混凝-超滤工艺去除水中复合污染物试验研究
2019-10-08赵纯金凡安叶孙志华郑怀礼
赵纯 金凡 安叶 孙志华 郑怀礼


摘要:研究分析腐殖酸对水溶液中纳米TiO2稳定性的影响,探究混凝剂投加量、pH、钙离子对混凝?超滤工艺去除水中腐殖酸纳米TiO2复合污染物的影响。结果表明,纳米TiO2与腐殖酸在水溶液中发生的静电吸附以及配位反应,将引起纳米TiO2有效粒径的减小,静电斥力增强,胶体分散更均匀,体系稳定性增加,易于迁移,从而给饮用水安全带来威胁。在单因素影响实验中,实验结果显示,混凝剂浓度为0.46 mmol/L,pH值在7~8之间(即弱碱性)时,能有效去除复合污染物,此时,膜通量较高,膜污染较轻,而水中钙离子的存在会加重膜污染。
关键词:纳米TiO2;腐殖酸;混凝;超滤;膜污染
中图分类号:X703.1
Abstract: The effect of humic acid (HA) on the stability of nano-TiO2 was analyzed, and the effects of coagulant dosage, pH and calcium(Ⅱ) concentration on the removal efficiency of HA- TiO2 composite pollutants by the coagulation-ultrafiltration process were investigated. The results showed that the electrostatic adsorption and coordination reaction occurred between nano-TiO2 and HA in the aqueous solution, which caused the decrease of effective particle size of nano-TiO2, the enhancement of electrostatic repulsion, more uniform dispersion of colloid, the increase of system stability and easy migration. These posed a threat to the safety of drinking water. The optimum parameter for HA-TiO2 composite pollutants removal was that the coagulant concentration is 0.46 mmol/L, and the initial pH value is between 7 and 8,the higher membrane flux and lighter membrane fouling was achieved under this condition. The calcium ion in the solution will lead to the increase of membrane fouling.
Keywords: nano-titanium dioxide; humic acid; coagulation; ultrafiltration; membrane fouling
近年来,随着居民生活质量的提高,饮用水安全问题备受关注,研究日趋深入。纳米材料作为一种广泛应用于化工制造、个人护理及食品工业等的新材料,进入天然水体后,天然有机物质(NOM,如腐植酸、富里酸等)与纳米粒子间的相互作用可能改变其毒性和稳定性,对其在环境中的迁移和转化有重要影响,给饮用水安全带来威胁[1-3]。学者们考察了纳米材料本身的性质,以及环境溶液的化学性质和物理因素对纳米材料在水体中的沉积、聚集及其潜在危害的影响,而关于纳米材料与NOM在水溶液中形成的复合污染物的研究却很少[4-5]。因此,研究NOM与纳米颗粒在水中的相互作用具有实际价值。
