Uremic clearance granule combined with Alprostadil in the treatment of chronic renal failure:A systematic review and meta-analysis.
2019-10-14JingGanYaFangGuoDongDongLiZiZhengZhouJiaBaoZhouJianDongGao
Jing Gan,Ya-Fang Guo,Dong-Dong Li,Zi-Zheng Zhou,Jia-Bao Zhou,Jian-Dong Gao
1.Department of Nephrology,Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine;TCM institute of kidney disease,Shanghai University of Traditional Chinese Medicine;Key Laboratory of Liver and Kidney Diseases (Shanghai University of Traditional Chinese Medicine),Ministry of Education;Shanghai Key Laboratory of Traditional Chinese Clinical Medicine (14DZ2273200),Shanghai,China.
2.Department of TCM,Shanghai Pudong Hospital,Fudan University Pudong Medical Center,Shanghai,China.
Abstract
Key words:Uremic clearance granule,Chronic Renal Failure,Alprostadil,Meta-Analysis
Background
Chronic renal failure (CRF) is now recognized to be a worldwide problem associated with significant morbidity and mortality and there is a steep increase in the number of patients reaching end-stage renal disease (ESRD) [1].CRF is a serious disease with many complications.It is a series of complex symptoms caused by renal dysfunction,which leads to confusion in the internal circulation of the human body [2].The clinical manifestations are anorexia,nausea and other symptoms.In the later stage,the corresponding clinical symptoms will appear in the blood system,cardiovascular system,neuromuscular system,respiratory system,endocrine system and other functions [3].If it is not found or controlled in time,it will threaten the life of the patient.Renal transplantation and dialysis are the main methods to treat the end stage of the disease,but there are some problems,such as high cost and the corresponding adverse reactions.Therefore,how to delay the progress of CRF and improve the quality of life of patients economically,early and effectively has become an important issue at present.Drug therapy is the main method for the treatment of early CRF,it is widely used because of its low medical cost and wide range of application.Alprostadil belongs to prostaglandin E1 preparation,which plays the role of renal vasodilation by directly replenishing prostaglandins lacking in human body,reducing glomerular contractility,vascular resistance and filtration rate,and increasing urine volume [4].Alprostadil can inhibit platelet aggregation,protect vascular endothelial cells,reduce vasoconstriction,stabilize lysosomal membrane,reduce blood viscosity and erythrocyte aggregation,improve erythrocyte deformability and other renal protective effects,which has been widely used in the treatment of CRF [5,6].
Uremic clearance granule is a commonly used traditional Chinese medicine (TCM) preparation,which is mainly made of Polygonum multiflorum,Codonopsis pilosula,Astragalus membranaceus and Atractylodes macrocephala.Its efficacy is to invigorate kidney and fill essence,activate blood circulation and remove blood stasis.Studies have shown that Uremic clearance granule can reduce the levels of urea nitrogen,serum uric acid and serum creatinine [7,8],which has a good effect on CRF.A number of RCTs [8] showed that the combination of Uremic clearance granule with Alprostadil was more effective,but its exact effectiveness and safety were still lack of evidence-based medical evidence.In this study,the systematic evaluation method was used to evaluate the efficacy and adverse reactions of Uremic clearance granule combined with Alprostadil,so as to provide a reference for clinical practice.
Methods
Search strategy
Electronic databases were searched using Pubmed,Embase,CNKI,Wanfang,Cochrane Library,and Web of Science from January 1990 to March 2019.The search strategy involved free-text words and medical subject heading terms.The search terms used individually or in combination were:"Uremic clearance granule" ,"Niaoduqing particles" and "Alprostadil","prostaglandin E1","PGE1","caverject" and "chronic renal failure","CRF","chronic kidney disease","CKD","chronic kidney failure" ,"chronic kidney insufficiency","chronic kidney dysfunction","chronic renal disease","chronic renal insufficiency","chronic renal dysfunction" "end stage renal disease","ESRD".The syntax used for Pubmed and Embase are provided in supplementary 1 and 2,and the search strategies used for the other databases were similar,with the necessary adaptions made.No restrictions were imposed on publication language.
