甘蔗NAD(P)H脱氢酶复合体O亚基基因克隆及其与甘蔗花叶病毒VPg互作
2019-09-10翟玉山邓宇晴程光远杨宗桃徐景升
翟玉山 赵 贺 张 海 邓宇晴 程光远 杨宗桃 王 彤 彭 磊 徐 倩 董 萌 徐景升
甘蔗NAD(P)H脱氢酶复合体O亚基基因克隆及其与甘蔗花叶病毒VPg互作
翟玉山 赵 贺 张 海 邓宇晴 程光远 杨宗桃 王 彤 彭 磊 徐 倩 董 萌 徐景升*
福建农林大学国家甘蔗工程技术研究中心 / 农业部福建甘蔗生物学与遗传育种重点实验室 / 教育部作物遗传育种与综合利用重点实验室, 福建福州 350002
NAD(P)H脱氢酶(NDH)复合体介导循环电子传递, 对于维持叶绿体高效的光合作用具有重要作用。甘蔗(spp. hybrid)中NDH复合体应答及参与甘蔗花叶病毒(, SCMV)的侵染尚未见报道。本研究克隆了甘蔗NDH复合体的O亚基, 命名为, 其开放读码框(open reading frame, ORF)长度为471 bp, 编码长度为156 aa的蛋白。生物信息学分析表明, ScNdhO为稳定的亲水性蛋白, 不存在信号肽, 无跨膜结构域; 蛋白二级结构元件多为无规则卷曲, 具有典型的NDH复合体O亚基结构域; 系统进化树分析表明, 该蛋白属于NDH复合体O亚基蛋白家族。实时荧光定量 PCR分析发现,基因的表达具有明显的组织特异性, 在成熟甘蔗叶片中的表达量最高, 在茎中的表达量最低, 在根中几乎不表达;基因在SCMV侵染早期上调表达, 后期下调表达。亚细胞定位分析表明, ScNdhO定位于叶绿体。酵母双杂交(yeast two hybrid, Y2H)和双分子荧光互补(bimolecular fluorescence complementation, BiFC)实验表明, ScNdhO与SCMV-VPg互作。推测ScNdhO被SCMV选择性利用, 可能参与SCMV基因组复制及花叶病症状的产生。
甘蔗; SCMV; 叶绿体; NAD(P)H脱氢酶复合体; O亚基
甘蔗(spphybrid)是我国乃至全世界最重要的糖料作物和能源作物之一, 甘蔗糖占我国食糖总量的92%, 占世界食糖总量的74%, 甘蔗乙醇占世界生物质燃料乙醇的60%[1-2]。甘蔗花叶病是严重危害甘蔗生产的病毒性病害之一, 在全世界蔗区包括我国甘蔗主产区广西、云南、广东、海南等地普遍发生[3-9]。甘蔗感染花叶病后, 叶片出现黄绿相间、大小不一的条纹或斑驳, 植株矮小, 分蘖减少,蔗糖结晶率下降, 甘蔗产量损失3%~50%, 蔗糖损失6%~14%[6,9-11]。甘蔗花叶病的病原主要为甘蔗花叶病毒(, SCMV)、高粱花叶病毒(, SrMV)和甘蔗条纹花叶病毒(, SCSMV), 均属于马铃薯Y病毒科()[6,8,12-19], 其中SCMV和SrMV属于马铃薯Y病毒属()[20-21], SCSMV是新发现的病原, 2012年国际病毒分类委员会(International Committee on Taxonomy of Viruses, ICTV)将其立为该科新属, 即禾病毒属()[9,22-23]。SCMV、SrMV和SCSMV都是单链正义RNA病毒, 长度约为10 kb, 编码2个多聚蛋白, 经自身编码的P1、HC-Pro和NIa酶解后形成11个功能成熟的蛋白, 分别为P1、HC-Pro、P3、P3N-PIPO、6K1、CI、6K2、VPg、NIa、NIb和CP[12-17,22-24]。此类病毒结构简单, 其基因组5′端没有帽子结构(m7GpppN, N为任意核酸), 病毒要完成复制, 必须通过其编码的VPg与真核翻译起始因子eIF4E共价连接, 才能启动病毒RNA翻译[25-26]。VPg是马铃薯Y病毒科病毒的多功能蛋白, 除了参与病毒的翻译, 还参与病毒在寄主体内的复制、胞间移动、长距离运输及自噬等过程[25,27-28]。
叶绿体中的光合系统由光合系统I (PSI)和光合系统II (PSII)组成。PSII反应中心被光能激发后, 裂解水分子释放氧气, 将来自水分子的电子经过PSII、质醌(PQ)、细胞色素b6/f复合体、质蓝素、PSI、铁氧还蛋白线性传递, 最终使NADP+还原, 并偶联产生跨类囊体膜质子梯度(ΔpH), 驱动ATP合成酶合成ATP[29]。除了这条主要的线性光合电子传递链, 叶绿体中还有一条与线粒体复合体I介导的呼吸电子传递链类似的由NAD(P)H脱氢酶(NDH)复合体和质子末端氧化酶共同参与的围绕PSI循环电子途径, 循环电子途径只产生ATP, 不积累NADPH[30-37]。叶绿体NDH复合体位于类囊体膜上[38-40], 在调节ATP合成促进碳同化产物形成、防止光系统过还原、减少活性氧、光保护抗逆等方面具有重要的生物学功能[41-47]。叶绿体NDH复合体至少有29个亚基, 构成NDH的5个亚复合体[25,48-56]。NDH复合体O亚基属于亚复合体A成员, 对于维持NDH复合体的稳定和正常功能具有重要作用[53,57]。
叶绿体是绿色植物光合作用的场所, 同时也是马铃薯Y病毒的复制场所, 马铃薯Y病毒的6K2诱导内质网形成囊泡, 移动到叶绿体形成叶绿体-6K2复合物, 为病毒基因组复制提供场所[58]。在前期研究工作中, 我们以SCMV编码蛋白VPg为诱饵, 利用酵母双杂交(yeast two hybrid, Y2H)技术从甘蔗叶片的cDNA酵母文库[59]中筛选到了一个具有完整开放读码框(open reading frame, ORF)的基因; 该基因的ORF为471 bp, 编码长度为156 aa的蛋白。根据Blastp同源比对分析推测该基因编码叶绿体NDH复合体的O亚基, 命名为。本研究克隆了该基因, 并进行了生物信息学、组织特异性与应答SCMV侵染的表达模式分析, 利用Y2H和BiFC研究了ScNdhO与SCMV-VPg的互作关系, 并对其在SCMV侵染中的作用做了探讨, 以期为甘蔗抗花叶病育种提供基础数据和实验依据。
