Discussion on Drug Procurement with Target Quantity in China and Its Problem
2019-09-06ZhengYongxiaYangYue
Zheng Yongxia, Yang Yue *
(School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China)
AbstractObjective To provide reference for improving drug procurement with target quantity in China. Methods Documents on drug centralized procurement such as the "Guidance on Drug Procurement with Target Quantity for 4+7 Cities" issued by National Healthcare Security Administration in November 2018 and "Notice on the National Organization of the Pilot Program for Drug Centralized Procurement and Use of Bidding Drugs" issued by the State Council were studied to summarize the mode and result of drug procurement with target quantity. Results and Conclusion Drug procurement with target quantity led by National Healthcare Security Administration solved some problems in the previous centralized drug procurement. For instance, quantity could not determine drug price, and medical institutions didn't pay to pharmaceutical companies on time. However, this new mode may also arouse new problems such as procurement monopoly, drug shortage, drug quality and inhibition of innovation.
Keywords: "4+7" quantity procurement; public medical institution; generic drug; consistency evaluation
1 Background
The reform of China's centralized drug procurement system began in 1993. It has gone through the beginning stage of centralized procurement, the trial stage of bidding procurement,the national implementation, the provincial or municipal (prefectural) level of centralized bidding procurement, and the online procurement on the level of provincial units[1]. The centralized drug procurement system plays an important role in standardizing the drug circulation order, adjusting the pattern of pharmaceutical industry and promoting the rational competition in the pharmaceutical industry[2]. However,as the centralized drug procurement agencies are subordinate to the provincial health administrative departments, they have to follow the orders from the health administrative departments. This results in such problems as they pay more attention to bidding than procurement, there is no linkage between quantity and price, and it takes long time for medical institutions to pay.
The procurement with target quantity refers to specifying the quantity of medicine in the process of bidding or negotiating in drug centralized procurement, so that pharmaceutical companies can quote for the quantity of drugs. Its purpose is to reduce drug price with quantity so that medical insurance costs can be controlled[3]. The "Guidance on Drug Procurement with Target Quantity for 4+7 Cities" issued by the National Healthcare Security Administration in November 15, 2018 marks the beginning of the national pilot drug procurement with target quantity[4]. This pilot program is the fi rst drug procurement policy made by National Healthcare Security Administration. The pilot program is also a major reform of the previous centralized drug procurement system. The trial areas are Beijing,Tianjin, Shanghai, Chongqing, Shenyang, Dalian,Xiamen, Guangzhou, Shenzhen, Chengdu and Xi'an.
2 The mode of pilot procurement with target quantity
2.1 The pilot drugs
The Alliance Procurement Office, which is composed of pilot areas, selected 31 generic drugs that have passed the consistency evaluation as the pilot drugs for procurement with target quantity in 4+7 cities. The pilot drugs are branded drugs and reference preparations, and generic drug approved according to the new chemical registration classification. The significant difference between this procurement with target quantity and the centralized drug at provincial level is to specify the purchasing quantity of each variety, which is estimated by public medical institutions in the pilot area according to the records of drug use in hospitals. The purchasing quantity accounts for 60%-70% of the annual use of drugs in the pilot areas and it accounts for about 30% of the market share in China[5]. It is stipulated that medical institutions must use the purchasing varieties with target quantity to complete the agreed amount of procurement.