传统的水处理工艺对纳米颗粒的去除并不理想,而超滤因能有效截留水中胶体、悬浮物、细菌等,在纳米颗粒去除方面展现出优势,但却面临膜污染等问题[6]。不过,将超滤与其他技术(混凝[7-8]、吸附[9-10]、预氧化[11-12]等)相结合,能有效减轻膜污染,其中,混凝?超滤因具有成本低、操作简单、处理效果好等优点而得到广泛应用。纳米颗粒经过混凝后凝聚,用膜过滤有很好的去除效果,且与传统的过滤方法相比,膜滤对水中的纳米颗粒表现出更好的去除效果[13-14]。
选取腐殖酸(HA)和纳米二氧化钛(纳米TiO2)作为研究对象,对纳米TiO2与腐殖酸之间的相互作用进行简单分析,探究混凝剂投加量、溶液pH及钙离子濃度对混凝?超滤工艺(C-UF)去除HA和纳米TiO2复合污染物(HA-T)的影响。
1实验材料与方法
1.1实验材料
实验所涉及的材料及药品信息具体见表1。
1.2 实验方法
1.2.1 聚合硫酸铁配制 混凝剂聚合硫酸铁(PFS)为实验室所制备[15],PFS母液配制浓度为2.857 mol/L,有效浓度为80%,稀释20倍,制得混凝剂PFS浓度为:C0=0.114 mol/L。
1.2.2原水配制 配制1.0 g/L HA储备液:将1.0 g HA和0.40 g NaOH溶解于1 000 mL超纯水中,室温(25 ℃)下搅拌24 h,用0.45 μm滤膜过滤后于4 ℃环境中保存备用。
配制1.0 g/L 纳米TiO2储备液:将0.10 g TiO2固体粉末溶解于100 mL超纯水中,并在超声机内(40 KHz)超声0.5 h以上,使其均匀分散,然后于4 ℃环境中保存备用。
2.3膜表面滤饼层形态分析
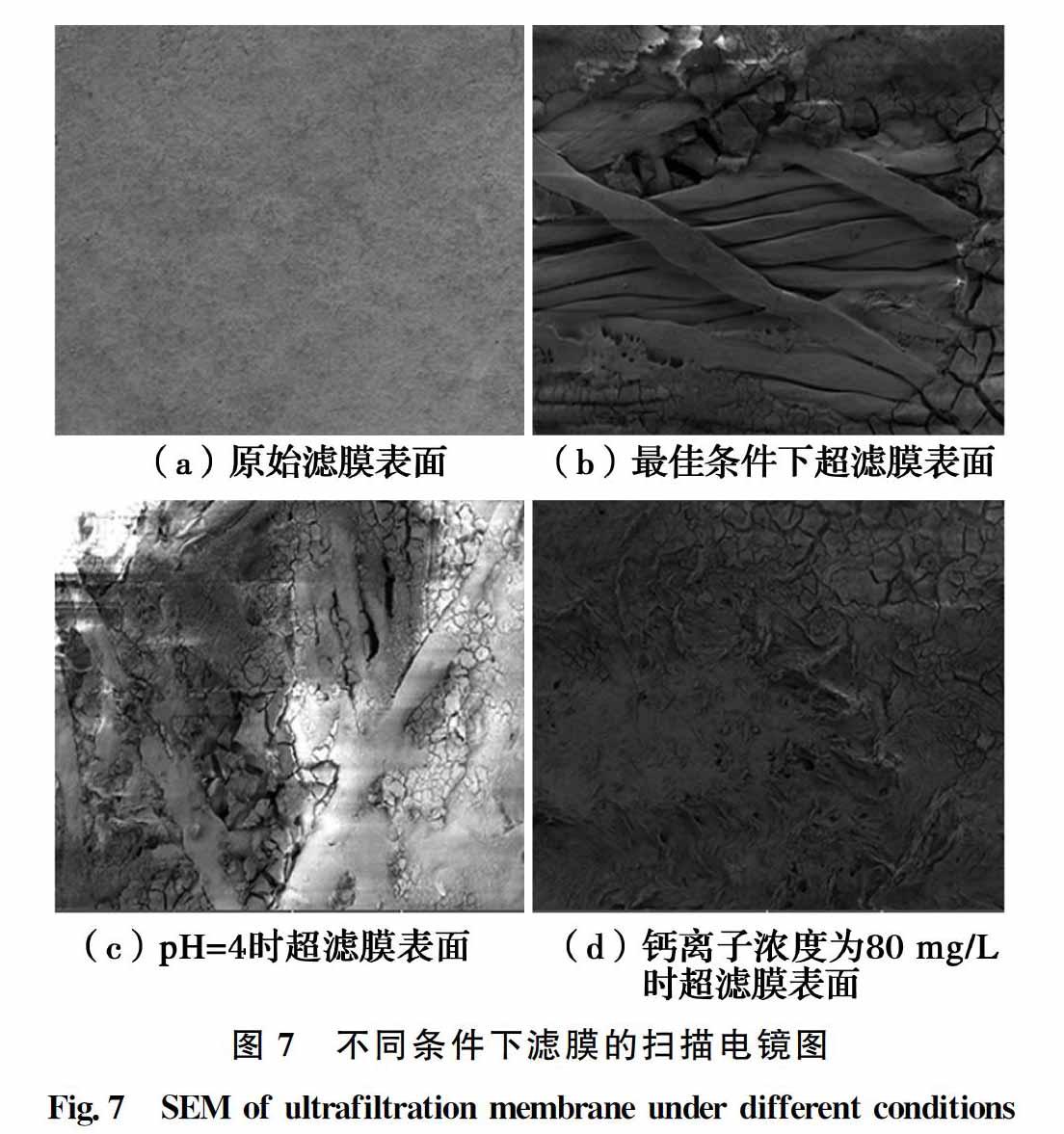
对实验后的滤膜进行SEM扫描,可以更加直观地观察膜表面滤饼层微观形态。图7(a)为原始滤膜表面,干净光滑;图7(b)为最优条件下(pH=8,混凝剂投加量为0.46 mmol/L,不添加Ca2+)滤膜表面滤饼层,可观察到其交连的大分子骨架结构,孔隙率高,膜堵塞情况较轻;图7(c)为将pH值调整为4时滤膜表面滤饼层,表面絮体密实,骨架结构分布不均匀,孔隙率低;图7(d)为80 mg/L Ca2+时滤膜表面滤饼层,其他条件同图7(b),与图7(b)的大分子骨架结构明显不同,絮体结构致密,孔隙小,说明Ca2+的存在会加重膜污染。根据XDLVO理论,pH值、PFS浓度、Ca2+浓度对膜表面的污染程度主要取决于污染物与膜材料、离子等相互作用的自由能,调低pH值、投加阳离子等措施都将导致自由能减少,膜污染加重[22-23]。
3结论
1)水溶液中纳米TiO2与HA会发生静电吸附以及配位反应,使纳米TiO2水溶液体系的有效粒径减小,静电斥力变大,纳米TiO2稳定性增强,胶体分散更均匀,易于迁移。
2)混凝剂投加量、pH值、Ca2+浓度都是影响C-UF工艺处理效果的重要因素。PFS浓度为0.46 mmol/L,弱碱性(pH值7~8)时,不添加钙离子,处理效果较好;其中,混凝剂投加量过低或过高,原水pH过低或过高,都会对C-UF工艺处理HA-T复合污染物产生不利影响,使超滤膜过滤通量减小,加重膜污染。
3)当混凝机理为以网捕卷扫、吸附架橋为主,以电性中和为辅时,膜污染较轻,膜通量较高。
参考文献
[1] CHEN Q Q, YIN D Q, ZHU S J, et al. Adsorption of cadmium(II) on humic acid coated titanium dioxide [J]. Journal of Colloid and Interface Science, 2012, 367(1): 241-248.