1 材料与方法
1.1 材料及处理方法
SCMV-FZ1病毒株系、甘蔗品种和本氏烟由福建农林大学农业部福建甘蔗生物学与遗传育种重点实验室提供。采用腋芽快繁技术培养组培苗。组培苗长至15~25 cm、完全展开叶出现4~5片时, 摩擦接种SCMV, 设置3个重复, 每个重复3株, 对照植株使用磷酸缓冲液(pH 7.0)摩擦接种, 使用基因特异引物(表1)检测接种是否成功。分别在接种后0 h、4 h、8 h、12 h、1 d、2 d、5 d、7 d取样, 研究目的基因应答SCMV侵染的表达模式。组培苗分蘖期时随机选取9株长势一致的植株, 分成3组, 每组3株。取其心叶、+1叶(甘蔗植株由上到下第一个有可见肥厚带的叶片)、+1叶的叶鞘、侧芽、蔗茎和白色嫩根, 用于研究目的基因的空间表达特性。取样后立即用液氮速冻, 置–80℃冰箱保存备用。

表1 本研究使用的引物
1.2 总RNA提取和cDNA合成
采用TRIzol (Invotrigen, USA)试剂按照说明书提取样品总RNA, 使用1.0%琼脂糖凝胶电泳检测RNA质量。使用Nanodrop (Thermo Scientific, USA)测定RNA的浓度, 按照Prime Script RT Reagent Kit使用说明书, 将RNA反转录成cDNA。
1.3 ScNdhO基因的克隆及生物信息学分析
挑取阳性酵母克隆测序, 获得目的基因的序列。利用NCBI的ORF finder (https://www.ncbi.nlm. nih.gov/orffinder/)软件, 对所获的基因序列进行开放阅读框分析。根据序列信息设计特异PCR引物, 以叶片的cDNA模板, 扩增该基因的ORF并测序验证。然后将该基因克隆至pMD19-T载体, 转化DH5α 感受态细胞。
利用ProtParam (http://expasy.org/tools/protparam. html)预测蛋白的一级结构、理化性质; 利用Prabi (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_hnn.html)、SignalP 4.1 (http://www. cbs.dtu.dk/services/SignalP/)和TMHMM 2.0 (http:// www.cbs.dtu.dk/services/TMHMM/)分别预测分析其二级结构、信号肽和跨膜特性; 通过NCBI 中的CDD (conserved domain database)数据库(https:// www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)预测蛋白保守结构域; 用 Blastp在线工具查找ScNdhO的同源氨基酸序列, 并使用DNAMAN7.0软件多重比对同源氨基酸序列, 使用ClustalX和MEGA 6.0的ML (maximum likelihood, LG+G)法构建系统进化树。
1.4 ScNdhO的RT-qPCR表达分析
根据基因的ORF序列, 设计特异性实时荧光定量PCR引物, 以供试材料的cDNA为模板, 以基因和基因(表1)为内参基因[60], 按照SYBR Green PCR Master Mix Kit (Roche)说明书配制反应体系。实时荧光定量PCR扩增程序为50℃ 2 min; 95℃ 10 min; 95℃ 15 s、60℃ 1 min, 循环40次。设置每个样品3次重复, 以无菌水作对照, 采用2–ΔΔCt算法进行基因表达水平分析, 使用统计软件SPSS 12.0对基因表达差异进行显著性分析。
1.5 载体构建
用目的片段, 以绿色荧光蛋白(green fluorescence protein, GFP)为标记, 构建ScNdhO蛋白的亚细胞定位载体ScNdhO-GFP。用SCMV的VPg编码序列, 以红色荧光蛋白(mCherry fluorescence protein, mCherry)为标记, 构建VPg的亚细胞定位载体VPg-mCherry。将基因构建到酵母表达载体 pGADT7中, 获得ScNdhO的捕获载体(GAL4 activation domain, AD) pGADT7-ScNdhO。利用Gateway技术, 将基因构建到BiFC的YC载体中, 得到ScNdhO-YC载体。所有构建的载体都经过测序验证。SCMV-VPg的Y2H诱饵载体(GAL4 DNA binding domain, BD) pGBKT7-SCMV-VPg、BiFC的YN载体SCMV-VPg-YN来自本课题组前期研究工作[17]。
1.6 ScNdhO的亚细胞定位
将含有ScNdhO-GFP植物表达载体的农杆菌注射入健康的本氏烟叶片, 48 h后在激光共聚焦显微镜(Leica TCS SP5II)下观察本氏烟叶片表皮细胞中ScNdhO蛋白的定位, GFP的激发光波长为488 nm, 捕获波长为505~555 nm。mCherry的激发光波长为552 nm, 捕获波长为590~630 nm。叶绿素自荧光的激发光波长为552 nm, 捕获波长为650~680 nm。
1.7 Y2H验证ScNdhO与SCMV-VPg的互作