2.2 The rules of drug bidding
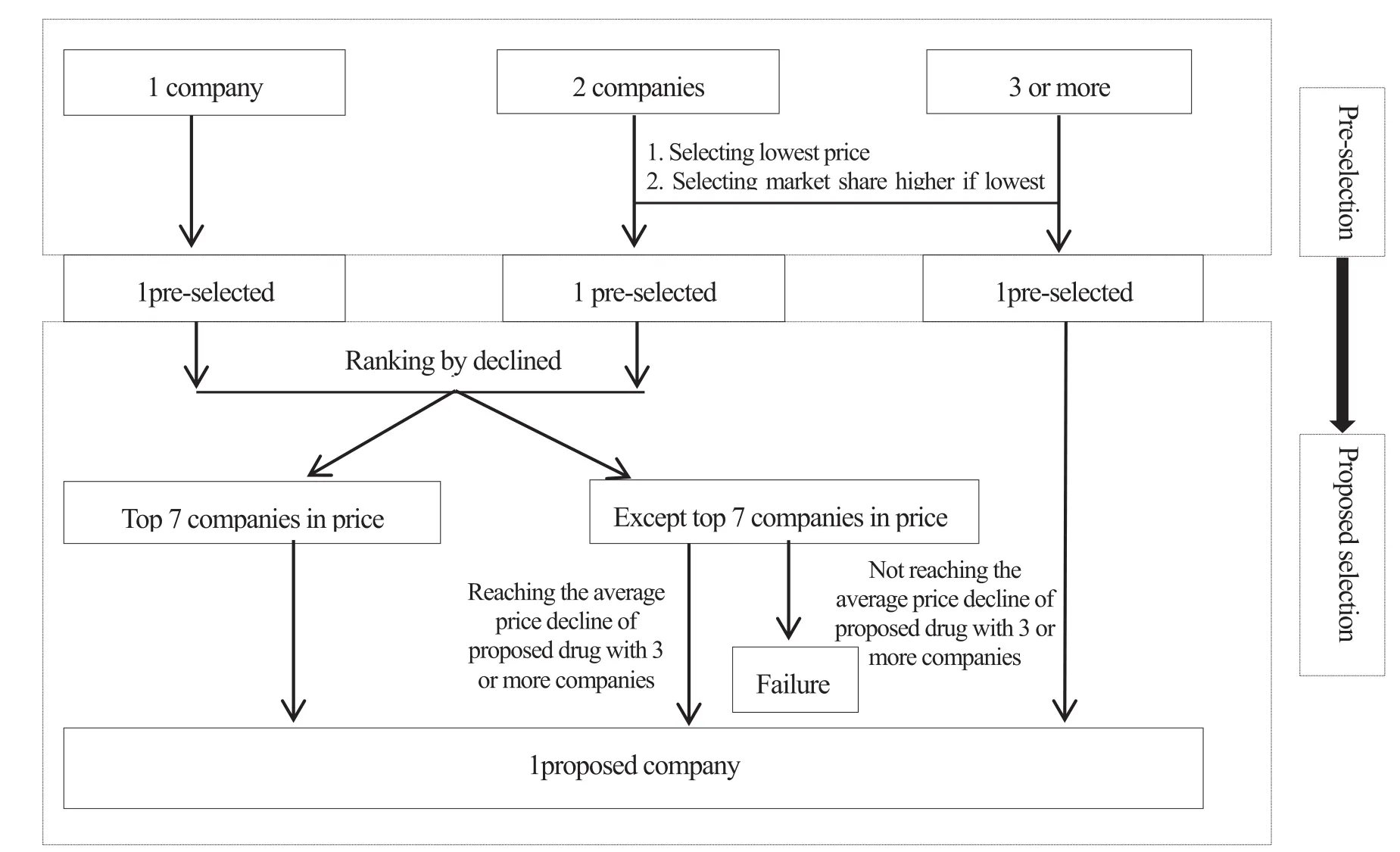
Fig.1 The bidding process of drug procurement with target quantity in 4+7cities
The bidding rules of drug procurement with target quantity adopt the principle of bidding fi rst and then bargaining, and selecting drugs with the lowest price. The drug bidding process is divided into four stages: pre-selection, candidate, price negotiation and fi nal determination. Pre-selection stage is the bidding to select drugs with the lowest price. If only one company is bidding for a drug variety, the company will get the qualification directly. If two or more companies participate, the company with the lowest quotation will get the pre-selection qualification. If two companies both have the lowest price, the one with high market share will get the pre-selection qualif i cation. The proposed selection is the bargaining stage. Drugs with the top 7 price decline are eligible for qualification of proposed selection, while other drugs with small drop rate are forced to reduce their price by referring to the drop rate of the competitive varieties. If the number of companies that meet the application conditions is less than two or equal to two,that the top 7 varieties with the largest drop rate will be eligible for the qualification. If more than three companies meet the quotation requirements, then the varieties with the lowest price will be chosen. After confirming the proposed selection companies, it will enter into the price negotiation procedure, and fi nally determine the final bidding drugs. Public medical institutions in pilot areas purchase drugs from the provincial drug centralized procurement platform. The specif i c bidding process is shown in Figure 1.
