黄土区沟道泥沙微生物群落变化特征及其影响因素
2019-10-23侯芳彬SalmanAli郭胜利
侯芳彬,王 蕊,Salman Ali,高 鑫,郭胜利,3*
黄土区沟道泥沙微生物群落变化特征及其影响因素
侯芳彬1,王 蕊1,Salman Ali2,高 鑫1,郭胜利1,3*
(1.西北农林科技大学水土保持研究所,黄土高原土壤侵蚀与旱地农业国家重点实验室,陕西 杨凌 712100;2.西北农林科技大学资源与环境学院,陕西 杨凌 712100;3.中国科学院、水利部水土保持研究所,陕西 杨凌 7 12100)
在黄土高原沟壑区,通过16S rRNA基因片段和ITS高通量测序,研究沟道泥沙中细菌和真菌群落在上-中-下游的变化特征.结果表明:与沟头相比,把口站的细菌群落中拟杆菌门(Bacteroidetes)与厚壁菌门(Firmicutes)的相对丰度分别增加6.6%和10.5%,而变形菌门(Proteobacteria)的相对丰度降低15.1%;真菌群落中担子菌门(Basidiomycota)的相对丰度增加7.7%,而子囊菌门(Ascomycota)降低30.2%;泥沙中黏粒含量与细菌丰富度(Chao1指数)和多样性(Shannon指数)之间显著负相关(<0.05),与真菌的丰富度和多样性无显著相关性;细菌和真菌群落多样性和丰富度的空间差异与SOC、Olsen-P的变化有关(<0.05).因此,泥沙中颗粒组成物和养分含量可能是影响沟道微生物群落变化的主要因素.
沟道泥沙;空间重分布;细菌;真菌;颗粒组成
陆地表面每年有高达75Gt的土壤发生迁移和重新分布,其中70%在陆地表面或相邻流域低洼处沉积[1-3].土壤的重新分布极大地影响了陆地生态系统的净初级生产力[4-6]以及空间异质性[3,7-10].土壤中栖息的微生物是陆地表面中最丰富与活跃的一类生物[11-13],是生物地球化学循环的重要驱动力[14].地表物质的侧向移动使得地貌[15-16]、土壤结构[10]、土壤养分[17-20]、微生物特性[21]等发生变化.
黄土高原沟壑区总面积3.56万km2,水土流失面积为3.06万km2,地形支离破碎,沟壑纵横,大体分为塬面、坡地和沟道,坡面和沟道合称为沟壑,例如高原沟壑区齐家川示范区内沟壑的面积可以占到56%以上[22].沟道不仅是坡面泥沙进入河流和湖泊的主要通道,也是泥沙沉积的主要区域.黄土高原沟道系统的产沙量高达整个黄土高原总侵蚀量的80%,坡面泥沙经过运移进入沟道系统[23-24],使得沟道中泥沙含沙量大于1000kg/m3 [25].沟道不仅是坡面泥沙进入河流和湖泊的主要通道,也是泥沙沉积的主要区域.黄土高原沟道系统的产沙量高达整个黄土高原总侵蚀量的80%,坡面泥沙经过运移进入沟道系统[23-24],使得沟道中泥沙含沙量大于1000kg/m3[26].泥沙迁移过程中轻(低密度)和细(黏粒和粉粒)的颗粒优先发生运移[25,27-28],更容易迁移到更远的地方[3,29-30].同时,泥沙颗粒的迁移、分布对土壤有机碳矿化和积累具有显著影响[5-6,31-32].
微生物是影响陆地表面生物地球化学循环的重要因素,已有研究表明微生物群落在坡位间存在显著差异[21,33],这主要是与坡位间底物供应和水分条件有关[21].Mohammadi等发现下坡位较高的土壤微生物量和活性与其较高的土壤水分和碳氮底物供应有关[34].坡面侵蚀-沉积区的土壤微生物量以及酶活性对土壤有机碳含量、湿度等因素的敏感程度不同[35-36].因此,侵蚀地形对水分和底物产生的变化会对坡面土壤微生物群落产生显著影响,但是,目前对沟道重分布过程中微生物群落分布的研究不清楚.因此,本文探讨的沟道泥沙微生物群落变化及其影响因素将有助于理解沟道泥沙的迁移分布对陆地生态系统物质循环的影响.
本研究选择黄土高原沟壑区典型治理小流域,基于该流域主沟道,从王东村到把口站等间距采集泥沙样品,利用高通量测序等技术获取不同位置泥沙中微生物群落的信息,分析泥沙中细菌和真菌群落的变化特征;在此基础上探讨了沟道中微生物群落变化的影响因素.
1 材料与方法
1.1 研究区概况
位于陕甘交界处的长武县王东沟小流域 (东经107°40′30″~107°42′30″,北纬35°12′~35°16′) (图1).该流域为“陕西长武农田生态系统国家野外科学观测研究站”的所在地,是我国重点水土流失治理小流域.土地面积8.3km2,塬、坡、沟约各占土地面积的1/3(27.7%36.4%35.9%)[37],沟壑密度为2.78km/km2,属典型的黄土高原沟壑类型区.塬面海拔1220m,从塬面到沟底的最大高差为280m.属于大陆季风气候,年均气温9.1℃,³10℃积温3029℃,多年平均雨量584mm,但季节性分布不均,降雨的60%以上都集中在6~9月,多以短期暴雨形式出现.该流域的主要土壤类型为黄墡土和黑垆土,母质为深厚的中壤质马兰黄土,土层深厚,土质疏松,质地均一,可蚀性高,为本实验的开展提供了条件.
1.2 沟道泥沙样品的采集
2018年5月中旬,沿王东沟流域主沟道(全长6.3km)等间距采样,间距2.1km左右(图1);选定的点依次为王东村(上游)、范家梁(中游)、杜家坪(中游)和把口站(下游).在沟道中随机采样,重复3次,共12个样品,考虑到沟道中的泥沙层较浅,每个采样点取0~10cm的泥沙样本.