Inclusion criteria
(i) Study type:RCTs,whether or not blind method was used.(ii) Participants:The serum creatinine (Scr) of the patients was 133~770umol/L,and no renal replacement therapy was performed.(iii) Intervention:Both groups were given routine treatment,including low salt,high quality and low protein diet control,control of blood pressure,maintenance of acid-base and electrolyte balance,and so on.Experimental group:Uremic clearance granule plus Alprostadil injection.Control group:Alprostadil injection.(iv) Outcome measures:Primary outcome:Total clinical effective rate,serum creatinine (Scr,μmol/L),urea nitrogen (BUN,mmol/L).Secondary outcome:changes of serum uric acid (UA,μmol/L),serum cystatin C (CysC,mg/L),endogenous creatinine clearance rate (Ccr,mL/min),24-hour urinary protein (24h-Upro,g).All outcome indicators should be unified in units,and those that can not be unified should be eliminated.
Exclusion criteria
(i) Non-RCT studies;(ii) RCTs with other methods of treatment as intervention measures;(iii) Inconsistent data;(iv) Animal studies,case reports,letters,reviews and conference abstracts.(v) Duplicate publication.
Data abstraction
Full text was read in detail to determine whether the literature meet the inclusion criteria.For similar articles of the same author appearing in different journals,the most complete (or recently published) article was selected for inclusion in the study.Data of all included literature were extracted according to the pre-designed tables.Detailed data abstracted from the trials included the name of first author,year of publication,study sample,sex and age of subjects,details of intervention,doses,effectiveness and ineffectiveness number,course of treatment,adverse events of treatment and the following reported outcomes:Scr,BUN,UA,CysC,Ccr,24h-Upro.Two investigators independently judged which literatures can be eligible in the meta-analysis,and the consistency was calculated by the Kappa value.They collected information from the included literatures independently,and the inconsistence were settled throuth discuss or by a third author.
Quality assessment
The quality of eligible studies was assessed according to the criteria from the Cochrane Handbook for Systematic Reviews of Intervention [9].Selection bias (random sequence generation and allocation concealment),performance bias (blinding of participants and personnel),detection bias (blinding of outcome assessment),attrition bias (incomplete outcome data),reporting bias (selective reporting),and other biases (determined according to sample size calculation method,inclusion/exclusion of criteria for patients' recruitment,comparability of baseline data,funding sources,and any other potential methodological flaw that might have influenced the overall assessment) were assessed.Three potential bias judgments:low risk,high risk,and unclear risk,were determined for each single trial during assessment.A judgment of low risk was made when all the seven items met the criteria as "low risk",a judgment of high risk of bias was made when at least one of the seven items was assessed as "high risk".
Data synthesis and analysis
Meta-analysis was carried out by Stata15.0 software.For count data and measurement data,standardized mean difference (SMD) or odds ratio (OR) with 95% confidence interval (CI) was calculated.The heterogeneity was assessed with Z test.The value of I2was used to determine the presence of heterogeneity.If p < 0.05 or I2>50%,heterogeneity was considered statistically significant,randomeffects model was used.Otherwise,it was determined that there be no heterogeneity,fixed-effects model was used.The Z test was used to compare the overall effects of treatment group and control group,and differences were considered to be statistically significant when p < 0.05.Funnel plot is a common method of measuring a qualitative publication bias [10].If the funnel plot asymmetrical on both sides of the distribution,suggesting the presence of bias;If symmetrical on both sides of the funnel plot,suggesting no bias.
Results
Description of studies
The combined literature retrieval yielded 213 citations initially,after reading the title and abstract of the identified studies,204 reports were excluded due to non-RCTs,nonrandomized,animal or basic research,duplicate publications,failure to get available data or review articles.Finally,a total of 9 RCTs enrolling 762 patients were identified as appropriate for inclusion in this analysis (Fig.1).The Kappa value is 0.654,which means that the systematic search consistency is strong.All the literature was published in Chinese,and all the research sites were in mainland China.Characteristics of the studies are summarized in Tab.1.
Methodological quality of included studies
Randomization:all studies mentioned randomization,only 2 articles [16,19] had a detailed description of random sequence generation.Allocation concealment:none of them discussed allocation concealment.Blinding:only one trial [14] reported "singleblind" for the patients,the remaining 8 studies did not mention blinding.Data integrity:no outcome data were missing from all studies.Selective publication:no selective results were reported.Other biases:it is impossible to judge whether there are other biases.For lacking of specific information,it can not be determined whether implementations were conducted adequately in the process of random sequence generation,blinding or allocation concealment,the quality of the included trials was relatively low.A description of the assessment of methodological quality in the studies is shown in Fig.2.