将pGADT7-ScNdhO、pGBKT7-SCMV-VPg组合, pGADT7-T、pGBKT7-53组合(阳性对照), pGADT7-T、pGBKT7-Lam组合(阴性对照), 分别转化酵母菌株, 30℃ 250 r min-1培养至OD546为0.6~0.8。将转化后的酵母细胞按照1、10-1和10-2梯度稀释, 分别在缺少亮氨酸(Leu)和色氨酸(Trp)的固体酵母合成限定基本培养基(SD/−Leu/−Trp)固体培养基上培养, 30℃倒置培养4~5 d。待菌落长到直径为3~4 mm左右后, 挑取单菌落转移到1 mL缺少亮氨酸(Leu)、色氨酸(Trp)、组氨酸(His)和腺嘌呤(Ade)的液体酵母合成限定基本培养基(SD/−Leu/− Trp/−His/−Ade)中培养。如果酵母生长, 将其转移到SD/−Leu/−Trp/−His/−Ade固体培养基上培养, 菌落生长表明蛋白之间存在互作。
1.8 BiFC验证ScNdhO与SCMV-VPg的互作
参照朱海龙等[18]的方法将ScNdhO-YC与SCMV-VPg-YN等比例共转农杆菌菌株, 将其注射入健康的本氏烟叶片中。正常条件下培养2~3 d后取样, 在激光共聚焦显微镜(Leica TCS SP5II)下观察拍照。YFP的激发光波长为514 nm, 捕获波长为530~590 nm。
2 结果与分析
2.1 ScNdhO基因的生物信息学分析
在前期研究中, 以SCMV-VPg为诱饵从甘蔗叶片cDNA酵母文库中筛选到1个具有全长ORF的基因, ORF长度为471 bp, 编码156个氨基酸。根据该基因序列设计特异PCR引物(表1), 从甘蔗中克隆了该基因, 测序结果与阳性酵母质粒测序结果相同。使用BlastP进行氨基酸序列比对, 发现该基因编码蛋白与高粱叶绿体NAD(P)H脱氢酶复合体O亚基(XP_002456968.1)同源性高达89%, 与玉米的O亚基(NP_001349312.1)同源性为89%, 因此将该基因命名为(GenBank登录号为MK280691)。
ProtParam分析表明, ScNdhO蛋白的分子量为16.93 kD, 等电点为9.30; 不稳定系数为39.54, 为稳定蛋白; 脂溶指数为88.46, 总平均疏水性是–0.164, 可能是亲水蛋白。二级结构预测表明, ScNdhO中无规则卷曲结构所占的比例最高, 为57.69%; 延伸链所占比例最低, 为18.59%; α-螺旋占23.72%。SignalP 4.1分析表明, ScNdhO蛋白不含信号肽, 是非分泌蛋白。TMHMM 2.0分析表明, ScNdhO蛋白无明显的跨膜区域。保守结构域分析表明,属于NDH复合体O亚基超基因家族, 佐证了ScNdhO为甘蔗NDH复合体的O亚基。
2.2 ScNdhO的氨基酸同源性分析和系统进化树分析
使用NCBI网站的Blastp搜索ScNdhO的同源序列, 对甘蔗及其他物种NDH复合体O亚基的氨基酸序列的同源性分析表明, ScNdhO蛋白的物种分化比较明显, 地钱()及单细胞的集胞蓝藻()与高等植物明显分离, 形成群I; 双子叶植物番茄()、烟草()、拟南芥()、葡萄()、三叶杨()、大豆()、苜蓿()形成群II; 单子叶植物高粱()、玉米()、谷子()、水稻()、二穗短柄草()、大麦()形成群III; 在单子叶植物群III中, C4植物形成明显的亚群III-2, C3植物形成亚群III-1 (图1)。这表明, ScNdhO在遗传进化上具有明显的种属差异性, 与物种进化程度相关。
2.3 ScNdhO的亚细胞定位
由图2可知, 对照GFP在烟草表皮细胞中大量表达, 绿色荧光信号在细胞质、质膜和细胞核中均有分布; ScNdhO-GFP融合蛋白的绿色荧光信号与叶绿体的自荧光信号重合, 表明其主要定位于叶绿体; ScNdhO-GFP与VPg-mCherry共定位于叶绿体, 表明SCMV-VPg有可能与ScNdhO-GFP互作于叶绿体。
2.4 ScNdhO基因的组织特异性表达及应答SCMV侵染的表达模式
图3表明,基因的表达具有明显的组织特异性, 在不同组织中的表达量差异显著: 在成熟叶片中相对表达量最高, 心叶和叶鞘中次之, 侧芽较少, 茎中微量表达, 根中几乎不表达。使用基因特异引物(表1)检测SCMV接种甘蔗, 扩增出目的片段, 表明接种成功。SCMV侵染对基因表达影响显著, 与对照相比,基因在侵染早期显著上调, 然后明显下调表达, 第7天的表达量低于对照(图4)。

图1 ScNdhO蛋白的氨基酸序列同源性分析和系统进化树分析
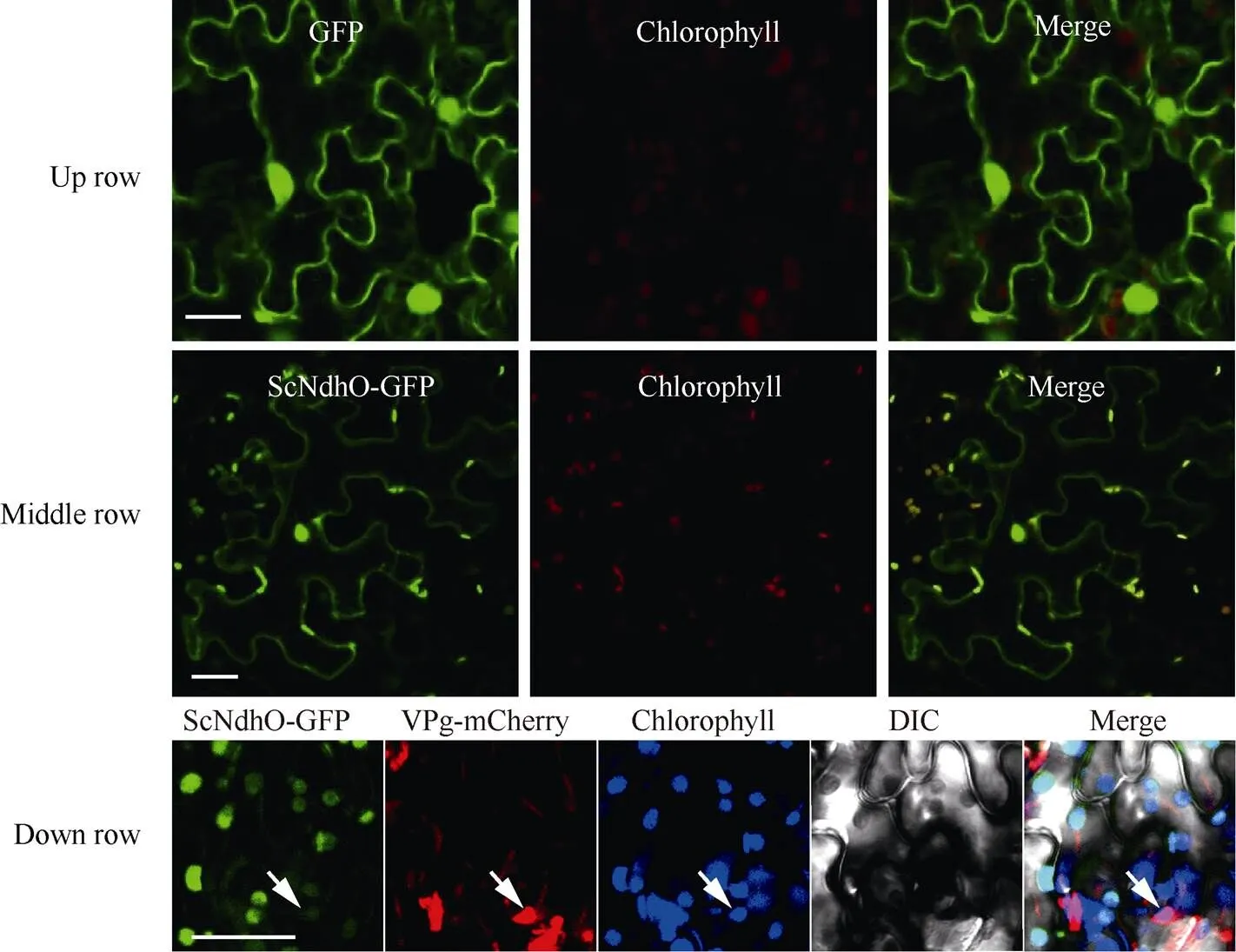
图2 ScNdhO-GFP在本氏烟表皮细胞中的定位
ScNdhO-GFP的定位如箭头所示; Up row: GFP对照; Middle row: ScNdhO-GFP定位; Down row: ScNdhO-GFP与VPg-mCherry共定位; bar = 50 μm。
The ScNdhO-GFP was labled by arrow; Up row: GFP control; Middle row: localization of ScNdhO-GFP; Down row: colocalization of ScNdhO-GFP with VPg-mCherry; bar = 50 μm.
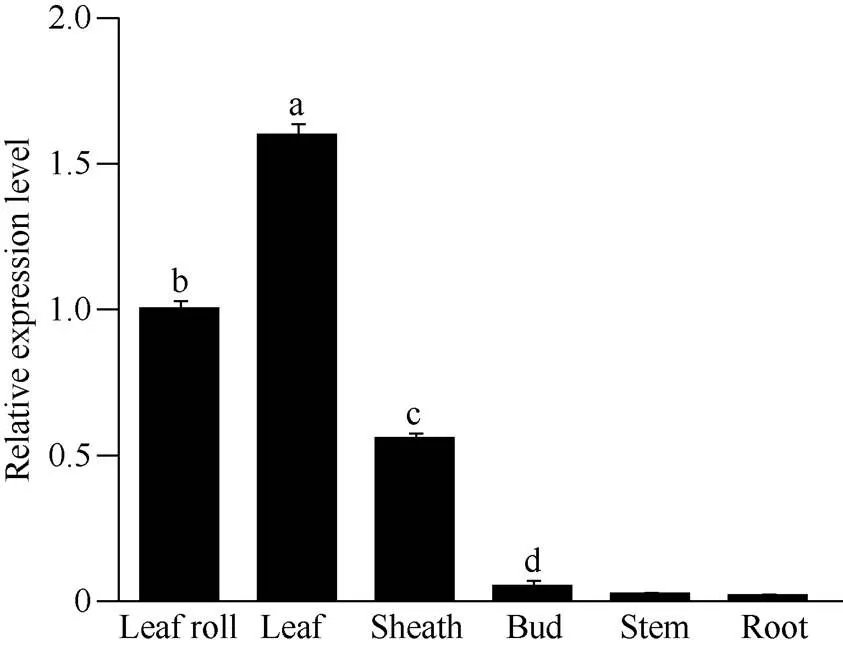
图3 ScNdhO基因在甘蔗不同组织中的表达模式
误差线为每组处理的标准误差(= 3)。Leaf roll: 心叶; Leaf: 正一叶; Sheath: 叶鞘; Bud: 腋芽; Stem: 茎; Root: 根。
The error bars represent the standard error of each treating group (= 3).