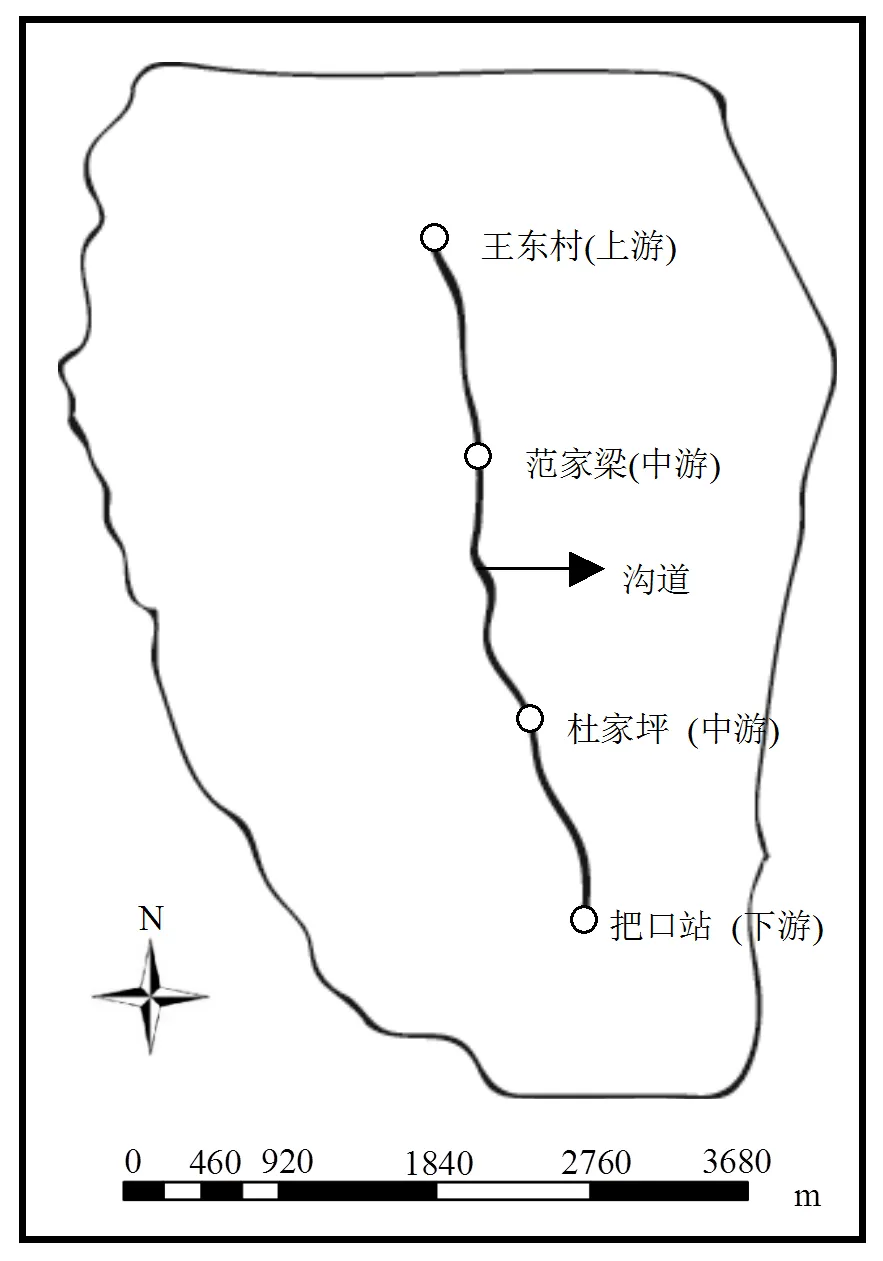
图1 王东沟流域采样位置示意
各样品混合均匀后分成两份:一份在室外置于4℃的保温箱内,之后立即转移至试验室−20℃的冷藏箱保存,以用于高通量测序;另一份样品风干,用于测试土壤颗粒、养分等理化指标.
1.3 DNA提取和高通量测序
采用Fast DNA SPIN Kit for soil试剂盒和MP FastPrep-24核酸提取仪提取土壤中的DNA.通过1%琼脂糖凝胶电泳和分光光度法(260nm/280nm处的光密度比)检查提取的DNA质量.将所有提取的DNA样品储存在−20℃下用于进一步分析.根据测序区域的选择,使用带 Barcode 的特异引物进行PCR扩增:338F (5'-ACTCCTACGGGAGGCAG-CAG-3')和806R(5'-GGACTACHVGGGTWTCTAAT-3')[38]扩增细菌16S rRNA基因的V3-V4区域,同时使用ITS1 (5'-CTTGGTCATTTAGAGGAAGTAA- 3')和ITS2(5'-GCTGCGTTCTTCATCGATGC-3')引物通过PCR扩增真菌ITS基因区域[39].
从2%琼脂糖凝胶中提取扩增子,并使用AxyPrep DNA凝胶提取试剂盒(Axygen Biosciences, Union City, CA, USA).根据制造商的说明纯化,然后使用GeneJET (Thermo Scientific公司)定量.将纯化的扩增子以等摩尔量合并,然后根据标准方案在Illumina MiSeq平台上进行配对末端测序(2´300).
首先使用QIIME包 (Quantitative Insights Into Microbial Ecology, v1.2.1)进行高质量序列的提取.之后使用以下标准对原始FASTQ文件进行解复用和质量过滤: (i)在10bp滑动窗口上获得平均质量分数<20的任何位点截短300bp,并丢弃短于50bp的读数; (ii)精确条形码匹配,引物匹配中的两个核苷酸错配,并除去含有模糊特征的读数; (iii)仅根据它们的重叠序列组装长于10bp的重叠序列.无法组装的读数被丢弃.使用UCLUST将唯一序列集分类为具有97%相似性阈值的操作分类单位(OTU).使用Usearch (版本8.0.1623)鉴定并除去嵌合序列.使用UCLUST对Silva119 16S rRNA数据库分析每个16S rRNA和ITS基因序列的分类,使用90%的置信度阈值.将细菌和真菌的原始读数共同存入NCBI序列读取存档(SRA)数据库.
1.4 理化性质分析
风干土样磨细,一部分过2mm筛(10目),分析土壤颗粒组成(MS-2000马尔文激光粒度仪)和pH值 (在土壤与溶液(1mol/L KCl)之比为1 : 2.5 (/)的上清悬浮液中测量).一部分过0.25mm筛(65目),测定样品中的有机碳含量(H2SO4-K2Cr2O7外加热法[40])和速效磷(Olsen法[41-42]).
1.5 数据分析方法
采用SAS (8.0, SAS Inst. 1998) 软件GLM程序包对沟道不同处理间的理化性质和微生物群落的Alpha、Beta多样性指数进行方差分析(ANOVA),当F检验显著时,再对四个位置处理间均值进行比较 (Duncan)检验(<0.05).通过Sigmaplot (12.5)软件绘制四个处理排名前十的微生物群落在门、纲、目、科、属水平的相对丰度图.采用R语言(3.5.1)对微生物数据进行整理,通过ape包和ggplot2包绘制主坐标分析图 (Principal Co-ordinates Analysis; PCoA),了解不同处理间门水平(phylum)上细菌和真菌群落的差异;通过vegan包绘制UPGMA聚类树(Unweighted Pair-group Method with Arithmetic Mean);通过vegan包和packfor包绘制冗余分析图(Redundancy Analysis; RDA).
2 结果与分析
2.1 沟道泥沙理化性状的变化特征
沟道泥沙颗粒的理化性状存在显著的空间差异(表1).沟道上游 (王东村) 泥沙的粉粒含量为68.4%,分别较中游(59.0%)、下游(53.3%)高出16%、28%.泥沙中黏粒含量变化最为明显,下游较上游增加54%(下游.上游:42.3%. 27.4%);砂粒含量无差异.土壤有机碳(SOC)含量从7.4g/kg (上游)增加到9.1g/kg (下游),增加幅度近23%.速效磷(Olsen-P)的变化趋势与SOC相似,与上游(17.3mg/kg)相比,中游(22.8mg/kg)和下游(27.2mg/kg)分别增加24.1%和36.4%.
