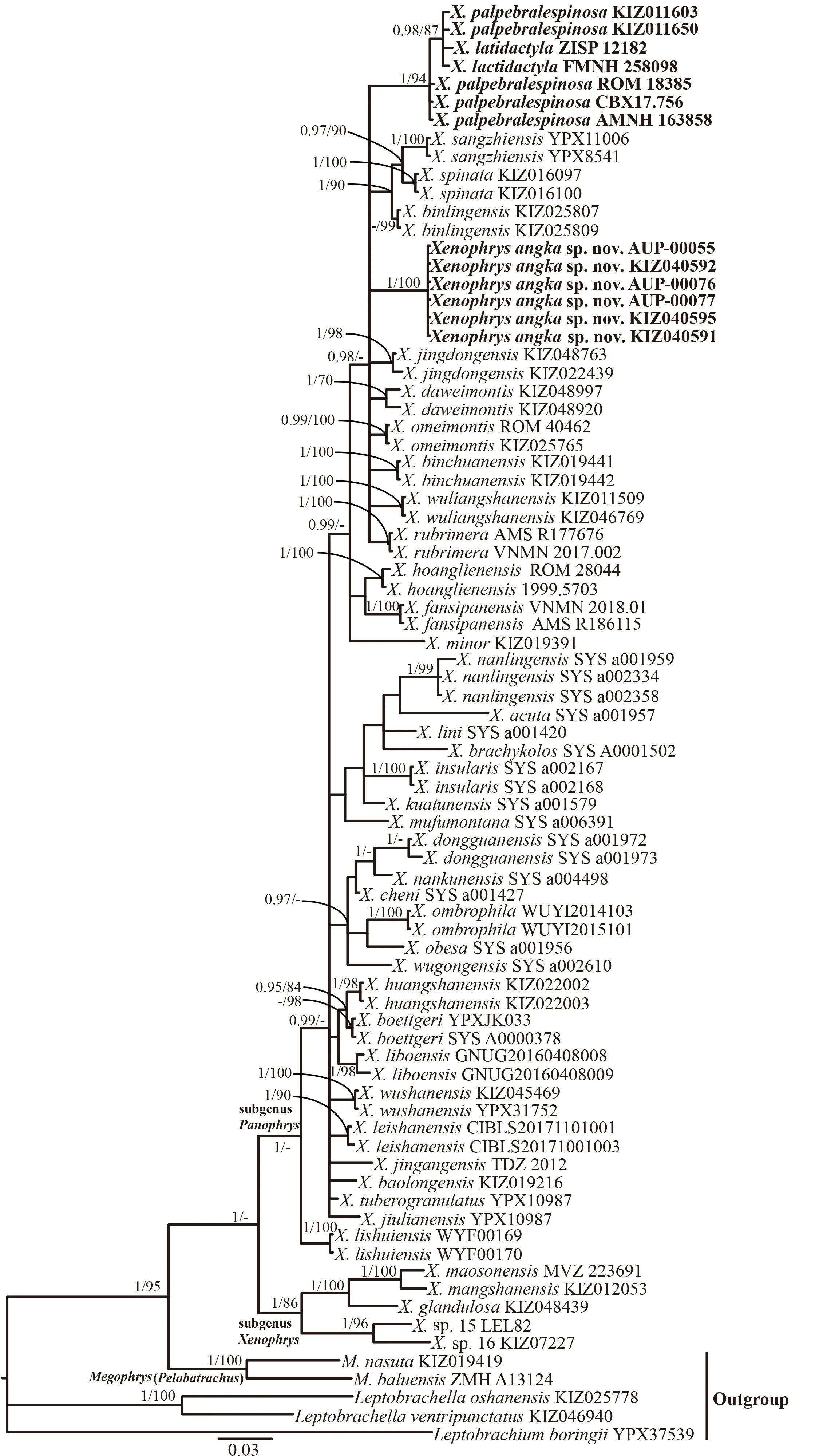
Figure 2 BI tree resulting from 541 bp length fragment of mitochondrial 16S rRNA gene for Xenophrys species and outgroups

Figure 3 Holotype Xenophrys angka sp.nov.(KIZ040591,field number AUP-00074,female)in life
Diagnosis:Xenophrys angka sp.nov.is a member of genus Xenophrys based on the following combination of morphological characters:head large,somewhat narrow and comparatively non-compressed,angular;tympanum distinct;vertical pupil;transverse skin fold at head basis absent;no large horny spines on dorsum;and two narrow glandular middorsal ridges present,forming X,H or Y-shaped figure(Chen et al., 2017). The following combination of characters diagnoses the new species:(1)small body size,adult snoutvent length(SVL)31.2-32.1 mm in males(n=2),37.5-39.2 mm in females (n=4); (2) tympanum distinct and circular;(3)vomerine ridges indistinct and vomerine teeth absent;(4) maxillary teeth present; (5) tongue heart-shaped, not notched posteriorly;(6)supratympanic fold distinct,extending from the posterior corner of eye to shoulder;(7)webbing between toes rudimentary;(8)lateral fringes on toes absent;(9)tibio-tarsal articulation reaching the area between eye and snout tip;(10)nuptial pads present on finger I;(11)subarticular tubercles present on the base of fingers I-II,but absent on fingers III-IV;(12)subarticular tubercle present at base of toes I,absent on toes II-IV;(13)heels meeting or overlapping when tibias positioned at right angle to body axis;(14)inner metatarsal tubercle big,outer metatarsal tubercle absent;(15)protruding projection posterior to cloaca of males present;(16)dorsal surface with a complete dark brown interorbital triangle with light blotch in the middle and two distinct thin opposing"V"-shaped reddish glandular ridges with ridge on dorsum; (17) orange coloration of groin contrasting with surrounding regions on males; and (18) inner metacarpal tubercle and outer metatarsal tubercles distinct reddish color.