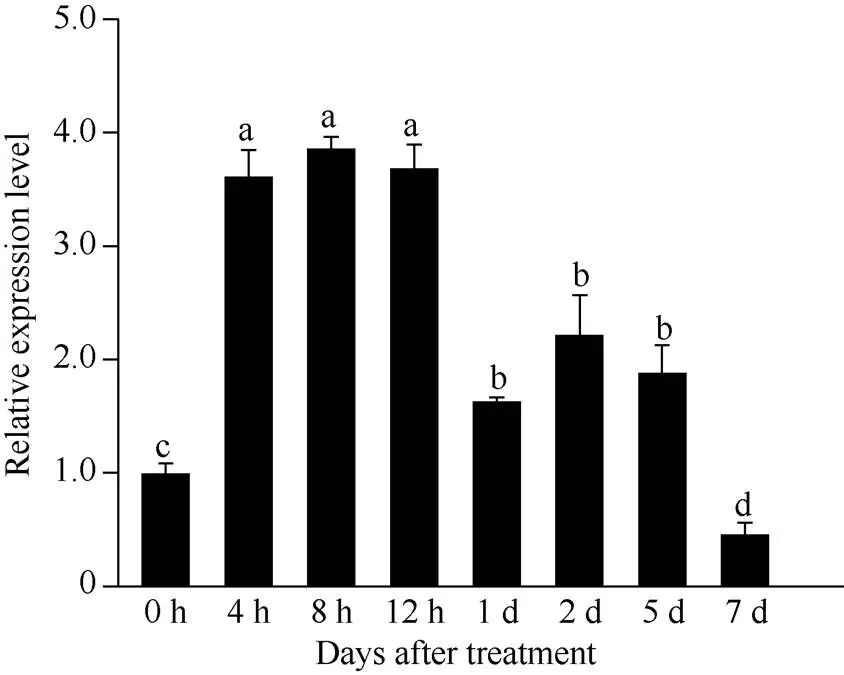
图4 ScNdhO基因应答SCMV侵染的表达模式
误差线为每组处理的标准误差(= 3)。
The error bars represent the standard error of each treating group (= 3).
2.5 ScNdhO与SCMV-VPg的互作验证
Y2H实验结果显示, 在四缺培养基上, pGADT7-ScNdhO和pGBKT7-SCMV-VPg共转的酵母菌株可以生长并呈现蓝色, 阳性对照可以正常生长并呈现蓝色, 阴性对照无菌落(图5), 说明ScNdhO与SCMV-VPg互作。BiFC实验结果与Y2H实验结果一致, ScNdhO-YC与SCMV-VPg-YN互作产生黄色荧光信号, 并与叶绿体的红色荧光信号重叠, 表明互作于叶绿体(图6)。
3 讨论
抗花叶病是甘蔗育种的重要指标之一[61-63], 但是有关甘蔗花叶病病原即SCMV、SrMV和SCSMV侵染甘蔗及诱发花叶病症状的分子机制研究却鲜见报道[12,17-18]。本研究首次克隆了甘蔗叶绿体NAD(P)H脱氢酶(NDH)复合体的O亚基基因, 首次报道了ScNdhO与SCMV-VPg互作并应答SCMV侵染。本研究对于阐明SCMV侵染甘蔗导致花叶病症状的分子机制研究具有重要意义。
叶绿体NDH复合体在碳水化合物同化、逆境应答等过程中具有重要作用[31,36,41-42,44,57]。线性电子传递中ATP/NADPH比值是固定的, 低于或接近卡尔文循环所需的比值1.5[64], 但是在逆境或者同化物大量合成的情况下, 循环电子途径必须介入供应ATP。对于C4植物而言, 光合作用所需的ATP/ NADPH比值至少要达到2.5。与C3光合作用相比, C4光合作用需要额外的2分子ATP用于1,5 -二磷酸核酮糖羧化酶/加氧酶(Rubisco)浓缩CO2, 这2分子额外的ATP就是由围绕PSI的循环电子传递提供的, 现已在玉米、黄顶菊等C4植物中鉴定了NDH复合体介导的循环电子传递和生产ATP[33-34,65-67]。进化分析也表明, C3植物与C4植物的NdhO分成不同的亚群。甘蔗叶片是主要的光合器官, 完全展开的正一叶是光合作用最旺盛的叶片, 本研究中在正一叶中的表达量最高, 推测叶绿体NDH复合体通过循环电子传递为CO2同化提供能量。NDH复合体O亚基对NDH复合体的正确组装和行使功能很重要, 敲除烟草或拟南芥的突变体, 亚复合体A无法组装, 进而完全瓦解NDH介导的循环电子传递[57,68]。在SCMV侵染早期上调表达, 一方面有可能通过这种互作使SCMV复制复合体定位于叶绿体, 完成病毒基因组复制; 另一方面则是寄主对SCMV侵染的抗性反应。在SCMV侵染后期下调表达, 本研究推测SCMV侵染影响了NDH复合体的稳定, 干扰了循环电子传递, 使光合作用处于逆境状态, 导致叶绿素减少[10], 产生花叶症状, 进而影响碳水化合物合成。
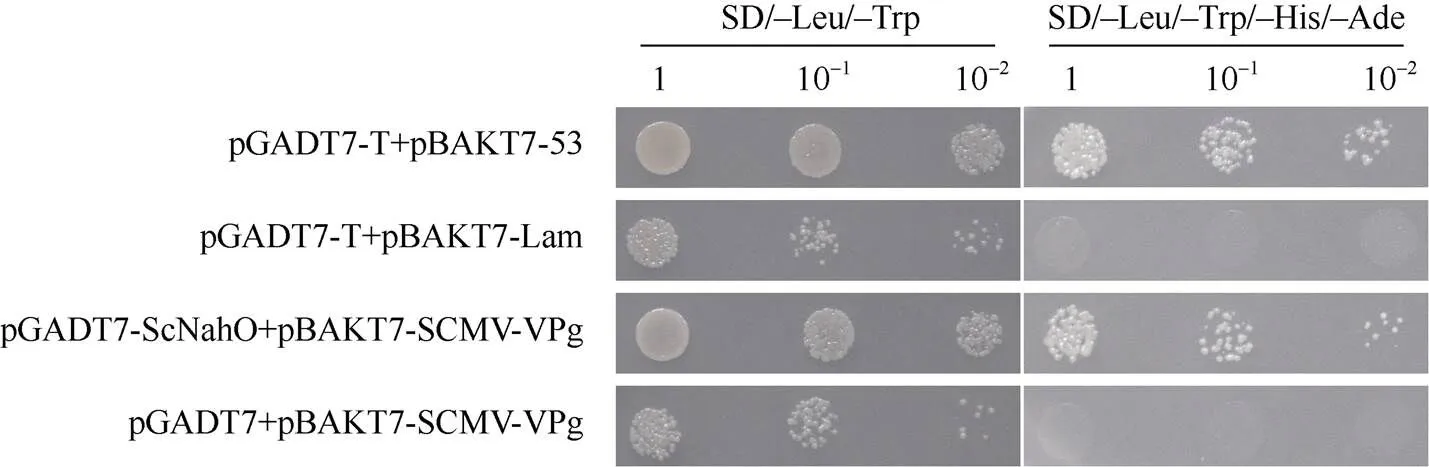
图5 Y2H检测ScNdhO与SCMV-VPg的互作
pGADT7-T和pGBKT7-53组合作为阳性对照, pGADT7-T和pGBKT7-Lam组合作为阴性对照。SD/−Leu/−Trp: 缺少亮氨酸(Leu)和色氨酸(Trp)的酵母合成限定基本培养基; SD/−Leu/−Trp/−His/−Ade: 缺少亮氨酸(Leu)、色氨酸(Trp)、组氨酸(His)和腺嘌呤(Ade)的酵母合成限定基本培养基。
The positive and negative controls are yeast cotransformants with pGADT7-T plus pGBKT7-53 and pGADT7-T plus pGBKT7-Lam, respectively. SD/−Leu/−Trp: synthetic defined yeast minimal medium lacking Leu and Trp; SD/−Leu/−Trp/−His/−Ade: synthetic defined yeast minimal medium lacking Leu, Trp, His, and Ade.