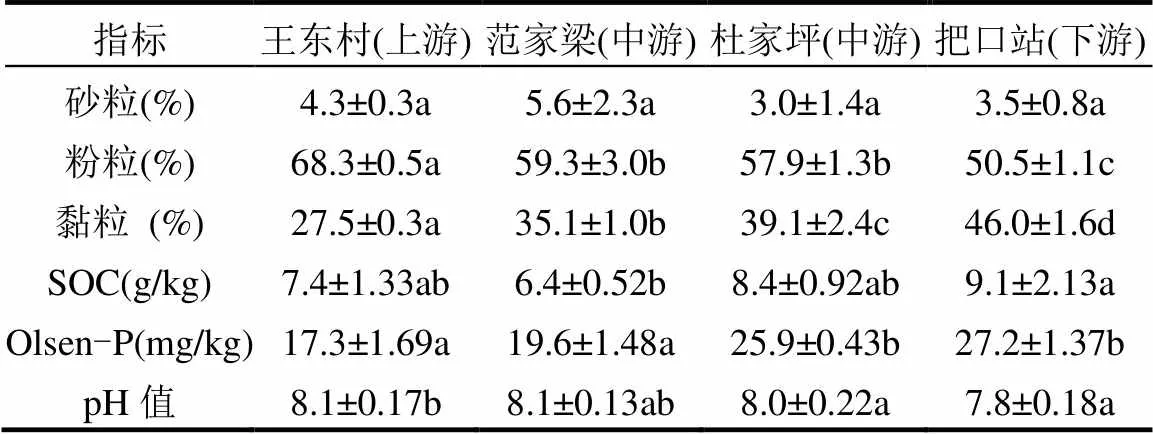
表1 沟道泥沙颗粒理化性状含量
注:同行不同字母表示空间位置变化差异性显著(<0.05).
2.2 不同空间位置细菌和真菌群落的组成差异
沟道泥沙样本中细菌和真菌群落分别获得34186和38902个高质量序列.从上游到下游,泥沙细菌群落的OTU数显著降低了23.2%,而真菌群落则呈现先降低后增加的趋势,且无显著性差异.沟道不同空间位置泥沙细菌的Chao1指数与Shannon指数具有显著差异(0.05)(表2),均表现为:上游(王东村)>中游(范家梁、杜家坪)>下游(把口站),表明从上游到下游,细菌群落的丰富度与多样性分别降低13.5%和10.4%.而真菌群落的Chao1指数与Shannon指数从王东村到把口站增加9.0%和5.4%,但均未达到显著水平.

表2 沟道泥沙微生物群落丰富度、多样性和OTU数(细菌和真菌有效序列分别为34186和38902条)
注:同行不同字母表示空间位置变化差异性显著(<0.05).
2.3 沟道泥沙细菌和真菌群落的变化特征
沟道内不同位置泥沙细菌和真菌群落在门、纲、目、科、属水平上的相对丰度存在一定差异 (图2、3).在细菌群落的门水平上,变形菌(Proteobacteria)、拟杆菌(Bacteroidetes)和厚壁菌(Firmicutes)在沟道不同处理中均为优势菌门,其中变形菌门的相对丰度降低了15.1%,但未达到显著水平;拟杆菌门和厚壁菌门较上游相比,相对丰度分别增加了6.6%和10.5%,但均未达到显著水平(图2a).纲水平上,-变形菌纲 (Gammaproteobacteria)在沟道不同位置的相对丰度占比最高且始终保持在35%以上.