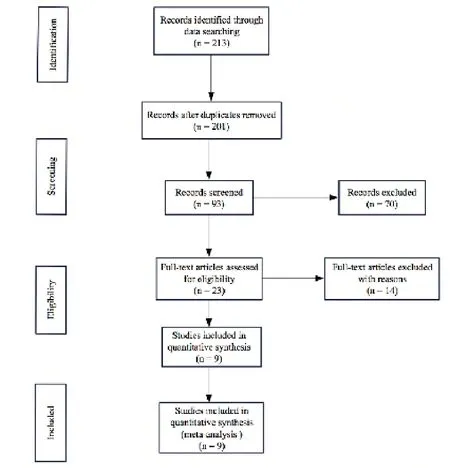
Figure 1.Study selection flow diagram
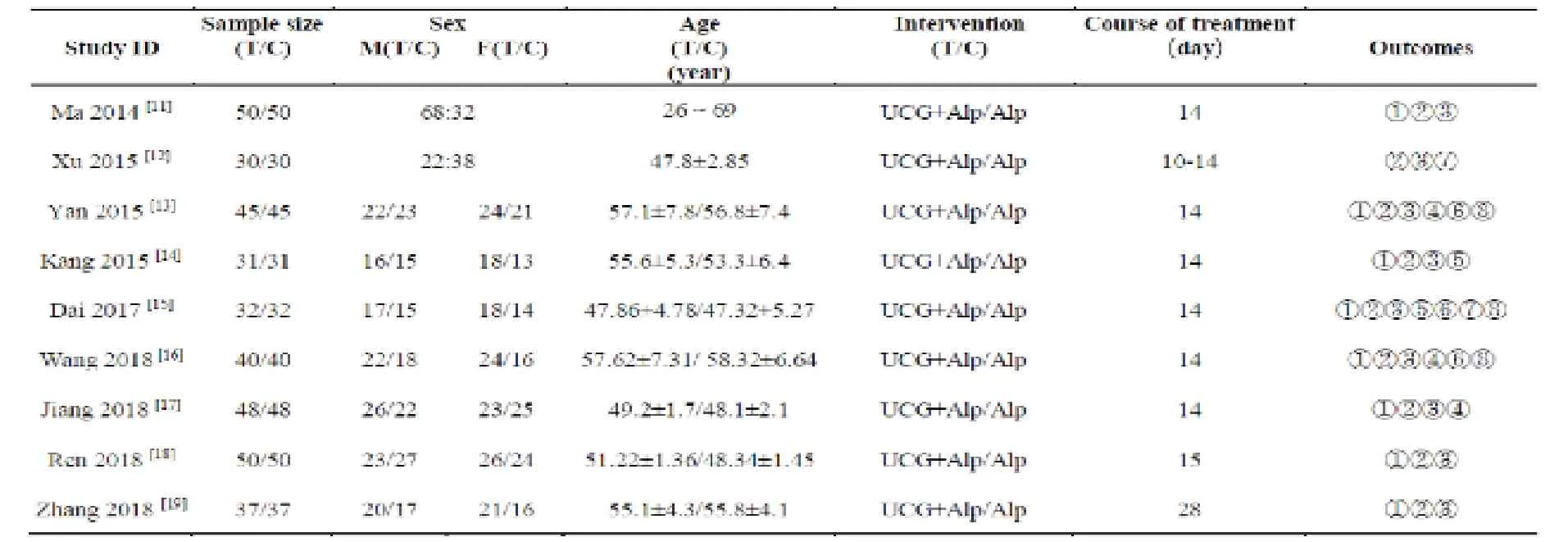
Table 1.Characteristics of 9 included trials
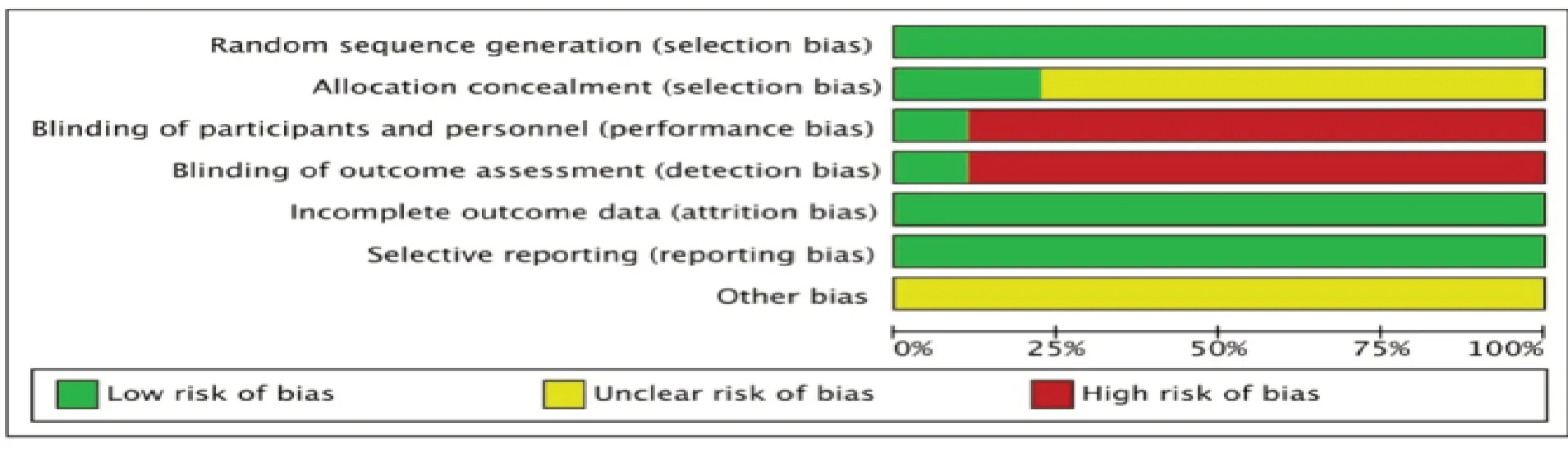
Figure 2.The risk of bias assessment in the studies
Treatment effect Primary outcome Total effective rate
A total of 8 studies included data on total effective rate [11,13-19].There was no heterogeneity in the statistical analysis (I2= 0.0%,P = 0.972),so a fixed-effects model was performed.Meta-analysis showed that the experimental group had advantages in improving the total effective rate [OR = 3.68 (2.44,5.55),P < 0.001] (Fig.3).
Scr
There are 9 studies [11-19] mentioned the change of Scr after treatment.Highly significant heterogeneity was found among these 9 studies (I2= 97.4%,P = 0.972),so a random-effects model was performed.Meta-analysis showed a significant improvement in Scr [SMD = -2.34 (-3.49,-1.19),P < 0.001] (Fig.4).
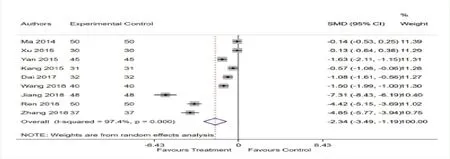
Figure 4:Forest plot of Scr
BUN
There are 9 studies [11-19] mentioned the change of BUN after treatment.Highly significant heterogeneity was found among these 9 studies (= 96.6%,P < 0.001),so a random-effects model was performed.Meta-analysis showed a significant improvement in Bun [SMD = -1.80 (-2.73,-0.87),P < 0.001] (Fig.5).