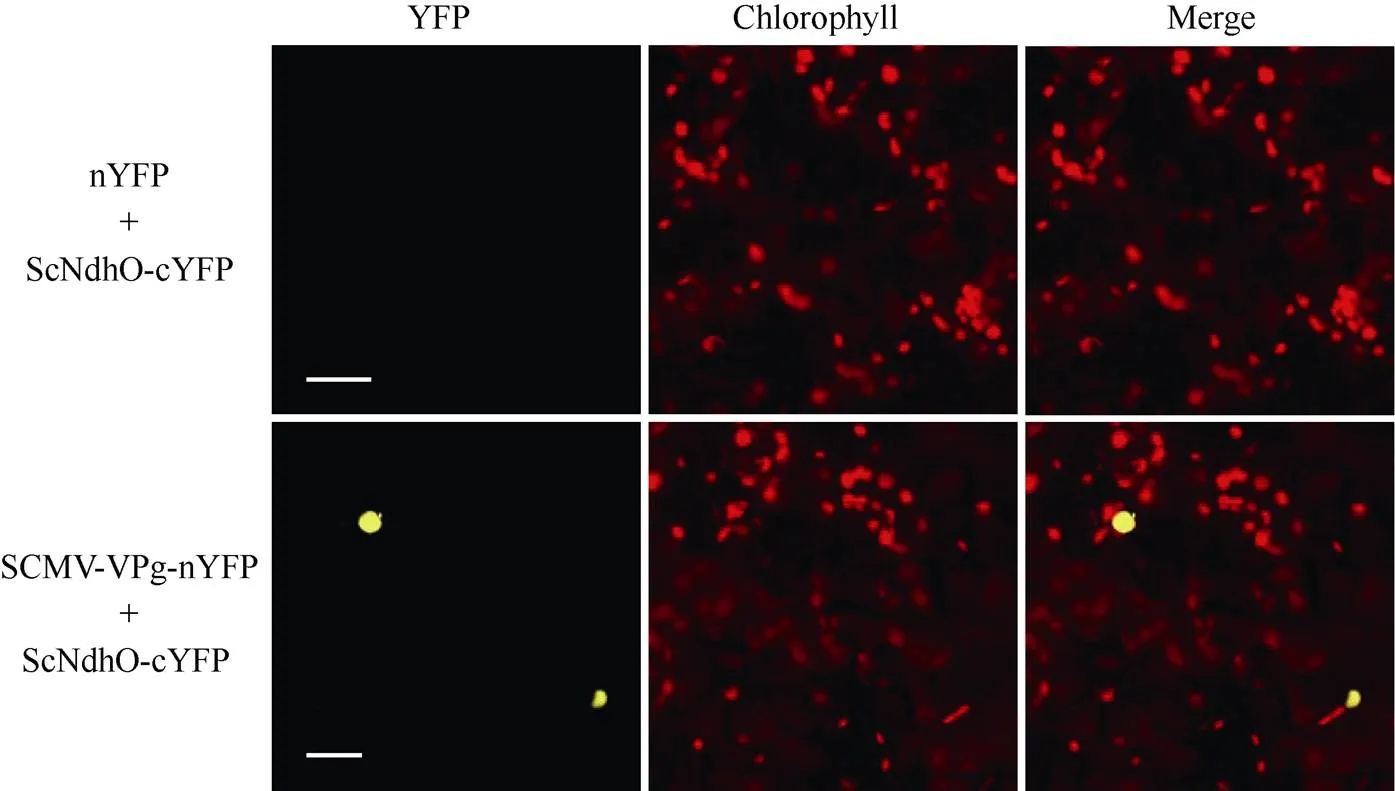
图6 BiFC检测ScNdhO与SCMV-VPg的互作
ScNdhO融合于YFP的C末端, SCMV-VPg融合于YFP的N末端, 然后在本氏烟叶片中瞬时表达, 48 h后激光共聚焦观察。bar = 50 μm。
ScNdhO was fused to the C-terminal half of YFP, while SCMV-VPg was fused to the N-terminal half of YFP. ScNdhO-YC and SCMV-VPg-YN were transiently coexpressed inleave. The fluorescent signal was monitored by confocal microscopy at 48 hpi. bar = 50 μm.
植物病毒结构简单, 必须与寄主因子互作才能完成系统性侵染[25-26]。本研究发现ScNdhO与SCMV-VPg互作, 因此推测ScNdhO被SCMV选择性利用。NDH复合体约有30个亚基, SrMV和SCSMV与SCMV的基因组结构非常相似, 他们的VPg有可能与ScNdhO或者其他亚基互作执行相应的生物学功能。病毒与寄主是协同进化的, 病毒对寄主侵染的最终目的是繁殖和传播[69]。由昆虫作为媒介传播的植物病毒中至少50%是由蚜虫以非持续性方式传播的[70-71]。蚜虫普遍嗜好发病植株, 比如雀麦花叶病毒属()的菜豆黄花叶病毒(, BYMV)和蚕豆斑驳病毒(, BBMV), 豌豆耳突花叶病毒属()的豌豆耳突花叶病毒(, PEMV), 侵染后造成叶片黄化, 吸引豌豆蚜[72], 与SCMV同属的小西葫芦黄花叶病毒(, ZYMV)侵染造成的叶片黄化植株吸引棉蚜[73]。SCMV是由多种蚜虫非持续性方式传播的[6], SCMV-VPg与ScNdhO互作并在侵染后期降低其表达, 可能对叶绿体产生胁迫, 造成叶片黄化, 吸引蚜虫便于病毒传播。病毒与寄主的互作是十分复杂的, 花叶病症状的产生可能是多方面因素的结果, 比如SCSMV-P3与甘蔗 Rubisco大亚基互作可能干扰Rubisco的功能而影响叶绿体的稳定性[18]。SCMV-VPg与ScNdhO互作的具体生物学功能还有待进一步研究。
4 结论
从甘蔗中克隆到叶绿体NAD(P)H脱氢酶(NDH)复合体的O亚基基因(GenBank登录号为MK280691), 其ORF长度为471 bp, 编码长度为156 aa的蛋白。ScNdhO蛋白定位于叶绿体, 在完全展开的功能叶片中表达量最高。ScNdhO蛋白与SCMV-VPg互作。基因响应SCMV侵染, 在侵染早期上调表达, 在侵染后期则下调表达。
[1] 翁卓, 黄寒. 中国制糖产业竞争力对比与政策建议——基于对巴西、印度、泰国考察的比较. 甘蔗糖业, 2015, (4): 65–72. Weng Z, Huang H. Comparative analysis on China’s sugar industry competitiveness: based onthe comparison of Brazil, India and Thailand sugar industry., 2015, (4): 65–72 (in Chinese with English abstract).