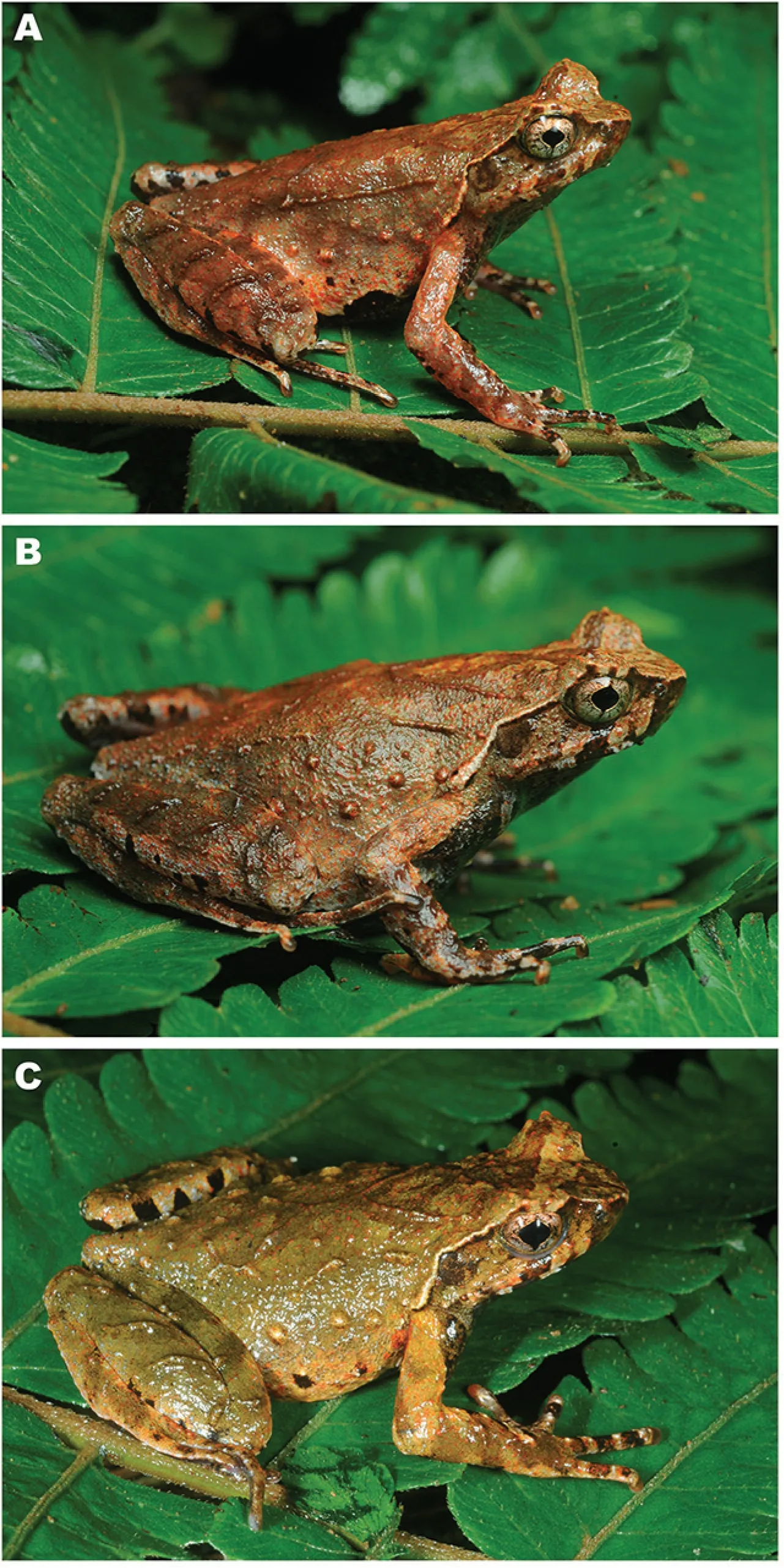
Figure 4 Type series of Xenophrys angka sp.nov.in life
Description of holotype (measurements in Table 1):KIZ040591, sexually mature female, body habitus stocky(Figure 3),body size small(SVL=38.2 mm);head large(head length(HDL)/SVL 33.8%,maximum head width(HDW)/SVL 32.2%), slightly longer than wide (HDW/HDL 95.3%);triangular in dorsal view;top of head flat;snout short(snout length(SNT)/HDL 32.6%)and wide(the distance between anterior orbital borders (IFE)/HDW 50.4%), snout obtusely pointed in dorsal view (Figure 3A), sharply protruding in profile,without rostral appendage,notably projecting beyond lower jaw (Figure 3C); loreal region vertical and concave;canthus rostralis distinct, sharp; dorsal region of snout flattened;eyes large(eye diameter(ED)/HDL 31.8%);slightly protuberant in dorsal view and notably protruding in profile(Figure 3), eye less than twice as long as maximum tympanum diameter(tympanum diameter(TD)/ED 56.1%)and subequal to snout length (ED/SNT 97.6%); eye-tympanum distance less than maximum tympanum diameter(tympanumeye distance(TED)/TD 87.0%);tympanum distinct,circular in shape,relatively small(TD/HDL 17.8%),eye diameter notably larger than tympanum diameter (ED/TD 178.3%); nostril rounded,laterally orientated,nostril closer to anterior corner of eye than to tip of snout(distance from nostril to eye(DNE)/snout-nostril distance(SN)85.7%);internarial distance equal to width of upper eyelid(internarial distance(IND)/width of upper eyelid(UEW)100.0%),slightly larger than interorbital distance (IND/interorbital distance (IOD) 103.0%); pineal ocellus not visible externally (Figure 3A); tongue heart-shaped, not notched posteriorly; vomerine ridges indistinct and vomerine teeth absent; maxillary teeth present; pupil diamond-shaped(Figure 3C),vertical.