2.3 The results of drug bidding
There are 25 of the 31 varieties successfully bidding in this pilot procurement with target quantity.According to the data released by National Health Commission of the People's Republic of China, the average price decline rate of the proposed selected drugs reached 52%, and some of them even exceeded 90%[6]. For example, both the price decline rate of Entecavir Dispersible Tablets from Tai-Tianqing Pharmaceutical Co. Ltd and Tenofovir Dipivoxil Fumarate Tablets from Beiye Pharmaceutical Co.Ltd reached 96%. Even the price of the branded drug Gefitinib Tablets was reduced 76%, and the price of Fosinopril Sodium Tablets was reduced 68%, which was lower than that of the surrounding countries and regions by more than 25%. The price reached the market expectation[7].
Only 2 branded drugs out of 25 successfully won the bid, namely AstraZeneca's Gefitinib and Fosinopril Tablets from Shanghai Squibb Pharmaceutical Co. Ltd[8]. The AstraZeneca's Gefitinib with a market share of 88% in 2017 is mainly used for the treatment of non-small cell lung cancer. There are also Ochinib Tablets, PD-L1 Monoclonal Antibody for treatment of non-small cell lung cancer in AstraZeneca and these drugs are highly synergistic so the selection of Gefitinib can occupy market share of other related varieties[7]. Shanghai Squibb Pharmaceutical Co., Ltd chose to reduce the price of Fosinopril Tablets to maintain brand image and market share because the number of its sales team decreased a lot. Fourteen original pharmaceutical companies, including Pfizer, Sanofi and Merk also participated in the pilot procurement with target quantity. They voluntarily declared that the price of drugs had dropped by about 10%-30%, but the price of drugs was still higher than that of domestic brands,so they failed in the pre-selection stage[9]. However,these original pharmaceutical companies are rich in drug varieties and it is easy for them to have access to hospitals, and most of them have opened up retail channels. Therefore, it is expected that the overall impact of pilot procurement with target quantity on the original pharmaceutical companies can be controlled under the circumstances of prescription outf l ow.
At the same time, after the procurement rules and time are clarified in the pilot areas, the procurement with target quantity starts. For example,the "Supplementary Document on Drug Procurement with Target Quantity in 4+7 Cities" for Shanghai further clarifies the rules and requirements for payment. Prepayment and special funds are used to guarantee payment of drugs. Meanwhile, the impact of procurement with target quantity is also expanding.Some pilot areas also require gradient price reduction for non-bidding varieties, otherwise their online promotion is not allowed. For example, the "Notice on the Implementation of the Results of the Drug Centralized Procurement in 4+7 Cities" and the"Gradient Price Reduction for Non-bidding Drugs"issued by Liaoning Province[10]clearly requires the gradient price reduction for non-bidding drugs.Chongqing Municipality demands pharmaceutical companies to lower quote prices for 207 drugs,otherwise they will be disqualified for bidding. The"Public Announcement on the Adjustment of Price with Drug Centralized Procurement of 4+7 cities in Xi'an, Shaanxi Province in 2019" issued by the Shaanxi Public Resources Trading Center requires the related products to adjust price according to the results of drug procurement with target quantity, otherwise the qualif i cation of drug bidding will be cancelled.
At present, 11 pilot areas have started drug procurement with quantity in an all-round way. As of April 14, 2019, the total purchase quantity of 25 selected varieties in 11 pilot areas reached 438 million pieces, with a total amount of 533 million yuan, and it completed 27.31% of the total purchases. The pilot procurement with target quantity has been carried out well[11].