[2] 刘燕群, 李玉萍, 梁伟红, 宋启道, 秦小立, 叶露. 国外甘蔗产业发展现状. 世界农业, 2015, (8): 147–152. Liu Y Q, Li Y P, Liang H W, Song Q D, Qin X L, Ye L. Current status and development of the abroad sugarcane industry., 2015, (8): 147–152 (in Chinese with English abstract).
[3] Filloux D, Fernandez E, Comstock J C, Mollov D, Roumagnac P, Rott P. Viral metagenomic-based screening of sugarcane from florida reveals occurrence of six sugarcane-infecting viruses and high prevalence of., 2018, 102: 2317–2323.
[4] Li W F, Zhang R Y, Shan H L, Yin J, Wang X Y, Luo Z M, Huang Y K, Shen K. Occurrence dynamics and control strategies of major pests and diseases of sugarcane in Yunnan., 2017, 18: 2490–2494.
[5] 徐正银, 吕榜丽, 李璞, 周龙武, 唐瑶, 唐君海, 秦碧霞, 蒙姣荣, 温荣辉, 陈保善. 广西甘蔗病毒病害调查及病原病毒鉴定. 南方农业学报, 2014, 45: 1957–1962. Xu Z Y, Lyu B L, Li P, Zhou L W, Tang Y, Tang J H, Qin B X, Meng J R, Wen R H, Chen B S. Disease survey and identification of viruses in sugarcane in Guangxi., 2014, 45: 1957–1962 (in Chinese with English abstract).
[6] Wu L, Zu X, Wang S, Chen Y.—Long history but still a threat to industry., 2012, 42: 74–78.
[7] 熊国如, 李增平, 赵婷婷, 蔡文伟, 王俊刚, 王文治, 冯翠莲, 张雨良, 张树珍. 海南蔗区甘蔗病害种类及发生情况. 热带作物学报, 2010, 31: 1588–1595. Xiong G R, Li Z P, Zhao T T, Cai W W, Wang J G, Wang W Z, Feng C L, Zhang Y L, Zhang S Z. Primary investigation to sugarcane on the diseases in Hainan Province., 2010, 31: 1588–1595 (in Chinese with English abstract).
[8] Xu D L, Park J W, Mirkov T E, Zhou G H. Viruses causing mosaic disease in sugarcane and their genetic diversity in southern China., 2008, 153: 1031–1039.
[9] Putra L K, Kristini A, Achadian E M A, Damayanti T A.in Indonesia: distribution, characterisation, yield losses and management approaches., 2014, 16: 392–399.
[10] 刘家勇, 赵培方, 赵俊, 崔洁, 陈学宽, 夏红明, 杨昆, 吴才文. 甘蔗花叶病对甘蔗叶片叶绿素含量的影响. 中国糖料, 2011, (4): 7–9. Liu J Y, Zhao P F, Zhao J, Cui J, Chen X K, Xia H M, Yang K, Wu C W. Effect ofon chlorophyll content of sugarcane leaves., 2011, (4): 7–9 (in Chinese with English abstract).
[11] Koike H, Gillaspie A G. Mosaic. In: Ricaud C, Egan B T, Gillaspie A G, Hughes C G, eds. Diseases of Sugarcane, Major Diseases. Amsterdam: Elsevier, 1989. pp 301–322.
[12] Cheng G Y, Dong M, Xu Q, Peng L, Yang Z T, Wei T Y, Xu J S. Dissecting the molecular mechanism of the subcellular localization and cell-to-cell movement of theP3N- PIPO., 2017, 7: 9868, doi: 10.1038/s41598-017- 10497-6.
[13] 郑艳茹, 翟玉山, 邓宇晴, 成伟, 程光远, 杨永庆, 徐景升. 甘蔗花叶病毒(SCMV)种群结构分析. 福建农林大学学报(自然科学版), 2016, 45: 135–140. Zheng Y R, Zhai Y S, Deng Y Q, Cheng W, Cheng G Y, Yang Y Q, Xu J S. The population structure of(SCMV).(Nat Sci Edn), 2016, 45: 135–140 (in Chinese with English abstract).
[14] 翟玉山, 彭磊, 杨永庆, 邓宇晴, 程光远, 郑艳茹, 徐景升. 甘蔗条纹花叶病毒 HC-Pro、P3N-PIPO、CP和VPg基因酵母双杂交诱饵表达载体的构建及自激活检测. 华北农学报, 2016, 31(1): 83–89. Zhai Y S, Peng L, Yang Y Q, Deng Y Q, Cheng G Y, Zheng Y R, Xu J S. Construction and self-activated detection of the baits of HC-Pro, P3N-PIPO, CP and VPg fromfor yeast two hybrid system., 2016, 31(1): 83–89 (in Chinese with English abstract).
[15] 邓宇晴, 杨永庆, 翟玉山, 程光远, 彭磊, 郑艳茹, 林彦铨, 徐景升. 甘蔗花叶病毒福州分离物全基因组克隆及种群分析. 植物病理学报, 2016, 46: 775–782. Deng Y Q, Yang Y Q, Zhai Y S, Cheng G Y, Peng L, Zheng Y R, Lin Y Q, Xu J S. Genome cloning of twoisolates from Fuzhou and phylogenetic analysis of SCMV., 2016, 46: 775–782 (in Chinese with English abstract).
[16] Dong M, Cheng G Y, Peng L, Xu Q, Yang Y Q, Xu J S. Transcriptome analysis of sugarcane response to the infection by(SCSMV)., 2017, 10: 45–55.
[17] Zhai Y S, Deng Y Q, Cheng G Y, Peng L, Zheng Y R, Yang Y, Xu J S. Sugarcane elongin C is involved in infection by sugarcane mosaic disease pathogens., 2015, 466: 312–318.
[18] 朱海龙, 程光远, 彭磊, 柴哲, 郭晋隆, 许莉萍, 徐景升. 甘蔗条纹花叶病毒 P3 蛋白与甘蔗Rubisco大亚基互作的研究. 西北植物学报, 2014, 34: 676–681. Zhu H L, Cheng G Y, Peng L, Chai Z, Guo J L, Xu L P, Xu J S. Interaction betweenP3 and rubisco large subunit from sugarcane., 2014, 34: 676–681 (in Chinese with English abstract).
[19] Ling H, Huang N, Wu Q, Su Y, Peng Q, Ahmed W, Gao S, Su W, Que Y, Xu L. Transcriptional Insights into the Sugarcane-Interaction., 2018, 11: 163–176.