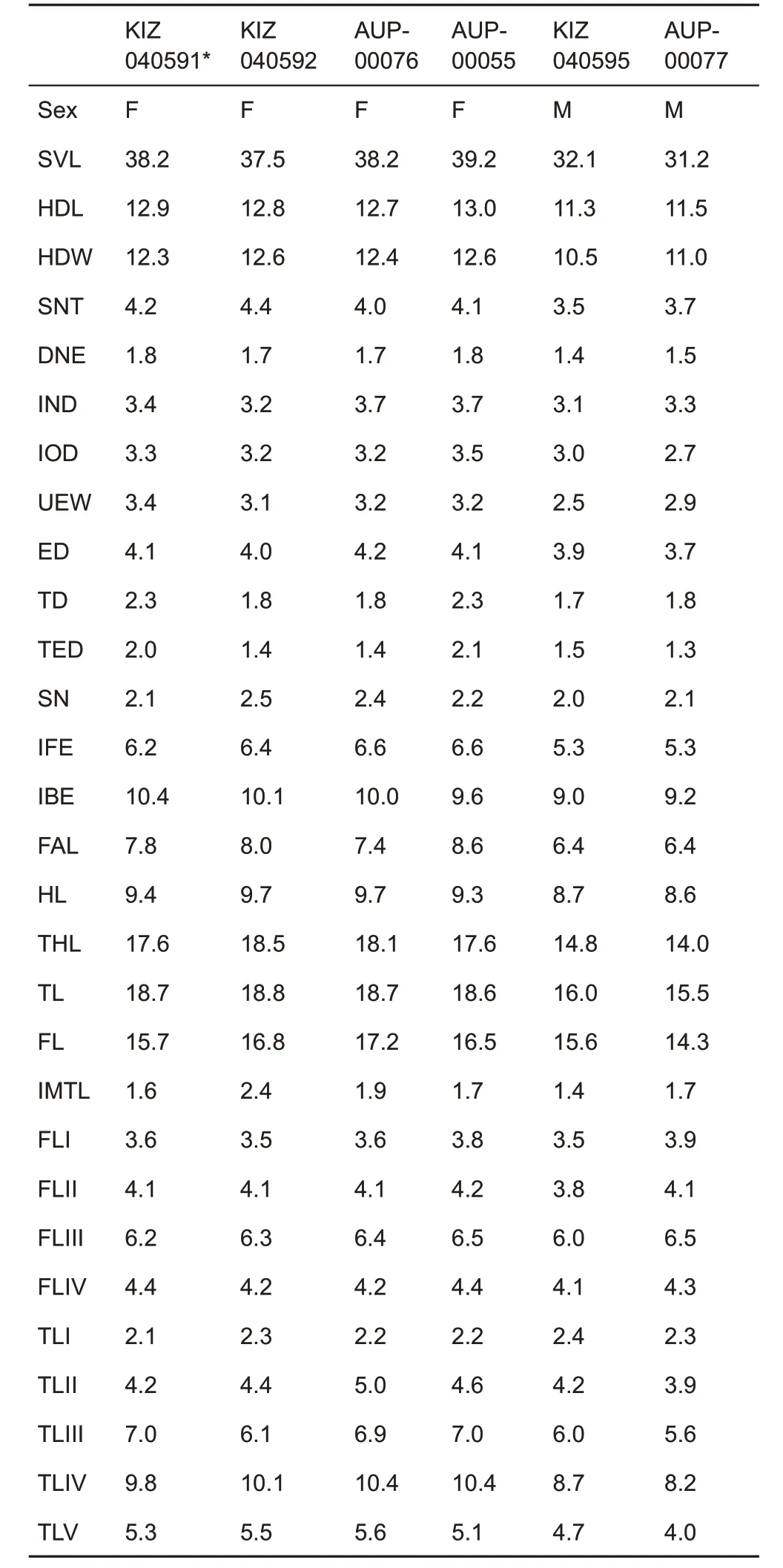
Table 1 Measurements (in mm) of type series of Xenophrys angka sp.nov.
Forelimbs moderately long and robust; forearm not enlarged, length shorter than hand length (forearm length(FAL)/hand length(HL)83.0%);fingers long and narrow,not flattened dorsoventrally, lateral fringes on fingers absent,relative finger lengths:I<II<IV<III;tips of all fingers rounded,slightly dilated relative to digit widths, with circular pads,terminal grooves absent;no webbing between fingers;a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV; more distal subarticular tubercles absent,replaced by low callous dermal ridges; supernumerary tubercles absent;inner metacarpal tubercle big,oval,outer metacarpal tubercle small,flattened(Figure 3D).