3 Discussions
3.1 The advantages of drug procurement with target quantity
Drug procurement with target quantity led by National Healthcare Security Administration solved some problems. For instance, quantity could not determine drug prices and medical institutions could not pay the money to pharmaceutical companies on time for previous centralized drug procurement.First of all, the pilot procurement with target quantity clarifies the quantity of purchasing and realizes price-quantity linkage. At the same time, the agreed purchasing quantity is about 60%-70% of the drug consumption in the pilot areas and it is clearly required that public medical institutions give priority to the use of procurement with target quantity. Secondly, the suきcient price competition can combine bidding with procurement. Under the premise of the same quality standards, some generic drugs that have passed the consistency evaluation of quality and efficacy are taken as the pilot drugs. This makes the branded drugs and generic drugs compete together which increases the pressure of price competition. Meanwhile,two rounds of bidding are adopted to determine the winning varieties. The winning varieties are determined through direct price competition. Then the proposed varieties are selected by the price reduction rate. Finally the payment of pilot drugs is guaranteed.The purchasing documents clearly stipulate that the public medical institutions in the pilot areas should give priority to the use of the purchasing varieties with quantity to ensure the successful procurement. In order to further guarantee drug repayment from medical institutions, Notice on the National Organization of the Pilot Program for Drug Centralized Procurement and Use of Bidding Drugs was issued by The State Council in 2019 which explicitly put forward that 30%of prepaid fund from the medical insurance should be used for drug payment by medical institutions on the basis of the total budget[12].
3.2 The monopoly of drug procurement caused by medical insurance
The newly established National Healthcare Security Administration integrates the functions of urban basic medical insurance, the new rural cooperative medical system and medical assistance in cities and towns, which were originally provided by the Ministry of Human Resources and Social Security, the former National Health and Family Planning Commission and the Ministry of Civil Affairs. Thus it makes the use of medical insurance funds more centralized. It is estimated that the budget of the medical insurance fund controlled by National Healthcare Security Administration will exceed 65%of the national medical expenditure[13]. At the same time, the National Healthcare Security Administration has the right to price, use and purchase medicines.It also integrates the functions of price management and purchasing of medicines and medical services as well as the expenses of medical institutions originally belonging to the National Development and Reform Commission, the former National Health and Family Planning Commission and the Ministry of Human Resources and Social Security. As a representative of insured personnel, the functions of National Healthcare Security Administration in drug procurement have been greatly strengthened, so that the state can organize centralized procurement with target quantity and pay by the medical insurance fund. According to this model, National Healthcare Security Administration is equivalent to a national GPO organization, which is easy to form a monopoly because of its strong purchasing power.
The centralized power of National Healthcare Security Administration determines does not conform to the law of drug use because it not only has the right to purchase drugs but also to use them. Drug use should be a process of feedback and adjustment by medical institutions according to actual clinical use. The most obvious purchasing concept of GPO organization in the United States is to guide drug purchasing by clinical use effect. Drugs must be tested in the hospitals by clinicians and experts to get the real clinical eあects, and then we can purchase them while taking drug use and price into account. In China, the centralized purchasing with target quantity weakens the evaluation of clinical effect, and prices can determine the bidding drugs, which mean they cannot meet the clinical needs. Therefore, for the pilot varieties, more attention should be paid to the real feedback of clinical use in medical institutions.Meanwhile, some alternative clinical drugs must be chosen.
In addition, the only winning bid with the lowest price and the price linkage may also lead to monopoly of product and price. A total of 189 varieties will pass the consistency evaluation in the future[14]. The pilot areas will strengthen the price linkage effect, so as to expand the regional market coverage. If the whole country adopts purchasing price and single winning bidder in 4+7 cities, the unsuccessful enterprises will lose market share soon. And the winning enterprises are more eager to further expand market share with scale eあect, thus forming monopoly. When the market mechanism is not perfect, monopoly will bring great harm to consumers. Therefore, we can choose 2 or 3 winning companies or give them a certain market share to prevent product monopoly.