下游与上游相比,拟杆菌纲(Bacteroidia)增加幅度为6% (18.6%24.2%);-变形菌纲 (Alphaproteobacteria) 的相对丰度降低近3倍(19.1%6.9%);梭状芽孢杆菌纲 (Clostridia) 的相对丰度增加近3倍 (3.3%9.4%) (图2b).科水平上,把口站处黄单胞菌科(Xanthomonadaceae)和甲壳虫菌科(Chitinophagaceae)的相对丰度均较上-中游显著降低(0.05) (图2d).
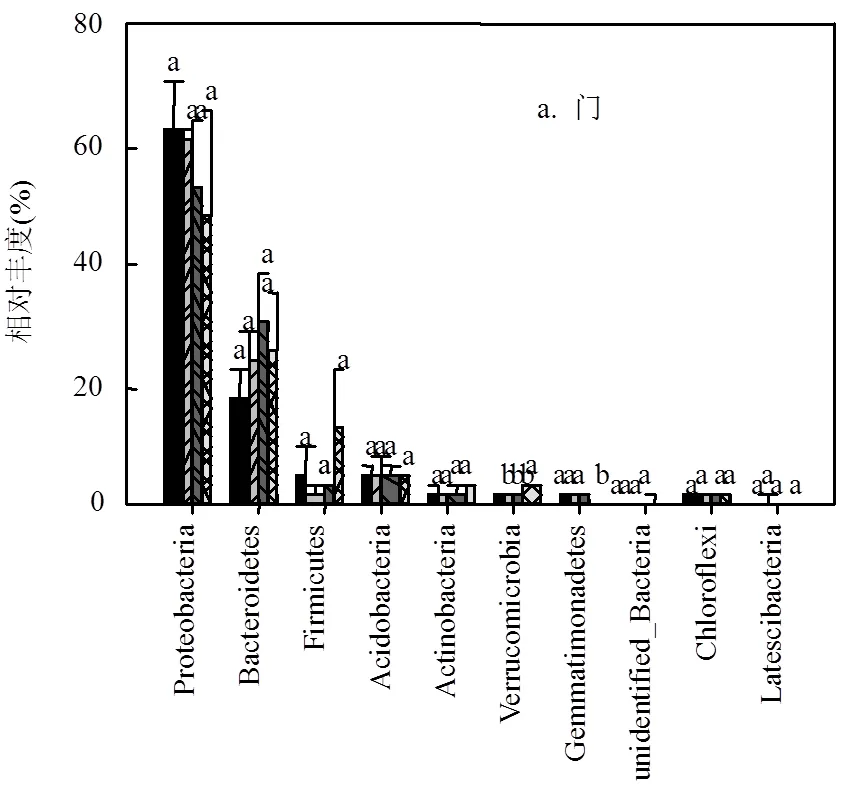

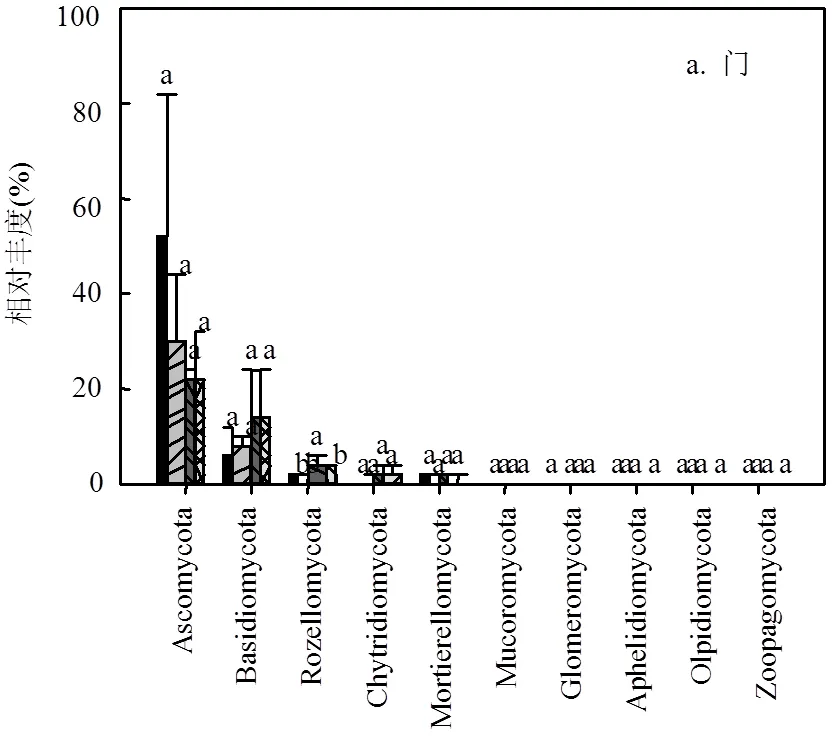
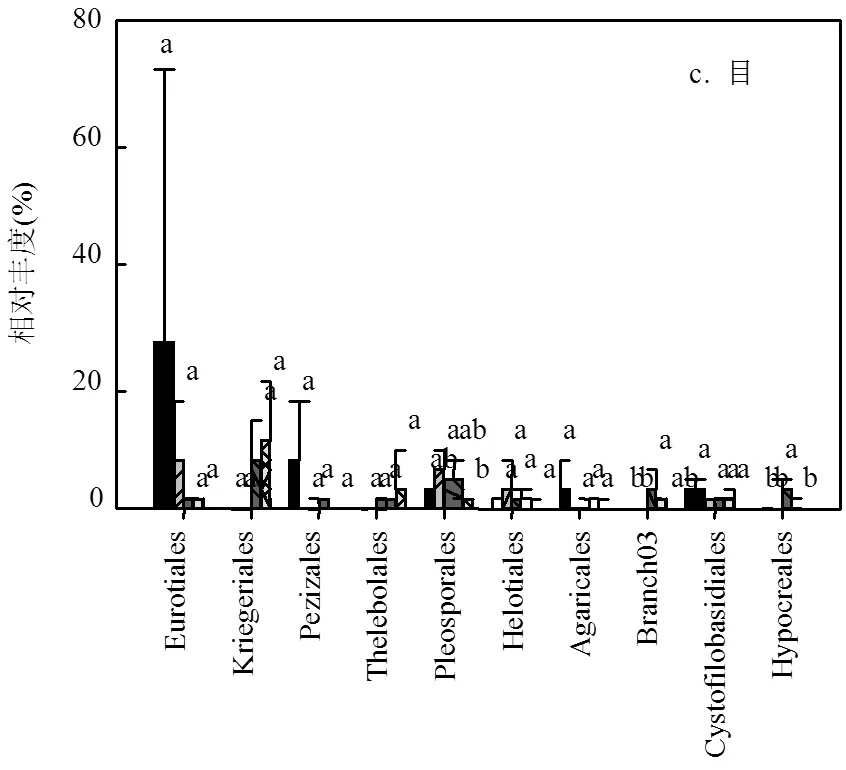
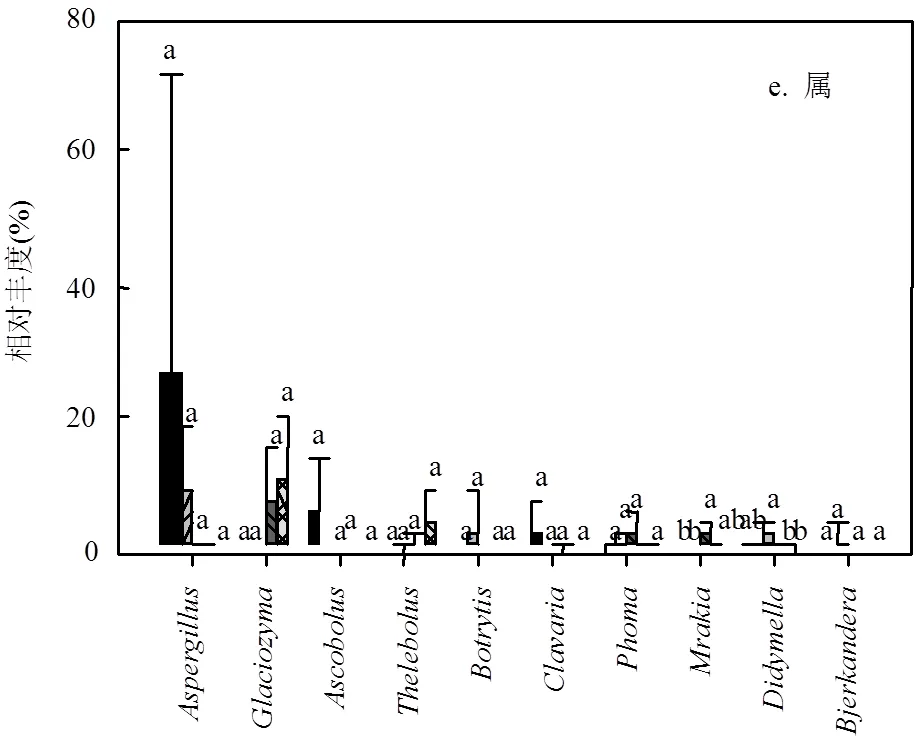
图3 沟道不同处理在门,纲,目,科,属水平上真菌群落的相对丰度 (P < 0.05)

真菌群落中,子囊菌门(Ascomycota)、担子菌门(Basidiomycota)和罗茨菌门(Rozellomycota)为优势菌门且均存在较大的变异性.子囊菌门在下游的相对丰度仅为上游相对丰度的41%(21.3%51.5%).与之相反,下游担子菌门的相对丰度为上游的2.18倍(14.2%6.5%),罗茨菌门为上游的1.62倍(3.1%1.2%).壶菌门(Chytridiomycota)和鞭毛菌门(Mortierellomycota)在沟道不同位置中有所差异,但平均相对丰度较小,分别为1.5%和1.1%(图3a).另外,除纲水平上座囊菌(Dothideomycetes)的相对丰度较上-中游显著降低外(0.05) (图3b),真菌群落在其它水平上均无显著差异.