Hindlimbs long and robust,thigh length shorter than tibia length(thigh length(THL)/tibia length(TL)94.1%),but greater than foot length (THL/foot length (FL) 112.1%); tibio-tarsal articulation of straightened limb reaching eye level; heels slightly overlapping when tibias positioned at right angles to body axis; toes long and slightly dorsoventrally flattened,relative toes lengths:I<II<V<III<IV;tips of all toes rounded,slightly dilated,terminal grooves absent;notably expanded relative to digit widths forming circular pads;terminal grooves absent;lateral dermal fringes on absent;rudimentary webbing present between all toes; tarsal fold absent; subarticular tubercle present at base of toes I,absent on toes II-IV,replaced by indistinct callous dermal ridges;inner metatarsal tubercle large,ca.1.5x longer than wide,oval-shaped;and outer metatarsal tubercle absent(Figure 3E).
Dorsal surface of body and both dorsal and lateral surfaces of head weakly granular;several large distinct warts scattered on flanks;horn-like tubercle and several smaller tubercles at edge of eyelids present; and supratympanic fold distinct,glandular,starting immediately at posterior corner of upper eyelid (palpebrum) and running posteriorly towards dorsal edge of tympanum, where it sharply curves ventrally becoming more prominent and swollen and gently continues towards axilla.Dorsolateral folds well-developed,glandular,almost straight, running from scapular region posteriorly towards sacral region becoming less distinct and interrupted posteriorly; two opposing "V"-shaped glandular skin folds present on dorsum joined by a ca.10 mm long dorsomedial fold in a hourglass-shape(Figure 3A);dorsal surfaces of limbs with small tubercles forming distinct transverse skin folds on hindlimbs and irregular reticulate folds on forelimbs;ventral surfaces of limbs, chest, abdomen and throat smooth;pectoral glands prominent,rounded,located closely to axilla(Figure 3D);femoral glands small,oval-shaped,positioned on posterior surface of thighs closer to groin than to the knee.
Color of the holotype in life:Coloration of holotype in life shown in Figure 3 and Figure 4A.In life,dorsal surface light brown with olive green tint, with a complete dark brown inverted triangle with light-ochre central blotch present between eyes;all small tubercles and glandular ridges on dorsal surfaces of head and body reddish-brown,hourglassshaped dorsal glandular skin ridges also reddish brown surrounded with darker grey-brown"X"-shaped marking with indistinct borders;flanks with irregular dark brown spots and reddish mottling getting denser ventrally(Figure 4A);lateral surfaces of head light brownish-gray to beige,upper lips with 3 to 4 dark reddish-brown vertical bars,the one below eye largest and most distinct;tympanum entirely dark brown;white supratympanic fold with black to dark-brown lower margin;loreal region dark-brown; the edge of upper eyelid(palpebrum)with 3 distinct reddish palpebral tubercles,the medial one largest forming horn-like projection; dorsally palpebral tubercles edged with dark brown;dorsal parts of limbs reddish-brown with dark brown crossbars and reddish mottling; dorsal surface of the fingers with dark brown crossbars;ventral surfaces of body and limbs primarily offwhite to grey,mottled with dense black and white blotches and flecks on belly; black and white marbling getting denser anteriorly;gular and chest dark grey and mottled with white;posterior part of belly off-white with large grey blotches;ventral surfaces of hindlimbs with contrasting black and white marbling;groin area,anterior surfaces of thighs and posterior surfaces of heels bright reddish-orange in life;pectoral and femoral glands small,cream-white in color;iris golden-bronze with dense black mottling;pupil edged with thin golden line;thenar and palmar surface of limbs dark grey; and inner metacarpal tubercle, outer metacarpal tubercle, and inner metatarsal tubercle bright pink.
Color of the holotype in preservative:For coloration see Supplementary Figure S1. After 2 years in preservative,dorsum faded to dark brown;a complete dark brown inverted triangle between eyes and crossbars on dorsal limbs and fingers still clear;two opposing"V"-shaped skin folds present on dorsum becoming less distinct;throat and chest turns dark brown; abdomen and ventral surface of limbs dark brown mottled with whitish-grey marbling;inner metacarpal tubercle and inner metatarsal tubercle become off-white, outer metacarpal tubercle brown;dark brown vertical bars present upper lips still clear;supratympanic folds dark brown;pectoral glands and femoral glands still distinct,cream;ventral side of the hands and toes brown and digit tips brown or cream.
Variation:Morphometric variation of types reported in Table 1.All 5 paratypes are very similar in morphology the holotype,but also show variation in color in life. Dorsal surface of holotype and paratype AUP-00055 light brown,but paratype AUP-00077 orange(Figure 4).
Male secondary sexual characteristics:The new species shows slight differences in body size between the sexes:females have slightly larger SVL (37.5-39.2 mm, mean 38.3 mm;n=4)than males(31.2-32.1 mm,mean 31.7 mm;n=2). All adult males have nuptial pads pinkish-red in life covering the dorsal surface of the base of FI. Male has external,single subgular vocal sac with slit-like openings at posterior corners of jaws and a prominent protruding projection posterior to cloaca(Figure 4).
Distribution and ecology:Xenophrys angka sp. nov. is currently known only from Doi Inthanon,Chiang Mai province,Thailand(Figure 1)at elevations from 1800 to 2200 m a.s.l.along forest cascade streams and waterfalls.Most males were located by calls made while sitting on vegetation 1-2 m from the stream;females were recorded in forest litter along the streams.Xenophrys angka sp.nov.appears to be a strict forest specialist,restricted to patches of undisturbed montane evergreen forests and is likely endemic to the Doi Inthanon-Thanon Thong Chai Range.It inhabits forest floor,leaf litter and the nearby undergrowth rocky mountainous surrounded by moist evergreen broadleaved forests.The new species was found in sympatry with Leptobrachium huashen Fei et Ye and Limnonectes taylori Matsui,Panha,Khonsue et Kuraishi.