[20] Hall J S, Adams B, Parsons T J, French R, Lane L C, Jensen S G. Molecular cloning, sequencing, and phylogenetic relationships of a new potyvirus:, and a reevaluation of the classification of the., 1998, 10: 323–332.
[21] Ward C W, Shukla D D. Taxonomy of potyviruses: current problems and some solutions., 1991, 32: 269–296.
[22] Li W F, He Z, Li S F, Huang Y K, Zhang Z X, Jiang D M, Wang X Y, Luo Z M. Molecular characterization of a new strain of(SCSMV)., 2011, 156: 2101–2104.
[23] Xu D L, Zhou G H, Xie Y J, Mock R, Li R. Complete nucleotide sequence and taxonomy of, member of a novel genus in the family., 2010, 40: 432–439.
[24] Olspert A, Carr J P, Firth A E. Mutational analysis of thetranscriptional slippage site utilized for expression of the P3N-PIPO and P1N-PISPO proteins., 2016, 44: 7618–7629.
[25] Wang A. Dissecting the molecular network of virus-plant interactions: the complex roles of host factors., 2015, 53: 45–66.
[26] Wittmann S, Chatel H, Fortin M G, Laliberté J F. Interaction of the viral protein genome linked of turnip mosaicwith the translational eukaryotic initiation factor(iso) 4E ofusing the yeast two-hybrid system., 1997, 234: 84–92.
[27] Heinlein M. Plant virus replication and movement., 2015, 479–480: 657–671.
[28] Cheng X, Wang A. The potyvirus silencing suppressor protein VPg mediates degradation of SGS3 via ubiquitination and autophagy pathways., 2017, 91: e01478-16.
[29] 王镜岩, 朱圣庚, 徐长法. 生物化学(下册)(第3版). 北京:高等教育出版社, 2002. pp 197–227. Wang J Y, Zhu S G, Xu C F. Biochemistry (Volume 2), 3rd edn. Beijing: Higher Education Press, 2002. pp 197–227 (in Chinese).
[30] Strand D D, Fisher N, Kramer D M. The higher plant plastid NAD(P)H dehydrogenase-like complex (NDH) is a high efficiency proton pump that increases ATP production by cyclic electron flow., 2017, 292: 11850–11860.
[31] 米华玲. 类囊体膜NAD(P)H脱氢酶复合体调控光合作用的研究进展. 植物生理学报, 2016, 52: 1457–1465. Mi H L. The regulation of NAD(P)H dehydrogenase complexes bound in thylakoid membranes in photosynthesis., 2016, 52: 1457–1465 (in Chinese with English abstract).
[32] Yamori W, Shikanai T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth., 2016, 67: 81–106.
[33] Ishikawa N, Takabayashi A, Sato F, Endo T. Accumulation of the components of cyclic electron flow around photosystem I in C4plants, with respect to the requirements for ATP., 2016, 129: 1–17.
[34] Ishikawa N, Takabayashi A, Noguchi K, Tazoe Y, Yamamoto H, Von C S, Sato F, Endo T. NDH-Mediated cyclic electron flow around photosystem I is crucial for C4photosynthesis., 2016, 57: 2020–2028.
[35] Xu M, Shi N, Li Q, Mi H. An active supercomplex of NADPH dehydrogenase mediated cyclic electron flow around photosystem I from the panicle chloroplast of., 2014, 46: 757–765.
[36] 李庆华, 何志辉, 米华玲. 叶绿体NAD(P)H脱氢酶(NDH)复合体的研究进展. 植物生理学报, 2013, 49: 401–409. Li Q H, He Z H, Mi H L. The research progress of chloroplast NAD(P)H dehydrogenase (NDH) complex., 2013, 49: 401–409 (in Chinese with English abstract).
[37] Wu Y X, Zheng F F, Ma W M, Han Z G, Gu Q, Shen Y G, Mi H L. Regulation of NAD(P)H dehydrogenase-dependent cyclic electron transport around PSI by NaHSO3at low concentrations in tobacco chloroplasts., 2011, 52: 1734–1743.
[38] Mi H, Endo T, Ogawa T, Asada K. Thylakoid membrane-bound, NADPH-Specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacteriumsp. PCC 6803., 1995, 36: 661–668.
[39] Mi H, Endo T, Schreiber U, Ogawa T, Asada K. NAD(P)H dehydrogenase-dependent cyclic electron flow around photosystem I in the cyanobacteriumPCC 6803: a study of dark-starved cells and spheroplasts., 1994, 35: 163–173.
[40] Mi H, Endo T, Schreiber U, Ogawa T, Asada K. Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacteriumPCC 6803., 1992, 33: 1233–1237.
[41] Zhao J, Gao F, Fan D Y, Chow W S, Ma W. NDH-1 is important for photosystem I function ofsp. strain PCC 6803 under environmental stress conditions., 2017, 8: 2183, doi: 10.3389/fpls.2017.02183.
[42] Xin C, He Z, Min X, Peng L, Mi H. NdhV subunit regulates the activity of type-1 NAD(P)H dehydrogenase under high light conditions in cyanobacteriumsp. PCC 6803., 2016, 6: 28361, doi: 10.1038/srep28361.
[43] He Z, Xu M, Wu Y, Lyu J, Fu P, Mi H. NdhM subunit is required for the stability and the function of NAD(P)H dehydrogenase complexes involved in CO2 uptake insp. strain PCC 6803., 2016, 291: 5902–5912.
[44] Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye J Y, Mi H L. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress., 2006, 141: 465–474.
[45] Yuri M, Mihoko H, Chikahiro M, Ken-ichi T, Tsuyoshi E, Masao T, Toshiharu S. Cyclic electron flow around photosystem I is essential for photosynthesis., 2004, 429: 579–582.
[46] Mi H, Klughammer C, Schreiber U. Light-induced dynamic changes of NADPH fluorescence inPCC 6803 and its ndhB-defective mutant M55., 2000, 41: 1129–1135.
[47] Endo T, Shikanai T, Takabayashi A, Asada K, Sato F. The role of chloroplastic NAD(P)H dehydrogenase in photoprotection., 1999, 457: 5–8.
[48] Kato Y, Sugimoto K, Shikanai T. NDH-PSI supercomplex assembly precedes full assembly of the NDH complex in chloroplast., 2018, 176: 1728–1738.
[49] Gao F, Zhao J, Wang X, Qin S, Wei L, Ma W. NdhV is a subunit of NADPH dehydrogenase essential for cyclic electron transport insp. strain PCC 6803., 2016, 170: 752–760.
[50] Gao F, Zhao J, Chen L, Battchikova N, Ran Z, Aro E M, Ogawa T, Ma W. The NDH-1L-PSI supercomplex is important for efficient cyclic electron transport in cyanobacteria., 2016, 172: 1451–1464.
[51] Zhao J H, Rong W Q, Gao F D, Ogawa T, Ma W M. Subunit Q is required to stabilize the large complex of NADPH dehydrogenase insp. strain PCC 6803., 2015, 168: 443–451.
[52] Zhang J S, Gao F D, Zhao J H, Teruo O, Wang Q X, Ma W M. NdhP is an exclusive subunit of large complex of NADPH dehydrogenase essential to stabilize the complex insp. strain PCC 6803., 2014, 289: 18770–18781.