2.4 细菌和真菌群落的Beta多样性分析及RDA分析
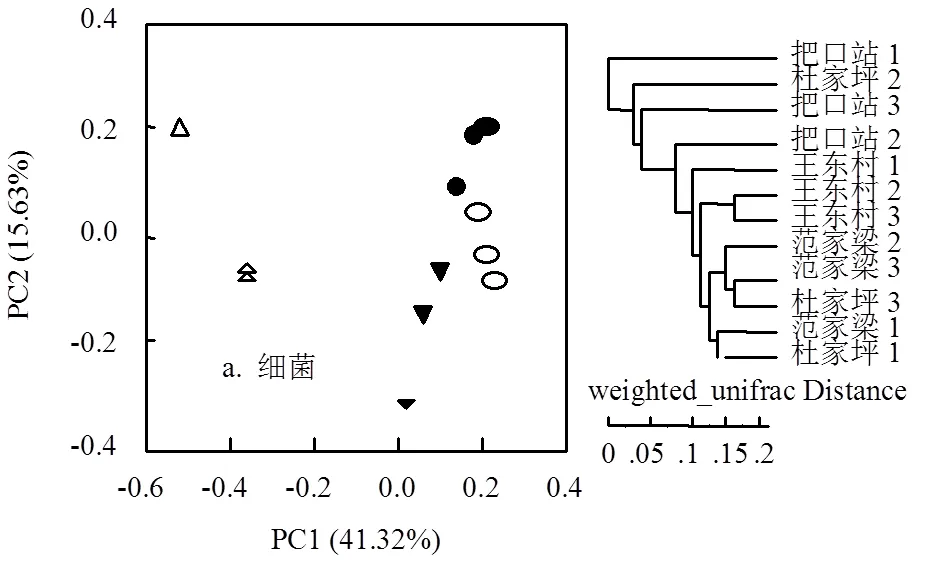
采用PCoA分析对沟道上-中-下游的泥沙样本进行分析,结果表明沟道微生物群落在分析结果中具有一定的空间差异(图4a、4b).细菌群落排序轴第1轴的贡献率为41.3%,排序轴第2轴的贡献率为15.6%,前2轴累计解释了变量的57.0%.其中,第一主成分轴上王东村、范家梁与杜家坪三点聚类明显,但与把口站差距明显.真菌群落排序轴第1轴的贡献率为21.1%,排序轴第2轴的贡献率为17.2%,前2轴累计解释了变量的38.3%.王东村、范家梁、杜家坪和把口站在第一与第二成分轴上均有差距.
构建UPGMA聚类树对沟道样品进行聚类分析,研究四个处理间的相似性.细菌群落的UPGMA聚类树表明:除杜家坪2外,把口站与其他3个处理的细菌群落区系明显属于两个不同的组(图4a);但是,真菌群落的UPGMA聚类树显示4个处理间的真菌群落区系区分不明显(图4b).

采用RDA分析对各环境因子(黏粒、粉粒、SOM和Olsen-P)与不同处理的泥沙微生物群落组成进行相关性分析(图5a、5b).结果显示细菌和真菌群落的分布与粒级含量、SOM和Olsen-P有较强的相关性.此外,在微生物群落,尤其是细菌群落的RDA分析中能够区分出把口站和上-中游两个明显的地理群.
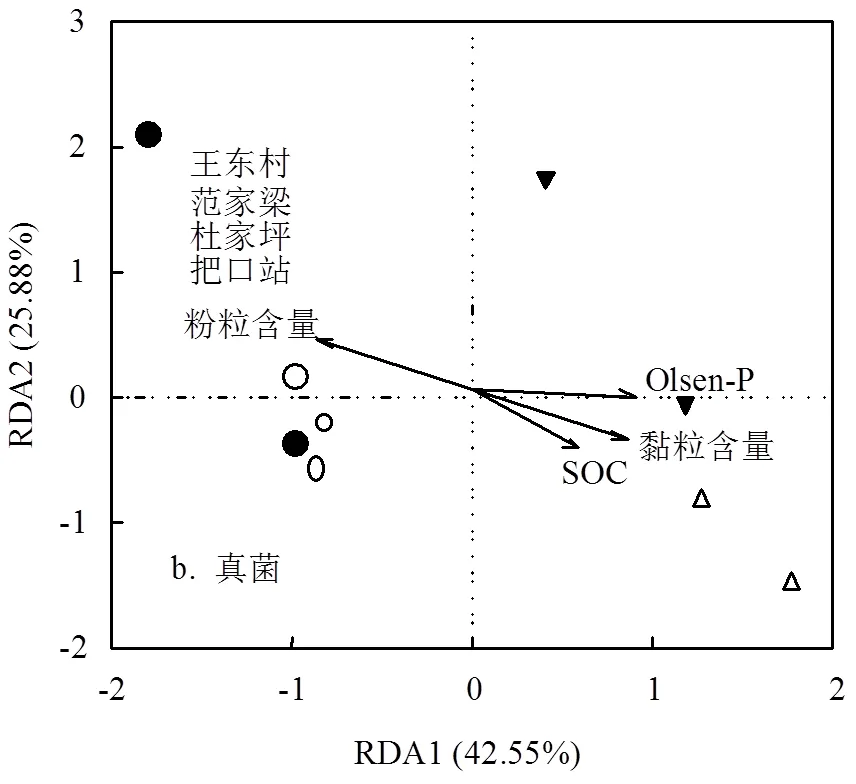
图5 沟道泥沙细菌和真菌群落在不同处理中的RDA分析

3 讨论
3.1 沟道泥沙颗粒分布的空间变化对微生物群落的影响
泥沙或土壤是微生物的栖息地,随着泥沙或土壤的运动,微生物群落会产生相应变化[43].从王东村(上游)到把口站(下游),沟道泥沙中黏粒比例增加,粉粒含量降低(表1);细菌和真菌群落的丰富度和多样性也存在差异(表2).通过粒级含量与Alpha多样性指数的回归分析发现:黏粒含量与细菌Chao1、Shannon指数显著负相关(<0.05);粉粒含量与细菌Chao1、Shannon指数显著正相关(<0.05).这些结果表明沟道细菌群落的空间分布差异可能与颗粒分布的空间变化有关.沟道内泥沙颗粒在迁移的过程中不断分选,黏粒含量不断提高,非毛管孔隙比例增加,使得细菌在该孔隙中活动困难[44],因而把口站处细菌群落的丰富度和多样性降低.除此之外,Yang等[45]在研究天然草地时发现与0.25~1mm和<0.25mm的颗粒相比, 2~4mm和1~2mm颗粒的细菌多样性更大;Sessitsch等[46]发现细砂、淤泥和黏土中相关细菌种群的组成、结构主要受粒径影响,细菌多样性呈现随粒径减小而增加的趋势.
与细菌不同,黏粒含量与真菌Chao1、Shannon指数显著正相关(<0.05);粉粒含量与真菌Chao1、Shannon指数显著负相关(<0.05).与细菌群落相关结果相比,泥沙粒级与真菌群落丰富度、多样性变化的相关性较弱,这是由于真菌菌丝的生长形式使它们比细菌具有更强的转移营养物和克服不良生长条件的能力[47-49].
3.2 沟道泥沙中微生物群落与养分含量的相关性
微生物群落的动态变化与侵蚀导致的土壤养分含量的改变密切相关[9,50-55].泥沙颗粒向下游分选的过程中黏粒含量比例增加,SOC、Olsen-P含量也有所提高.由RDA分析,SOC和Olsen-P沿沟道上-中-下游的含量变化是影响细菌与真菌群落组成和活性的因素(图5a、5b).SOC、Olsen-P与细菌丰富度、多样性显著相关(<0.05),表明SOC和Olsen-P能够影响细菌群落的组成.根据微生物营养对策分类,富营养菌可利用活性较高的碳源快速生长,寡养菌对贫瘠养分条件抗性能力更强[56].本研究中变形菌门[57]和拟杆菌门[56]均属于富养菌,因此这两种菌在养分含量丰富的把口站相对丰度较高.