Comparisons:We compared Xenophrys angka sp.nov.with their 66 known congeners on the basis of morphology.Comparisons with each subgenus are discussed separately below.
Subgenus Panophrys: Xenophrys angka sp. nov. differs from the following large-sized species by having a smaller adult male size,SVL 31.2-32.1 mm(vs.adult-male 42.0-45.0 mm,n=5,in X.baolongensis,Ye et al.,2007;45.0-51.0 mm,n=3,in X.binlingensis,Fei et al.,2009;81.3 mm,n=1,in X.caudoprocta,Fei et al.,2009;53.0-56.5 mm,n=2,in X.jingdongensis,Fei et al.,2009;56.0-59.5 mm,n=10,in X.omeimontis, Fei et al., 2009; 54.7 mm, n=1, in X.sangzhiensis,Jiang et al.,2008;99.8-115.6 mm,n=6,in X.shuichengensis,Tian et al.,2000;and 47.2-54.4,n=18,in X.spinata,Fei et al.,2009);from X.liboensis by having a smaller adult female body size,SVL 37.5-39.2 mm(vs.60.8-70.6 mm,n=8,in X.liboensis,Zhang et al.,2017);further from X.acuta by meeting or overlapping when tibias positioned at right angle to body axis(vs.not meeting in X.acuta,Li et al.,2014),and head slightly longer than wide(vs.head length slightly shorter than head width in X.acuta,Li et al.,2014);from X. binchuanensis by lateral dermal fringes on toes absent (vs. lateral dermal fringes on toes wide in X.binchuanensis,Fei et al.,2009),and horn-like tubercle above eyelids present(vs.absent in X.binchuanensis,Fei et al.,2009);from X.boettgeri by tongue not notched behind(vs.tongue notched behind in X.boettgeri,Fei et al.,2009),lateral dermal fringes on absent(vs.present in X.boettgeri,Fei et al.,2009),and male with external single subgular vocal sac(vs. male with internal single subgular vocal sac in X.boettgeri, Fei et al., 2009); from X. brachykolos by head slightly longer than wide(vs.head width larger than head length in X.brachykolos,Fei et al.,2009),male with external single subgular vocal sac (vs. male with internal single subgular vocal sac in X.brachykolos,Fei et al.,2009),and heels meeting or overlapping (vs. not meeting in X.brachykolos,Fei et al.,2009);from X.cheni by lateral fringes on fingers and toes absent(vs.lateral fringes on figures and toes wide in X.cheni,Wang et al.,2014),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.subarticular tubercles indistinct in X.cheni,Wang et al.,2014),golden-bronze(vs.iris dark brown in X.cheni,Wang et al., 2014), and tongue not notched behind (vs. margin of tongue notched behind in X.cheni,Wang et al.,2014);from X.daweimontis by vomerine ridges indistinct and vomerine teeth absent(vs.present in X.daweimontis,Rao&Yang,1997),and male with external single subgular vocal sac(vs.male with internal vocal sac in X.daweimontis,Rao&Yang,1997);from X. dongguanensis and X. nankunensis by vomerine ridges indistinct and vomerine teeth absent (vs. strong vomerine ridge bearing vomerine teeth in X.dongguanensis and X.nankunensis,Wang et al.,2019),subarticular tubercle present at base of toes I,absent on toe II-IV(vs.subarticular tubercles only present at base of each toe in X.dongguanensis and X.nankunensis,Wang et al.,2019),and heels meeting or overlapping(vs.heels not meeting in X.dongguanensis and X.nankunensis,Wang et al.,2019);from X.fansipanensis and X.hoanglienensis by vomerine ridges indistinct and vomerine teeth absent (vs. present in X.fansipanensis and X. hoanglienensis,Tapley et al., 2018),inner and outer metacarpal tubercles present(vs.absent in X.fansipanensis and X.hoanglienensis,Tapley et al.,2018),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.absent in X.fansipanensis and X.hoanglienensis,Tapley et al.,2018),inner metatarsal tubercle large (vs. inner metatarsal tubercle very weakly in X.fansipanensis and X.hoanglienensis,Tapley et al.,2018),and protruding projection posterior to cloaca of males present(vs.absent in X.fansipanensis and X.hoanglienensis,Tapley et al.,2018);from X.huangshanensis by tympanum distinct(vs.indistinct in X.huangshanensis,Fei et al.,2009),male with external single subgular vocal sac (vs. male with internal single subgular vocal sac in X.huangshanensis,Fei et al.,2009),subarticular tubercle present at base of toes I,absent on toe II-IV(vs.present in X.huangshanensis,Fei et al.,2009), and heels meeting or overlapping (vs. heels not meeting in X. huangshanensis, Fei et al., 2009); from X.insularis by vomerine ridges indistinct and vomerine teeth absent(vs.vomerine ridge strong with vomerine teeth in X.insularis,Wang et al.,2017),subarticular tubercle present at base of toes I,absent on toe II-IV(vs.subarticular tubercle only present at base of each toe in X.insularis,Wang et al.,2017),heels meeting or overlapping(vs.not meeting in X.insularis, Wang et al., 2017), and tibio-tarsal articulation reaching area between eye and snout tip (vs. reaching forward to posterior edge of tympanum in X.insularis,Wang et al.,2017);from X.jinggangensis by subarticular tubercle present at base of toes I,absent on toe II-IV(vs.a large subarticular tubercle at base of each toe in X.jinggangensis,Wang et al.,2012),relative finger lengths:I<II<IV<III(vs.II<I<IV<III in X.jinggangensis,Wang et al.,2012),and lateral fringes on fingers absent(vs.present in X.jinggangensis,Wang et al.,2012);from X.jiulianensis by tongue not notched posteriorly (vs. tongue weakly notched posteriorly in X.jiulianensis,Wang et al.,2019),subarticular tubercle present at base of toes I, absent on toe II-IV (vs. subarticular tubercles only present at base of toe I and II in X.jiulianensis,Wang et al.,2019);from X.kuatunensis by male with external single subgular vocal sac (vs. male with internal single subgular vocal sac in X.kuatunensis,Fei