3.3 The shortage of drugs
Drug shortage is a complex and objective problem. There are many causes, including manufacturing problems, quality problems and the time needed for new supplier identification. The pilot procurement with target quantity in 4+7 cities adopts the principle of one winning pharmaceutical company. The bidding company undertake 60%-70% of the drug supply in 11 pilot cities. If the winning company has problems in raw materials or production process, it may affect the drug supply.For example, Nimesulide Dispersible Tablets of Kangzhi Pharmaceutical Co., Ltd. withdrew from procurement with target quantity of Shanghai in January 2019[15]. Some drug shortages problems also exist in the drug centralized procurement, 43 pharmaceutical manufacturers in 2019 applied to cancel the online promotion for 108 drugs in Inner Mongolia due to the shortage problem[16]. Drug shortage is a challenging factor in the ever-changing health care system. The only winning company in the procurement with target quantity increases the company's determination, but at the same time, it may also lead to drug shortage. The only winning company monopolizes the whole market, which is not conducive to a healthy competition in the long run and may lead to drug shortages. Drugs are recognized as special commodities, which have strict quality requirements and market access conditions. All drug production links need to follow strict conditions and facilities requirements because any error in any process may lead to unqualified products. There will be supply risk if the only bidding company has the monopoly of active pharmaceutical ingredients which will have a serious impact on drug production. Therefore,diあerent market shares can be allocated to candidate bidding drugs to ensure eあective market competition while dealing with the possible drug shortages. We can allow 2 or 3 companies to win at the same time to promote drug price reduction in stages according to the supply and quality of drugs. Meanwhile, we should strengthen the supervision of drug quality.
3.4 The quality of drug bidding
In order to occupy the market share, the bidding companies may lower the price of drugs, or even sell them at cost price, so there may be drug quality risk[14]. The price and quality of drugs are negatively correlated, and reducing the production cost of drugs will inevitably have an impact on the quality of drugs. Therefore, we should pay attention to the clinical eきcacy of the products which have passed the consistency evaluation. Quality and efficacy should go fi rst, and the attention should be paid to the safety of sudden adjustment of medication for long-term patients[17].
3.5 Inhibition of drug innovation
Firstly, the pilot procurement with target quantity may aあect the enthusiasm of enterprises in evaluating the consistency of generic drugs. The varieties selected for procurement are generic drugs which have passed the consistency evaluation of quality and efficacy.Therefore, after investing a lot of resources to pass the consistency evaluation, companies can occupy 30% of the market share with price reduction. However, the unsuccessful bidding varieties must compete with the drugs that have not passed the consistency evaluation for the remaining 70% of the market share. So it is obviously unfair for the drugs that have passed the consistency evaluation because of the small market share. Consistency evaluation of generic drugs is based on the fact that the quality of generic drugs in China is diあerent from that of original drugs. It is of great significance to improve the quality of generic drugs to ensure the safety and eあectiveness of drug use in China. The price reduction of the bidding drugs is so large that the winning companies are worried about whether the cost of consistency evaluation of generic drugs will be returned. Besides, the unsuccessful companies will consider whether they will spend more on generic drug consistency evaluation in the future.
Secondly, it aあects the investment of companies in new drug research and development and the improvement of product quality. Because companies invest a lot of money in drug consistency evaluation,failure to win the bid means that their previous investment will be lost. Therefore, many companies may produce drugs with lower costs instead of paying attention to the needs of patients and the quality of drugs. In addition, the current pilot purchasing with target quantity doesn't focus on the evaluation of drug quality, it only pay attention to the price of drugs,which may encourage companies to pursue lower costs while ignoring the innovation and improvement of drug quality in the process of research and development, production and sales. If this policy is popularized nationwide, it can affect the enthusiasm of enterprises in developing new drugs and passing consistency evaluation. Therefore, we should further refine the quality evaluation system of procurement and incorporate the innovation and quality level of products into the evaluation system. While guiding and promoting companies to reduce drug prices, we should urge them to improve the quality of products,and constantly innovate new products to meet the needs of patients.
杂志排行
亚洲社会药学杂志的其它文章
- A Study of Cognition and Demand of Pharmacy Services in Hospitals from the Views of Doctors, Pharmacists and Patients
- Reducing the Error Rate of Drug Delivery in Hospital Pharmacies: From the Angle of Pharmacy Quality Control Circle
- The Regulating Eあect of Complementary Assets on the lncremental lnnovation of Traditional Chinese Medicine Enterprises
- SWOT Analysis and Development Suggestions on HK Drugs of G Pharmaceutical Firm
- Ref l ections on the Management of Preventing lnterest Conf l ict among Pharmacopoeia Members