真菌群落同样与土壤养分含量密切相关.SOC、Olsen-P与真菌丰富度、多样性正相关(<0.05).这可能与真菌具有良好的分解能力有关:子囊菌门和担子菌门能够分解大多数有机物质和植物残骸[58-59],因而沟道真菌群落的丰富度和多样性与养分含量有关.另外,PCoA分析中(图4b),随养分含量的增加,不同处理间真菌群落差距明显也可以印证这一点.另外,Trivedi等[50]研究发现侵蚀区SOC的流失能够抑制土壤微生物群落(特别是真菌)的快速生长;Thormann[51]发现门水平上不同真菌群落在泥炭地碳循环中发挥重要作用.因此,研究认为细菌和真菌群落在沟道泥沙中的动态变化与养分含量变化有关.
3.3 沟道的厌氧环境对微生物群落的影响
厌氧环境能够影响细菌群落的丰富度和多样性[55].由PCoA分析和UPGMA聚类树,把口站与王东村、范家梁、杜家坪3个处理差距明显(图4a),表明把口站由于长期积水产生的厌氧环境影响了细菌群落.由图2b,-变形菌纲(好氧菌)[60]在把口站的相对丰度较其他3个处理降低了近2/3(6.9% vs18.0%),拟杆菌纲(厌氧菌)[61]则增加6%(24.2% vs. 18.6%).由此,厌氧环境使把口站与上-中游细菌群落差异明显.
此外,结合王东沟流域塬面与坡面的已有研究成果,沟道Chao1指数(2408.2)和Shannon指数(8.45)显著低于塬面(4732.0, 10.09)和坡面(4589.4, 9.84) (< 0.05)[21,33],表明细菌群落的Alpha多样性指数在塬-坡-沟的地形变化中呈降低趋势.塬面和坡面的优势菌门中除变形菌(兼性厌氧菌)外,酸杆菌和放线菌均为好氧菌[60,62];沟道的优势菌门中,变形菌(兼性厌氧菌),拟杆菌(厌氧菌)和厚壁菌(厌氧菌)等厌氧菌富集(图2a)[61].从黄土高原沟壑区地貌组成角度来看,塬面和坡面是来水来沙区[63-65],沟道是水流和泥沙的汇集区[24],因而沟道中水分条件良好,形成的厌氧环境有利于厌氧菌的生存,进而影响了沟道细菌群落的分布.
沟道的厌氧环境尽管对细菌影响显著,但对真菌群落的影响不显著.对真菌群落而言,坡面土壤中的优势菌门为接合菌门(Zygomycota)、担子菌门和子囊菌门[21];沟道中为子囊菌门、担子菌门和罗茨菌门(图3a).有学者在接合菌门,子囊菌门和担子菌门中发现了17种需氧真菌种[66].但是,也有研究发现子囊菌门中有些菌纲,如粪壳菌能够适应厌氧环境 (图3b)[67-69];罗茨菌门也可以同时生存于土壤、淡水以及海洋沉积物等环境(图3a)[70-71].同时,UPGMA聚类树(图4b)聚类不明显可以证明这一点.
4 结论
4.1 门水平上,与上游相比,把口站的细菌群落中拟杆菌门与厚壁菌门的相对丰度分别增加6.6%、10.5%,变形菌门降低15.1%;真菌群落中担子菌门的相对丰度增加7.7%,而子囊菌门降低30.2%.
4.2 细菌群落的丰富度、多样性降低,而真菌群落增加,这可能与颗粒的空间分布有关.
4.3 沟道的理化环境影响微生物群落的分布.除此以外,沟道内厌氧环境在一定程度上影响了细菌群落的分布.
[1] Robert F Stallard. Terrestrial sedimentation and the carbon cycle: Coupling weathering and erosion to carbon burial [J]. Global Biogeochemical Cycles, 1998,12(2):231-257.
[2] Chaplot V, Jean Poesen. Sediment, soil organic carbon and runoff delivery at various spatial scales [J]. Catena, 2012,88(1):46-56.
[3] Asemeret Asefaw Berhe, Rebecca T Barnes, Johan Six, et al. Role of soil erosion in biogeochemical cycling of essential elements: carbon, nitrogen, and phosphorus [J]. Annual Review of Earth and Planetary Sciences, 2018,46(1):521-548.
[4] Harden J W, Sharpe J M, Parto W J, et al. Dynamic replacement and loss of soil carbon on eroding cropland [J]. Global Biogeochemical Cycles, 1999,13(4):885-901.
[5] Lal R. Soil erosion and carbon dynamics [J]. Soil & Tillage Research, 2005,81:137-142.
[6] Berhe A A, Harte J, Harden J W, et al. The significance of the erosion-induced terrestrial carbon sink [J]. Bioscience, 2007,57(4): 337-346.
[7] Gregorich E G, Greerb K J, Anderson D W, et al. Carbon distribution and losses — erosion and deposition effects [J]. Soil & Tillage Research, 1998,47:291-302.
[8] Nannipieri P, Ascher J, Ceccherini M T, et al. Microbial diversity and soil functions [J]. European Journal of Soil Science, 2003,54:655-670.
[9] Stavi I, Lal R. Variability of soil physical quality in uneroded, eroded, and depositional cropland sites [J]. Geomorphology, 2011,125: 85-91.
[10] Zhang J H, Wang Y, Jia L Z, et al. An interaction between vertical and lateral movements of soil constituents by tillage in a steep-slope landscape [J]. Catena, 2017,152:292-298.
[11] Whitman W B, Coleman D C, William J Wiebe. Prokaryotes: The unseen majority [J]. Proc. Natl. Acad. Sci., 1998,5: 6578-6583.
[12] Torsvik V, Øvreås L, Frede T Thingstad. Prokaryotic diversity- magnitude, dynamics, and controlling factors [J]. Science, 2002, 296(5570):1064-1066.
[13] Craig J Venter, Remington Karin, Heidelberg John F, et al. Environmental genome shotgun sequencing of the Sargasso Sea [J]. Science, 2004,304(5667):66-74.
[14] Paul G Falkowski, Tom Fenchel and Edward F Delong. The microbial engines that drive Earth’s biogeochemical cycles [J]. Science, 2008,320(5879):1034-1039.
[15] Liu Huanyao, Zhou Jiaogen, Feng Qingyu, et al. Effects of land use and topography on spatial variety of soil organic carbon density in a hilly, subtropical catchment of China [J]. Soil Research, 2017,55(2): 134.
[16] Dialynas Y G, Bastola S, Bras R L, et al. Topographic variability and the influence of soil erosion on the carbon cycle [J]. Global Biogeochemical Cycles, 2016,30(5):644-660
[17] Berhe A A, Barnes R T, Six J, et al. Erosional redistribution of topsoil controls soil nitroge dynamics [J]. Biogeochemistry, 2017,132:37-54.