et al.,2009),and heels meeting or overlapping (vs. not meeting in X.kuatunensis,Fei et al.,2009);from X.leishanensis by male with external single subgular vocal sac(vs.male with internal single subgular vocal sac in X.leishanensis,Li et al.,2018),relative finger lengths: I<II<IV<III (vs. II<I<V<III in X.leishanensis,Li et al.,2018),and head slightly longer than wide(vs.head width slightly larger than head length in X.leishanensis, Li et al., 2018); from X. lini by subarticular tubercle present at base of toes I,absent on toe II-IV(vs.subarticular tubercle distinct at base of each toe in X.lini,Wang et al.,2014),and lateral dermal fringes on fingers and toes absent(vs.wide in X.lini,Wang et al.,2014);from X.lishuiensis by male with external single subgular vocal sac(vs.male with internal single subgular vocal sac in X.lishuiensis,Wang et al.,2017);from X.minor by a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.absent in X.minor,Fei et al.,2009),subarticular tubercle present at base of toes I,absent on toe II-IV(vs.absent in X.minor,Fei et al.,2009),lateral dermal fringes on toes absent(vs.absent in X.minor,Fei et al.,2009),male with external single subgular vocal sac (vs. male with internal single subgular vocal sac in X.minor,Fei et al.,2009),and horn-like tubercle above eyelids present(vs.absent in X.minor,Fei et al.,2009);from X.mufumontana by relative finger lengths:I<II<IV<III(vs.II=IV<I<III in X.mufumontana,Wang et al.,2019),subarticular tubercle present at base of toes I,absent on toe II-IV(vs.subarticular tubercles only present at base of each toe in X.mufumontana,Wang et al.,2019),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.presence of a subarticular tubercle at base of each finger in X.mufumontana,Wang et al.,2019),and outer metacarpal tubercle small,flattened(vs.indistinct in X.mufumontana,Wang et al.,2019);from X.nanlingensis by vomerine ridges indistinct and vomerine teeth absent (vs.vomerine ridge and vomerine teeth present in X.nanlingensis,Wang et al.,2019),tongue not notched posteriorly(vs.tongue notched posteriorly in X.nanlingensis,Wang et al.,2019),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV (vs. presence of a subarticular tubercle at base of each finger in X.nanlingensis,Wang et al.,2019),subarticular tubercle present at base of toes I,absent on toe II-IV(vs.presence of a subarticular tubercle at base of each toe in X.nanlingensis,Wang et al.,2019),and adult males have nuptial pads pinkish-red in life covering dorsal surface of base of FI(vs.nuptial pads and nuptial spines invisible in males during breeding season in X.nanlingensis,Wang et al., 2019); from X. obesa by vomerine ridges indistinct(vs.two vomerine ridges moderately developed in X.obesa,Li et al.,2014),heels meeting or overlapping(vs.not meeting in X.obesa,Li et al.,2014),and head slightly longer than wide(vs.head width slightly larger than head length in X.obesa,Li et al.,2014);from X.ombrophila by heels meeting or overlapping(vs.not meeting in X.ombrophila,Messenger et al., 2019), and tibio-tarsal articulation reaching area between eye and snout tip(vs.reaching posterior corner of eye in X. ombrophila, Messenger et al., 2019); from X.palpebralespinosa by vomerine teeth absent(vs.present in X.palpebralespinosa,Fei et al.,2009),lateral dermal fringes on fingers and toes absent(vs.slight lateral fringes on fingers,lateral fringes wide on toes in X.palpebralespinosa,Fei et al.,2009),and male with external single subgular vocal sac(vs.male with internal single subgular vocal sac in X.palpebralespinosa,Fei et al.,2009);from X.rubrimera by vomerine teeth absent(vs.present in X.rubrimera,Tapley et al.,2017),a large subarticular tubercle present at base of fingers I-II, absent on fingers III-IV (vs. absence of subarticular tubercles on fingers in X.rubrimera,Tapley et al.,2017),and head slightly longer than wide(vs.head width slightly larger than head length in X.rubrimera,Tapley et al.,2017);from X.tuberogranulata by male with external single subgular vocal sac (vs. male with internal single subgular vocal sac in X.tuberogranulata,Mo et al.,2010),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.indistinct in X.tuberogranulata,Mo et al.,2010),and relative finger lengths:I<II<IV<III(vs.II<IV=I<III in X.tuberogranulata,Mo et al.,2010);from X.wugongensis by heels meeting or overlapping (vs. not meeting in X.wugongensis,Wang et al.,2019),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.presence of a subarticular tubercle at base of each finger in X.wugongensis, Wang et al., 2019), subarticular tubercle present at base of toes I,absent on toe II-IV(vs.presence of a subarticular tubercle at base of each toe in X.wugongensis,Wang et al., 2019); from X. wuliangshanensis by a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.indistinct in X.wuliangshanensis,Fei et al.,2009),male with external single subgular vocal sac(vs.male with internal single subgular vocal sac in X.wuliangshanensis,Fei et al.,2009),horn-like tubercle at edge of eyelids present(vs.absent in X.wuliangshanensis,Fei et al.,2009),and relative finger lengths: I<II<IV<III (vs. IV<III<II=I in wuliangshanensis,Fei et al.,2009);from X.wushanensis by horn-like tubercle and some smaller tubercles at edge of eyelids present (vs. absent in X. wushanensis, Fei et al.,2009),male with external single subgular vocal sac(vs.male with internal single subgular vocal sac in X.wushanensis,Fei et al.,2009),and lateral dermal fringes on toes absent(vs.male with wide lateral fringes in X.wushanensis,Fei et al.,2009).