[2] WANG Y, XUE N, CHU Y B, et al. CuO nanoparticle–humic acid (CuONP–HA) composite contaminant removal by coagulation/ultrafiltration process: The application of sodium alginate as coagulant aid [J]. Desalination, 2015, 367: 265-271.
[3] GHAEMI N, MADAENI S S, DARAEI P, et al. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles [J]. Chemical Engineering Journal, 2015, 263: 101-112.
[4] 鲁晶, 刘冬梅, 刘世光, 等. 纳米TiO2颗粒与腐殖酸和SDBS的相互作用机制[J]. 哈尔滨工业大学学报, 2015, 47(8): 21-24.LU J, LIU D M, LIU S G, et al. Interaction mechanisms of the TiO2 nanoparticles with humic acid and SDBS [J]. Journal of Harbin Institute of Technology, 2015, 47(8): 21-24.(in Chinese)
[5] LI L, SILLANP?? M, RISTO M. Influences of water properties on the aggregation and deposition of engineered titanium dioxide nanoparticles in natural waters [J]. Environmental Pollution, 2016, 219: 132-138.
[6] 范小江, 张锡辉, 苏子杰, 等. 超滤技术在我国饮用水厂中的应用进展[J]. 中国给水排水, 2013, 29(22): 64-70.FAN X J, ZHANG X H, SU Z J, et al. Application of ultrafiltration technology in drinking water treatment plants in China [J]. China Water & Wastewater, 2013, 29(22): 64-70.(in Chinese)
[7] FENG R Q, YUE Q Y, GAO B Y, et al. Effect of pH on floc properties and membrane fouling in coagulation–ultrafiltration hybrid process with different Al-based coagulants [J]. Desalination and Water Treatment, 2016, 57(54): 26041-26049.
[8] DONG H Y, GAO B Y, YUE Q Y, et al. Floc properties and membrane fouling of different monomer and polymer Fe coagulants in coagulation-ultrafiltration process: The role of Fe (III) species [J]. Chemical Engineering Journal, 2014, 258: 442-449.