[18] Hu Yaxian, Berhe Asmeret Asefaw, Fogel Marilyn L, et al. Transport-distance specific SOC distribution: Does it skew erosion induced C fluxes [J]. Biogeochemistry, 2016,128(3):339-351.
[19] Zhang Haicheng, Liu Shuguang, Yuan Wenping, et al. Inclusion of soil carbon lateral movement alters terrestrial carbon budget in China [J]. Science Reports, 2014,4(7247):1-6.
[20] Kirkels F M S A, Cammeraat L H, Kuhn N J. The fate of soil organic carbon upon erosion, transport and deposition in agricultural landscapes — A review of different concepts [J]. Geomorphology, 2014,226:94-105.
[21] Sun Qiqi, Hu Yaxian, Wang Rui, et al. Spatial distribution of microbial community composition along a steep slope plot of the Loess Plateau [J]. Applied Soil Ecology, 2018,130:226-236.
[22] 毕华兴,刘立斌,刘斌.黄土高塬沟壑区水土流失综合治理范式 [J]. 中国水土保持科学, 2010,8(4):27-33. Bi Huaxing, Liu Libin, Liu Bin. Paradigm of integrated management on soil and water losses in LoessPlateau-gullyRegion [J]. Science of Soil and Water Conservation, 2010,8(4):27-33.
[23] 费祥俊,邵学军.泥沙源区沟道输沙能力的计算方法 [J]. 泥沙研究, 2004,1:1-8. Fei Xiangjun, Shao Xuejun. Sediment transport capacity of gullies in small watersheds [J]. Journal of Sediment Research, 2018,130:226- 236.
[24] 王光谦,李铁键,薛 海,等.流域泥沙过程机理分析 [J]. 应用基础与工程科学学报, 2006,14(4):455-462. Wang Guangqian, Li Tiejian, Xue Hai, et al. Mechanism analysis of watershed sedmient processes [J]. Journal of Basic Science and Engineering, 2006,14(4):455-462.
[25] 钱 婧,张丽萍,王文艳.红壤坡面土壤团聚体特性与侵蚀泥沙的相关性 [J]. 生态学报, 2018,38(5):1590-1599. Qian Jing, Zhang Liping, Wang Wenyan. The relationship between soil aggregates and eroded sediments from sloping vegetated red soils of South China [J]. Acta Ecologica Sinica, 2018,38(5):1590-1599.
[26] 王兴奎,钱 宁,胡维德.黄土丘陵沟壑区高含沙水流的形成及汇流过程 [J]. 水利学报, 1982,7(4):26-35. Wang Xingkui, Qian Ning, Hu Dewei. The formation and process of confluence of the flow with hyperconcentration in the Gullied- Hilly Loess Areas of the Yellow River Basin [J]. Shuili Xuebao, 1982,7(4): 26-35.
[27] Bajracharya R M, Lal R, Kimble J M. Erosion effects on carbon dioxide concentration and carbon flux from an ohio alfisol [J]. Soil Science Society of America Journal, 2000,64(2):694-700.
[28] Walling D E. The sediment delivery problem [J]. Journal of Hydrology, 1983,65:209-237.
[29] Beusen A H W, Dekkers A L M, Bouwman A F, et al. Estimation of global river transport of sediments and associated particulate C, N, and P [J]. Global Biogeochemical Cycles, 2005,7(5):345-361.
[30] G C Starr, R Lal, R Malone, et al. Modeling soil carbon transported by water erosion processes [J]. Land Degrad. Dev., 2000,11:83-91.
[31] Lal R, Pimentel D. Soil erosion: a carbon sink or source [J]. Science, 2008,319:1040-1042.
[32] Jacinthe P A, Lal R. A mass balance approach to assess carbon dioxide evolution during erosional events [J]. Land Degradation & Development, 2001,12(4):329-339.
[33] Wang Rui, Sun Qiqi, Wang Ying, et al. Contrasting responses of soil respiration and temperature sensitivity to land use types: Cropland vs. apple orchard on the Chinese Loess Plateau [J]. Science of the Total Environment, 2018,621:425-433.
[34] Mohammadi M F, Jalali S G, Kooch Y, et al. The effect of landform on soil microbial activity and biomass in a hyrcanian oriental beech stand [J]. Catena, 2017,149:309-317.
[35] 杨佳佳,安韶山,张 宏,等.黄土丘陵区小流域侵蚀环境对土壤微生物量及酶活性的影响 [J]. 生态学报, 2015,35(17):5666-5674. Yang Jiajia, An Shaoshan, Zhang Hong, et al. Effect of erosion on soil microbial biomass and enzyme activity in the Loess Hills [J]. Acta Ecological Sinica, 2015,35(17):5666-5674.
[36] 覃 乾,朱世硕,夏 彬,等.黄土丘陵区侵蚀坡面土壤微生物量碳时空动态及影响因素 [J]. 环境科学, 2019,40(4):1973-1980. Qin Qian, Zhu Shishuo, Xia Bin, et al. Temporal and spatial dynamics of soil microbial biomass carbon and its influencing factors on an eroded slope in the Hilly Loess Plateau Region [J]. Environmental Science, 2019,40(4):1973-1980.
[37] Li Y, Su S. Comprehensive Study of Efficient and ecological economic system in Wangdonggou of Changwu County [M]. Xinjiang: Science and Technology Document Press, 1991.
[38] Li Y, Zhang Qian, Zhang Fangfei, et al. Analysis of the microbiota of black stain in the primary dentition [J]. Plos One, 2015,10(9).
[39] Ruibo Sun, Melissa Dsouza, Jack A Gilbert, et al. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter [J]. Environ Microbiol, 2016,18(12):5137-5150.
[40] Nelson D W, Sommers L E. Total carbon, organic carbon, and organic matter: laboratory method. Methods of Soil Analysis, Part 3 [M]. Madison:Soil Science Society of America, 1996:961-1010.
[41] Olsen S R, Cole V, Watenable F S, et al. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Cir. no. 939analysis, part 2 [J]. Am Soc Agron, 1954,9:914-926.
[42] Murphy J, Riley J P. A modified single solution method for the determination of phosphate in natural waters [J]. Anal. Chim. Acta, 1962,27:31-36.
[43] R K Väisänen, Roberts M S, Garl, J L. Physiological and molecular characterisation of microbial communities associated with different water-stable aggregate size classes [J]. Soil biology & biochemistry, 2005,37(11):2007-2016.
[44] 耿增超,戴 伟.土壤学 [M]. 北京:科学出版社, 2011:79-85. Geng Zengchao, Dai Wei. Soil Science [M]. Beijing: Science Press, 2011:79-85.
[45] Yang Chao, Liu Nan, Zhang Yingjun. Soil aggregates regulate the impact of soil bacterial and fungal communities on soil respiration [J]. Geoderma, 2019,337:444-452.
[46] Meagan E Schipanski, Elena M Bennett. The influence of agricultural trade and livestock production on the global phosphorus cycle [J]. Ecosystems, 2011,15(2):256-268.
[47] Jennings D H. Translocation of Solutes in fungi [J]. Bio. Rev., 1987, 62:215-243.