Subgenus Xenophrys: Xenophrys angka sp. nov. differs from the following large-sized specie by having a smaller adult male size,SVL 31.2-32.1 mm(vs.adult-male 55.8-62.4 mm,n=6,in X.aceras,Wang et al.,2017;39.1-45.0 mm,n=8,in X. ancrae, Mahony et al., 2013; 76.7 mm, n=20, in X.auralensis,Ohler.,2002;57.1 mm,n=1,in X.damrei,Mahony,2011;56.9-68.4 mm,n=4,in X.flavipunctata,Mahony et al.,2018;76.3-81.0 mm,n=10,in X.glandulosa,Fei et al.,2009;68.0-73.5 mm,n=7,in X.himalayana,Mahony et al.,2018;55.6-66.6 mm,n=8,in X.lekaguli,Stuart et al.,2006;47.0 mm,n=1,in X.longipes,Wang et al.,2017;65.5 mm,n=1,in X.major,Fei et al.,2009;62.5 mm,n=1,in X.mangshanensis,Fei et al.,2009;58.0-76.0 mm,n=6,in X.maosonensis,Yang et al.,2018;57.2-68.0 mm,n=16,in X.medogensis,Fei et al.,2009;71.3-93.8 mm,n=12,in X.periosa,Mahony et al.,2018;73.5-83.1 mm,n=6,in X.robusta,Mahony et al.,2018;45.9-53.4 mm,n=7,in X.megacephala,Mahony et al.,2011;47.4-53.0 mm,n=33,in X.takensis,Mahony,2011);from X.oreocrypta by having a smaller adult female body size,SVL 37.5-39.2 mm(vs.94.9 mm,n=1,in X.oreocrypta,Mahony et al., 2018); further from X. monticola by vomerine ridges indistinct(vs.distinct in X.monticola,Mahony et al.,2018),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.absent in X.monticola,Mahony et al.,2018),inner and outer metacarpal tubercles distinct(vs.absent in X. monticola, Mahony et al., 2018), and inner metatarsal tubercle large (vs. indistinct in X. monticola,Mahony et al.,2018);from X.oropedion by vomerine ridges indistinct and vomerine teeth absent (vs. present in X.oropedion,Mahony et al.,2013),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.absent in X. oropedion, Mahony et al., 2013), protruding projection posterior to cloaca of males present(vs.absent in X. oropedion, Mahony et al., 2013), and inner and outer metacarpal tubercles distinct (vs. absent in X. oropedion,Mahony et al.,2013);from X.pachyproctus and X.parva by vomerine ridges indistinct and vomerine teeth absent (vs.present in X.pachyproctus and X.parva,Fei et al.,2009),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.absent in X.pachyproctus and X.parva,Fei et al.,2009),and male with external single subgular vocal sac(vs.male with internal single subgular vocal sac in X. pachyproctus and X. parva, Fei et al., 2009); from X.serchhipii by toes with rudimentary webbing(vs.at least onefourth webbing in X.serchhipii,Mathew&Sen,2007),relative finger lengths:I<II<IV<III(vs.2nd and 4th fingers subequal in length in X.serchhipii,Mathew&Sen,2007),and vomerine teeth absent(vs.present in X.serchhipii,Mathew&Sen,2007); from X. vegrandis by a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.absent in X. serchhipii, Mathew & Sen, 2007), protruding projection posterior to cloaca of males present(vs.absent in X.serchhipii,Mathew&Sen,2007),and pupil vertical,iris golden-bronze(vs.pupil horizontally orientated,iris metallic yellowish-orange in X.serchhipii,Mathew&Sen,2007);from X.zhangi by vomerine ridges indistinct and vomerine teeth absent(vs.present in X.zhangi,Fei et al.,2009),a large subarticular tubercle present at base of fingers I-II,absent on fingers III-IV(vs.absent in X.zhangi,Fei et al.,2009),lateral dermal fringes on toes absent(vs.present in X.zhangi,Fei et al.,2009),and male with external single subgular vocal sac(vs.male with internal single subgular vocal sac in X.zhangi,Fei et al.,2009);from X.zunhebotoensis by horn-like tubercle and a number of smaller tubercles at edge of eyelids present(vs. absent in X. zunhebotoensis, Mathew & Sen, 2007),tongue not notched behind(vs.tongue notched behind in X.zunhebotoensis,Mathew&Sen,2007),and vomerine teeth absent(vs.present in X.zunhebotoensis,Mathew&Sen,2007).
From species not yet assigned to a subgenus:Xenophrys angka sp.nov.differs from X.feii by lateral dermal fringes on toes absent(vs.lateral fringes on toes moderate to wide in X.feii,Yang et al.,2018),lateral fringes on fingers absent(vs.moderate lateral fringes present on outer most three fingers in X.feii,Yang et al.,2018),groin coloration in life contrasting with surrounding regions on males(vs.groin coloration not contrasting with surrounding regions on males in X.feii,Yang et al.,2018),and nuptial pads present on finger I(vs.absent in X.feii,Yang et al.,2018).