[53] Zhang J S, Gao F D, Zhao J H, Teruo O, Wang Q X, Ma W M. NdhO, a subunit of NADPH dehydrogenase, destabilizes medium size complex of the enzyme insp. strain PCC 6803., 2014, 289: 26669–26676.
[54] Dai H L, Zhang L L, Zhang J S, Mi H L, Ogawa T, Ma W M. Identification of a cyanobacterial CRR6 protein, Slr1097, required for efficient assembly of NDH-1 complexes insp. PCC 6803., 2013, 75: 858–866.
[55] Battchikova N, Wei L, Du L, Bersanini L, Aro E M, Ma W. Identification of novel Ssl0352 protein (NdhS), essential for efficient operation of cyclic electron transport around photosystem I, in NADPH:plastoquinone oxidoreductase (NDH-1) complexes ofsp. PCC 6803., 2011, 286: 36992–37001.
[56] Kentaro I, Tsuyoshi E, Toshiharu S, Eva-Mari A. Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits., 2011, 52: 1560–1568.
[57] Rumeau D, Bécuwe-Linka N, Beyly A, Louwagie M, Garin J, Peltier G. New subunits NDH-M, -N, and -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants., 2005, 17: 219–232.
[58] Wei T Y, Zhang C W, Hou X L, Wang A M. The SNARE protein Syp71 is essential forinfection by mediating fusion of virus-induced vesicles with chloroplasts., 2013, 9: e1003378.
[59] 朱海龙, 翟玉山, 程光远, 郭晋隆, 许莉萍, 徐景升. 感染甘蔗花叶病毒甘蔗叶片cDNA文库的构建及评价. 西北农业学报, 2014, 23(11): 79–84. Zhu H L, Zhai Y S, Cheng G Y, Guo J L, Xu L P, Xu J S. Construction and evaluation of yeast two hybrid cDNA library for sugarcane leaf infected with(SCMV)., 2014, 23(11): 79–84 (in Chinese with English abstract).
[60] Guo J L, Ling H, Wu Q B, Xu L P, Que Y X. The choice of reference genes for assessing gene expression in sugarcane under salinity and drought stresses., 2014, 4: 7042, doi: 10.1038/srep07042.
[61] Yao W, Ruan M, Qin L, Yang C, Chen R, Chen B, Zhang M. Field performance of transgenic sugarcane lines resistant to., 2017, 8: 104, doi: 10.3389/fpls.2017.00104.
[62] 杨川毓, 施肖堃, 张铃, 郭莺, 阮妙鸿, 陈如凯, 张木清. 抗花叶病转基因甘蔗的活性氧代谢分析. 热带作物学报, 2012, 33: 1101–1106. Yang C Y, Shi X K, Zhang L, Guo Y, Ruan M H, Chen R K, Zhang M Q. Evaluation on yield and sugar characteristics in transgenic sugarcane mediated withgene from., 2012, 33: 1101–1106 (in Chinese with English abstract).
[63] 郭莺, 阮妙鸿, 吴杨, 刘佳, 杨川毓, 张木清. 甘蔗转基因的研究. 热带作物学报, 2010, 31: 965–971. Guo Y, Ruan M H, Wu Y, Liu J, Yang C Y, Zhang M Q.gene transformation in sugarcane., 2010, 31: 965–971 (in Chinese with English abstract).
[64] David M K, John R E. The importance of energy balance in improving photosynthetic productivity., 2011, 155: 70–78.
[65] Naoya N, Megumi I, Michel H, Akiho Y, Yuri Nakajima M. Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4photosynthesis in the genus., 2013, 199: 832–842.
[66] Munekage Y N, Eymery F, Rumeau D, Cuiné S, Oguri M, Nakamura N, Yokota A, Genty B G P. Elevated expression of PGR5 and NDH-H in bundle sheath chloroplasts in C4flaveria species., 2010, 51: 664–668.
[67] Darie C C, Pascalis L D, Mutschler B, Haehnel W. Studies of the Ndh complex and photosystem II from mesophyll and bundle sheath chloroplasts of the C4-type plant., 2006, 163: 800–808.
[68] Peng L W, Yoichiro F, Masayuki F, Toshiharu S. Multistep assembly of chloroplast NADH dehydrogenase-like subcomplex A requires several nucleus-encoded proteins, including CRR41 and CRR42, in Arabidopsis., 2012, 24: 202–214.
[69] Fraile A, Garcíaarenal F, Carr J P, Loebenstein G. The coevolution of plants and viruses: resistance and pathogenicity., 2010, 76: 1–32.
[70] Ng J C K, Perry K L. Transmission of plant viruses by aphid vectors., 2004, 5: 505–511.
[71] Nault L R. Arthropod transmission of plant viruses: a new synthesis., 1997, 90: 521–541.
[72] Simon H, Glen P. Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance?, 2008, 37: 1573–1581.
[73] Salvaudon L, Mescher M C. Outcomes of co-infection by two potyviruses: implications for the evolution of manipulative strategies., 2013, 280: 20122959, doi: 10.1098/rspb. 2012.2959.
Cloning of NAD(P)H complex O subunit gene and its interaction with VPg of
ZHAI Yu-Shan, ZHAO He, ZHANG Hai, DENG Yu-Qing, CHENG Guang-Yuan, YANG Zong-Tao, WANG Tong, PENG Lei, XU Qian, DONG Meng, and XU Jing-Sheng*
SugarcaneResearch & Development Centre, China Agricultural Technology System, Fujian Agriculture and Forestry University/Key Laboratory of Sugarcane Biology and Genetic Breeding, Ministry of Agriculture / Key Laboratory of Ministry of Education for Genetics, Breeding and Multiple Utilization of Crops, Fuzhou 350002, Fujian,China
NAD(P)H dehydrogenase (NDH) complex mediates cyclic electron transports, playing key role in efficient photosynthesis in chloroplast. The involvement of NDH complex in(SCMV) infection of sugarcane (spp. hybrid) has not been reported. In this study, we isolated the coding sequence of the subunit of the NAD(P)H dehydrogenase complex from sugarcane and designated it as. The open reading frame (ORF) ofis 471 bp and encodes a 156 aa length protein. Bioinformatics analysis showed that ScNdhO is a stable hydrophilic protein with no signal peptide and transmembrane domain. The secondary structure of ScNdhO is composed of mostly random coilα-helices, with a typical domain of NDH complex O subunit. Phylogenetic tree analysis showed that ScNdhO belongs to the NDHO supperfamily. Real-time quantitative PCR analysis showed thatgene was tissue specific in sugarcane, with the lowest expression level in roots or stem, and the highest in leaf. The expression ofwas upregulated in the early stage of SCMV infection, but downregulated with time going. Subcellular location assays showed that ScNdhO was located in chloroplast. ScNdhO interacted with the VPg from SCMV as demonstrated by yeast two hybrid and bimolecular fluorescence complementation assays. We proposed that ScNdhO should be selectively employed by SCMV and involved in the mosaic symptom.
sugarcane; SCMV; chloroplast; NAD(P)H dehydrogenase complex; O subunit
本研究由国家自然科学基金项目(31371688)资助。
This study was supported by the National Natural Science Foundation of China (31371688).
徐景升, E-mail: xujingsheng@126.com
E-mail:yushanzhai@126.com
2018-12-31;
2019-01-19;
2019-03-08.
10.3724/SP.J.1006.2019.94002
URL: http://kns.cnki.net/kcms/detail/11.1809.S.20190307.1645.004.html