[48] Markus N T. Diversity and function of fungi in peatlands a carbon cycling perspective [J]. Soil. Sci., 2006,86:281-293.
[49] Yuste J C, En˜unlas J P, Estiarte M, et al. Drought-resistant fungi control soil organic matter decomposition and its response to temperature [J]. Global Change Biology, 2011,17(3):1475-1486.
[50] Xiao Haibing, Li Zhongwu, Chang Xiaofeng, et al. The mineralization and sequestration of organic carbon in relation to agricultural soil erosion [J]. Geoderma, 2018,329:73-81.
[51] Huang Wei, Chen Xing, Jiang Xia, et al. Characterization of sediment bacterial communities in plain lakes with different trophic statuses [J]. Microbiologyopen, 2017,6(5).
[52] Lu Sidan, Sun Yujiao, Zhao Xuan, et al. Sequencing Insights into Microbial Communities in the Water and Sediments of Fenghe River, China [J]. Archives of Environmental Contamination & Toxicology, 2016,71(1):122-132.
[53] Diab W, Toufailya J, Villieras F, et al. Study of physicochemical properties of colloidal sediments of Litani River in Lebanon [J]. Physics Procedia, 2014,55:251-258.
[54] Terrence G, Acosta-Martinez V, Francisco J C. Pyrosequencing reveals bacteria carried in different wind-eroded sediments [J]. Journal of environmental quality, 2012,41(3):744-753.
[55] Jiang Hongchen, Dong Hailiang, Zhang Gengxin, et al. Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China [J]. Applied and environmental microbiology, 2006,72(6):3832-3845.
[56] Noah Fierer Mark A Bradford, Robert B J. Toward an ecological classification of soil bacteria [J]. Ecology, 2007,88(6):1354-1364.
[57] Brajesh K S, Richard D B, Pete Smith, et al. Microorganisms and climate change: terrestrial feedbacks and mitigation options [J]. Nature Reviews Microbiology, 2010,8(11):779-790.
[58] Francois Lutzoni F, Frank Kauff, Cymon J Cox, et al. Assembling the fungal tree of life: Progress, classification and evolution of subcellular traits [J]. American Journal of Botany, 2004,91(10):1446-1480.
[59] Baldrian P, Kolarik M, Stursova M, et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition [J]. The ISME Journal, 2012,6,248-258.
[60] Wells C L, Wilkins T D. Clostridia: Sporeforming Anaerobic Bacilli. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX) [M]. Galveston: University of Texas Medical Branch, 1996: Chapter 18.
[61] Flynn T M, Koval J C, Greenwald S M, et al. Parallelized, aerobic, single carbon-source enrichments from different natural environments contain divergent microbial communities [J]. Front Microbiol, 2017, 8:1-14.
[62] Eichorst S A, Breznak J A, Schmidt T M. Isolation and characterization of soil bacteria that define Terriglobus gen. nov., in the phylum Acidobacteria [J]. Appl Environ Microbiol, 2007,73(8): 2708-2717.
[63] 徐雪良.韭园沟流域沟间地、沟谷地来水来沙量的研究 [J]. 中国水土保持, 1987(8):23-26. Xu Xueliang. Study on the amount of water coming from the ditch and valley in the Gengyuangou Basin [J]. Soil and Water Conservation In China, 1987(8):23-26.
[64] 陈 浩.降雨特征和上坡来水对产沙的综合影响 [J]. 水土保持学报, 1992,6(2):17-23. Chen Hao. The synthetic effect of rainfall characteristics and runoff from upper slope on sediment generation [J]. Journal of Soil and Water Conservation, 1992,6(2):17-23.
[65] 陈 浩,王开章.黄河中游小流域坡沟侵蚀关系研究 [J]. 地理研究, 1999,18(4):363-372. Chen Hao, Wang Kaizhang. A study on the slope-gully erosion relationship on small basins in the loess areas at the middle reaches of the Yellow River [J]. Geographical Research, 1999,18(4):363-372.
[66] Young D, Dollhofer V, Callaghan T M, et al. Isolation, identification and characterization of lignocellulolytic aerobic and anaerobic fungi in one- and two-phase biogas plants [J]. Bioresour Technol, 2018,268: 470-479.
[67] Spatafora J W. Ascomal evolution of filamentous ascomycetes: evidence from molecular [J]. Canadian Journal of Botany, 1995, 73(S1):811-815.
[68] Neuveglise C, Brygoo Y, Vercambre B, et al. Comparative-analysis of molecular and biological characteristics of strains of Beauveria brongniartii isolated from insects [J]. Mycological Research, 1994, 98(3):322-328.
[69] Berbee M L, Taylor J W. Two ascomycete classes based on fruiting- body characters and ribosomal DNA sequence [J]. Molecular Biology and Evolution, 1992,38(5):1590-1599.
[70] Turner M. The evolutionary tree of fungi grows a new branch [N]. Nature News, 2014-05-31.
[71] Ghosh P. Missing link fungi found in Devon pond [N]. BBC News, 2014-10-31.
致谢:本实验的野外采样工作由李伟佳、贺瑶等同学提供帮助,在此表示感谢.
Variations of soil microbial communities along a valley bottom of the loess plateau and the influencing factors.
HOU Fang-bin1, WANG Rui1, Salman Ali2, GAO Xin1, GUO Sheng-li1,3*
(1.State Key Laboratory of soil Erosion and Dryland Farming on the Loess Plateau, Institute of Soil and Water Conservation, Northwest A&F University, Yangling 712100, China;2.College of Resource and Environment, Northwest A&F University, Yangling 712100, China;3.Institute of Soil and Water Conservation, Chinese Academy of Sciences and Ministry of Water Resource, Yangling 712100, China)., 2019,39(10):4350~4359
Sediments were collected from up- to down-stream along a valley bottom on the Loess Plateau. Physicochemical properties of the collected sediments were measured and the characteristics of bacterial and fungal communities in the sediment samples were also determined using the high-throughput sequencing of 16S rRNA gene fragment and ITS. From up- to down-stream of the studied valley, the relative abundance of Bacteroidetes and Firmicutes in the collected sediments increased by 6.6% and 10.5% respectively, and the relative abundance of Proteobacteria decreased by 15.1%. The relative abundance of Basidiomycota increased by 7.7%, while the relative abundance of Ascomycota decreased by 30.2%. The bacterial richness (Chao1index) and diversity (Shannon index) were negatively correlated with sediment clay content (< 0.05), while the correlations between clay content and fungal were not significant. The richness and diversity of bacterial and fungal communities were also correlated with sediment SOC and Olsen-P (< 0.05). Therefore, sediment compositions and nutrient content appeared to be a crucial factor influencing the spatial variability of sediment microbial communities and diversity.
sediments;spatial re-distribution;bacteria;fungi;sediment compositions
X172
A
1000-6923(2019)10-4350-10
侯芳彬(1995-),女,河南新乡人,西北农林科技大学硕士研究生,主要研究方向为土壤生态.
2019-03-07
国家自然科学基金资助项目(41371279)
* 责任作者, 研究员, slguo@ms.iswc.ac.cn