The diversity of Xenophrys is underestimated greatly(Chen et al.,2017;Liu et al.,2018).In the last three years,19 new species were described mostly from China,Vietnam and India(Li et al.,2018;Mahony et al.,2018;Messenger et al.,2019;Tapley et al.,2017,2018Wang et al.,2017;Wang et al.,2017;Wang et al.,2019;Yang et al.,2018;Zhang et al.,2017).Compared to other countries in the Indochinese region,the diversity of Xenophrys in Thailand attracted less attention and no new species were described for the country within the last seven years.Herein,we describe a new species of Xenophrys from northern Thailand based on morphological and molecular analyses.Our discovery increases the number of amphibian species recorded in Thailand to 194,and the species number of Xenophrys to 27.The diversity of amphibians known from Thailand has increased remarkably from 125(Khonsue and Thirakhupt, 2001) to 193 (Frost, 2019) and the diversity remains underestimated(Chan et al.,2018;Chen et al.,2017,2018; Grismer et al., 2016; Laopichienpong et al., 2016;Matsui et al.,2018;Pawangkhanant et al.,2018;Poyarkov et al., 2018; Sheridan & Stuart, 2018; Suwannapoom et al.,2017;Suwannapoom et al.,2018).Further field surveys and taxonomic studies on the Thai herpetofauna will likely result in further discoveries of yet unknown lineages and species of amphibians.
Our study also elucidates taxonomic status of Xenophrys latidactyla(Orlov et al.,2015),which a recently described from northern Vietnam. It was described as a member of Megophrys sensu lato based on a single male specimen collected from Pu Mat National Park in south-western Nghe An Province.Orlov et al.(2015)indicated that this species is most closely resembles X. palpebralespinosa, inhabiting montane areas of northern Vietnam(recorded from provinces Lao Cai,He Giang,Cao Bang,Vinh Phuc,Son La,Thanh Hoa,Nge An)and northern Laos,eastern part of Yunnan and the westernmost part of Guangxi,China(Bourret,1937,1942;Fei et al.,2009,2010Orlov et al.,2015;Tapley et al.,2017).The main morphological differences of these two species were the presence of very wide dermal fringes along all length of the toes in X.latidactyla(vs.broad dermal fringes absent in X.palpebralespinosa), presence of prominent subarticular tubercles in X. latidactyla (vs. absent), and differences in tympanum size.Our mtDNA genealogy unambiguously places two specimens of X.latidactyla,including the type specimen ZISP 12182, within radiation of X. palpebralespinosa from northern Vietnam and Laos.The genetic divergence between X.latidactyla and X.palpebralespinosa is small,ranging from 0.3%-1.4%,suggesting that these two taxa might be conspecific. Morphological differences that were used by Orlov et al.(2015)to diagnose these species might be subject to significant variation,which was not assessed due to small sample size(n=1):feet webbing and dermal fringes on toes in X.palpebralespinosa are normally significantly reduced out of the breeding season and get enlarged during the reproduction(personal observation); the reported distinct subarticular tubercles in X. latidactyla might be the result of partial dehydration of limbs due to preservation in ethanol.Thus,no unambiguous morphological or molecular characters distinguishing these two species.Therefore,we transfer X.latidactyla to the synonymy of X. palpebralespinosa as a subjective junior synonym.Further,this result indicates that molecular analyses are essential in assessing species diversity of Xenophrys,and megophryids in general.
NOMENCLATURAL ACTS REGISTRATION
The electronic version of this article in portable document format represents a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone(see Articles 8.5-8.6 of the Code).This published work and the nomenclatural acts it contains have been registered in ZooBank,the online registration system for the ICZN.The ZooBank LSIDs(Life Science Identifiers)can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefixhttp://zoobank.org/.
Publication LSID:urn:lsid:zoobank.org:pub:599B00C7-BCD7-4C24-ACB6-0AC2B508BADA.
Xenophrys angka LSID:urn:lsid:zoobank.org:act:44272F1C-0019-4EE5-83E9-28C3286BCA4F.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Specimen collection protocols were approved by the Institutional Ethical Committee of Animal Experimentation of the University of Phayao(certificate number UP-AE59-01-04-0022 issued to Chatmongkon Suwannapoom) and the Institute of Animal for Scientific Purposes Development Thailand(No.U1-01205-2558).
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS'CONTRIBUTIONS
J.C.,C.S.,and Y.H.W.designed the study.C.S.,P.P.,and N.A.P collected specimens in the field.J.Q.J.performed molecular experiments.Y.H.W.and K.X.measured the specimens.Y.H.W.performed data analyses.Y.H.W.,C.S.,N.A.P.,R.W.M.,and J.C.wrote the manuscript.All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We appreciate Tang Van Duong and Hisanori Okamiya for assisting in the lab and in the field.We would like to thank the Laboratory Animal Research Center,University of Phayao.We would like to thank the Department of National Parks,Wildlife and Plant Conservation in Thailand for permission to Chatmongkon Suwannapoom work in the field.
杂志排行
Zoological Research的其它文章
- Diversity scaling of human vaginal microbial communities
- Direct sunlight exposure reduces hair cortisol levels in rhesus monkeys(Macaca mulatta)
- Ongoing green peafowl protection in China
- First record of the ferret-badger Melogale cucphuongensis Nadler et al.,2011(Carnivora:Mustelidae),with description of a new subspecies,in southeastern China
- A new species of the genus Raorchestes(Anura:Rhacophoridae)from Yunnan Province,China
- Sinocyclocheilus sanxiaensis,a new blind fish from the Three Gorges of Yangtze River provides insights into speciation of Chinese cavefish