[9] 董秉直, 张庆元, 冯晶, 等. 粉末活性炭预处理对超滤膜通量的影响[J]. 环境科学学报, 2008, 28(10): 1981-1987.DONG B Z, ZHANG Q Y, FENG J, et al. Influence of powered activated carbon (PAC) pretreatment on ultrafiltration membrane flux[J]. Acta Scientiae Circumstantiae, 2008, 28(10): 1981-1987.(in Chinese)
[10] 冯萃敏, 张欣蕊, 孙丽华, 等. PAC-UF工艺的膜污染特性及膜污染物质研究[J]. 给水排水, 2015, 51(3): 125-131.FENG C M, ZHANG X R, SUN L H, et al. Study on the characteristics of membrane fouling and the composition of matters for membrane fouling in PAC-UF process [J]. Water & Wastewater Engineering, 2015, 51(3): 125-131.(in Chinese)
[11] 陈卫, 袁哲, 徐林, 等. 高锰酸钾预氧化对有机物构型与超滤膜污染的影响[J]. 中南大学学报(自然科學版), 2012, 43(1): 389-394.CHEN W, YUAN Z, XU L, et al. Effect of KMnO4 pre-oxidation on organic configuration and ultrafiltration membrane fouling [J]. Journal of Central South University, 2012, 43(1): 389-394.(in Chinese)
[12] 俞海祥, 伊学农, 杨光炜, 等. 臭氧超滤去除微污染水中农药残留物[J]. 水处理技术, 2015, 41(12): 110-113.YU H X, YI X N, YANG G W, et al. Ozone cooperated with ultrafiltration for the removal of pesticide residues from slight-polluted water[J]. Technology of Water Treatment, 2015, 41(12): 110-113.(in Chinese)
[13] MENG F G, CHAE S R, DREWS A, et al. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material [J]. Water Research, 2009, 43(6): 1489-1512.
[14] ZHANG Y, CHEN Y S, WESTERHOFF P, et al. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles[J]. Water Research, 2009, 43(17): 4249-4257.
[15] 乔永生, 沈腊珍, 李晓琛, 等. Fe3 O4/SiO2复合粒子的制备及对Cd2+ 的吸附性能研究[J]. 中北大学学报(自然科学版), 2015, 36(3): 343-347,353.QIAO Y S, SHEN (L|X)(Z), LI X C, et al. Preparation of Fe3 O4/SiO2 composite particles for cadmium(II) adsorption from aqueous solution [J]. Journal of North University of China (Natural Science Edition), 2015, 36(3): 343-347,353.(in Chinese)
[16] 罗敏. 紫外分光光度法测定聚酯纤维中二氧化钛含量[J]. 应用化工, 2006, 35(2): 144-146.LUO M. Determination of titanium dioxide content in polyester fiber by UV-spectrophotometry [J]. Applied Chemical Industry, 2006, 35(2): 144-146.(in Chinese)
[17] ERHAYEM M, SOHN M. Effect of humic acid source on humic acid adsorption onto titanium dioxide nanoparticles[J]. Science of the Total Environment, 2014, 470/471: 92-98.
[18] 刘媛媛, 潘纲. 吸附模式对有机物光催化降解的影响 1.H-酸在TiO2表面的吸附模式[J]. 环境化学, 2006, 25(1): 1-5.LIU Y Y, PAN G. EFFECT OF ADSORPTION MODES ON THE PHOTOCATALYTIC DEGRADATION OF ORGANIC MATTERS 1. ADSORPTION MODES OF H-ACID ON TiO2 [J]. Environmental Chemistry, 2006, 25(1): 1-5.(in Chinese)
[19] ZHU M, WANG H T, KELLER A A, et al. The effect of humic acid on the aggregation of titanium dioxide nanoparticles under different pH and ionic strengths [J]. Science of the Total Environment, 2014, 487: 375-380.
[20] LUO M X, HUANG Y X, ZHU M, et al. Properties of different natural organic matter influence the adsorption and aggregation behavior of TiO2 nanoparticles [J]. Journal of Saudi Chemical Society, 2018, 22(2): 146-154.
[21] ERHAYEM M, SOHN M. Stability studies for titanium dioxide nanoparticles upon adsorption of Suwannee River humic and fulvic acids and natural organic matter [J]. Science of the Total Environment, 2014, 468/469: 249-257.
[22] 張冬, 董岳, 周东菊, 等. 基于XDLVO理论的超滤膜污染机理研究[J]. 中国给水排水, 2016, 32(21): 66-70.ZHANG D, DONG Y, ZHOU D J, et al. Study on fouling behavior of ultrafiltration membrane based on XDLVO theory [J]. China Water & Wastewater, 2016, 32(21): 66-70.(in Chinese)
[23] WANG J W, MO Y B, MAHENDRA S, et al. Effects of water chemistry on structure and performance of polyamide composite membranes [J]. Journal of Membrane Science, 2014, 452: 415-425.
(编辑:王秀玲)