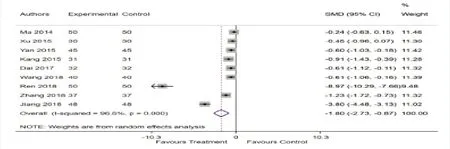
Figure 5.Forest plot of BUN
Secondary outcome
Meta-analysis showed a significant improvement in Ccr [SMD = 0.71 (0.44,0.97),P < 0.001].Meta-analysis showed no statistical significance between the groups in UA [SMD = -1.75 (-3.59,0.08),P = 0.061],CysC [SMD = -1.33(-2.68,0.02),P = 0.053] and 24h-Upro [SMD = -0.15 (-0.50,0.20),P = 0.402] (Tab.2).

Table 2.Meta-analysis of Secondary outcome
Safety profile and adverse events
Due to the limited number of literature included in this clinical study,only 4 literature [13-16] reported adverse reactions,and 3 of them [13,15,16] specified the observation methods and items of adverse reactions.The other 5 studies [11-12,17-19] did not mention adverse reactions.There was no statistical heterogeneity between the groups (I2= 0.0%,P = 0.823),so a fixed-effects model was used.Meta-analysis showed that there was no statistically significant difference between the two groups [SMD = 0.61 (0.31,1.17),P = 0.138] (Fig.6).

Figure 6.Forest plot of adverse events
Publication bias
The funnel plot analysis based on the total effective rate showed the evidence of publication bias.It can be seen that the distribution of each study is relatively asymmetric,suggesting publication bias (Fig.7).
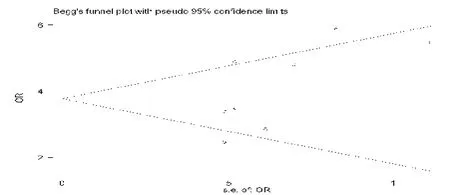
Figure 7:Funnel plot of the total effective rate
Discussion
Ureic clearance granule (Niaoduqing particles) is a common traditional Chinese herbal medicine that manufactured according to the guideline of TCM,and its effective constituents are astragaloside,emodin and salvianolic acid A [8].Both fundamental research and clinical studies have confirmed that Ureic clearance granule can improve renal function,alleviate CKDassociated complications,reduce urinary protein,improve renal anemia,and correct the disorder of lipid metabolism,glucose metabolism and calcium-phosphorus metabolism,reduce glomerular pathological changes,prevent glomerular sclerosis and renal interstitial fibrosis and so forth [20,21].The total effective rate of clinical treatment of CRF is 60%~90% [22,23].According to the current molecular research progress,its pharmacological mechanism is mainly related to anti-fibrosis,repair of podocytes,improvement of hemorheology,protection of endothelial function,improvement of microinflammation,antioxidant stress and so on [24].Therefore,whether CRF patients with dialysis or not can get multiple benefits from using Ureic clearance granule in addition to basic western medicine treatment.In China,Ureic clearance granule has been used clinically for more than 20 years and their mechanism of action has been comprehensively investigated [25].
The results of our meta-analysis showed that Ureic clearance granule and Alprostadil was superior to the Alprostadil alone in improving the total effective rate and Ccr,reducing the levels of Scr and BUN in the patients.At the same time,the results of meta-analysis did not change after excluding some literature,which indicated that the results were stable and reliable.In reducing UA,CysC and 24h-Upro,the results were inconsistent with those of descriptive analysis,so it could not confirm the efficacy of Ureic clearance granule,this may be related to the fact that the literature included in this study has very few analysis of UA,CysC and 24h-Upro.More clinical studies are needed to confirm it.However,there was considerable clinical heterogeneity among these included trials,the reasons of the underlying heterogeneity may due to the different course of treatment (10 to 28 days),inconsistency of measurement methods applied in each study,low methodology.In terms of adverse reactions,there is no serious adverse reactions reported in all literature,while only three literature [13,15,16] reported the specific contents of adverse reactions.The most adverse reactions reported are mild dizziness,headache and gastrointestinal reactions like vomiting,abdominal pain,abdominal distension and diarrhea in both groups.Patients could tolerate the symptoms and relieved themselves without symptomatic treatment.Compared with alprostadil alone,there was no significant difference between the two groups,which showed that the addition of Ureic clearance granule did not increase the adverse reactions and had good safety.
This study had several limitations.Firstly,this meta-analysis has not been registered online.Secondly,the number of literature included in this study is small,all of the included trials were conducted and published in China,the subjects were Chinese,and the extrapolation of the results was restricted,thus the Egger test and Begg test manifested that there was potential publication bias among the included studies.Meanwhile,the quality of methodology of the studies was generally low.For those studies without detail explanation of quality control measures,we can not rule out the possibility of a selective bias,implementation bias and measurement bias,most items were assessed as unclear risk,which may influence the validity of overall findings and overestimate the effectiveness of Ureic clearance granule to some degree.Thirdly,all the included studies did not mention about withdrawal and dropouts.Almost half of the included trials did not report adverse events.Fourthly,CRF is a chronic disease with different stages of development and the admission time of patients was varied,which makes it difficult to adopt uniform random and blind design.
In conclusion,this study showed that a combination of Ureic clearance granule and Alprostadil was beneficial for total effective rate,Scr,and BUN.Besides,it also can promote Ccr.Ureic clearance granule is security and no obvious adverse reactions.However,it is important to use rigorously appropriate randomization,allocation concealment and double-blinded experimental method to carry out high-quality,multi-center RCTs with large sample size.
Acknowledgments
This work is supported by Shanghai Three-Year Project of Traditional Chinese Medicine (ZYSNXD-CCYJXYY and ZY3-CCCX-3-4002).
杂志排行
Medical Theory and Hypothesis的其它文章
- The mechanism and traditional Chinese medicine research of bone destruction and hypercalcemia in multiple myeloma
- Discussion on the clinical significance of moxibustion in improving cancer pain
- Principle and operation of Jing-well point temperatures' test
- A preliminary study on the properties of red traditional Chinese medicine
