Predicting differentiation potential of human pluripotent stem cells: Possibilities and challenges
2019-08-28LiPingLiuYunWenZheng
Li-Ping Liu,Yun-Wen Zheng
Li-Ping Liu,Yun-Wen Zheng,Institute of Regenerative Medicine,Affiliated Hospital of Jiangsu University,Jiangsu University,Zhenjiang 212001,Jiangsu Province,China
Li-Ping Liu,Yun-Wen Zheng,University of Tsukuba Faculty of Medicine,Tsukuba,Ibaraki 305-8575,Japan
Yun-Wen Zheng,Yokohama City University School of Medicine,Yokohama 236-0004,Japan
Abstract
Key words: Human pluripotent stem cells; Induced pluripotent stem cells; Embryonic stem cells; Differentiation potential; Prediction; Pluripotency; Malignant potential; Embryoid bodies; Lineage-specific differentiation; Teratoma
INTRODUCTION
The capability of human pluripotent stem cells (hPSCs) to differentiate into any cell type has revolutionized medical research.Their widely known potential applications include the study of complex diseases,cell-based drug screening,and transplantation therapy[1].With the development of organoid technology,hPSCs also play a critical role to mimicin vivotissues and organs at the three-dimensional level and provide a unique opportunity to model human organ development and study various diseases[2].In the near future,integration of multiple patient-specific hPSC-derived organoids into a dynamic four-dimensional system by organ-on-chip technology will contribute to the study of the systematic interactions among different tissues and organs in the body[3].All these applications require the selection and characterization of cell lines that reliably,efficiently,and stably differentiate into disease-relevant cell types.However,significant variation has been observed in the differentiation potential and efficiency of various human induced pluripotent stem cell (iPSC)lines[1,4,5]and embryonic stem cells (ESCs)[6-8],and no single cell line can uniformly differentiate into all lineages.Differences among hPSC lines mainly include DNA methylation[9-13]and gene expression[1,8],which have functional implications for both ESC and iPSC lines[1].Particularly,for iPSCs,the variations may be donordependent[14,15]or original cell type-dependent[9],while,in other studies,this relationship was not found[10,16].Moreover,the characteristics of hPSCs may differ depending on the number of passages[17],culture medium components[18],and feeder conditions[19].As a result,understanding the variability among different cell lines is necessary for ensuring the efficacy and safety of hPSC applications.Additionally,a long period (up to weeks or months) is typically required to complete hPSC differentiation into a specific cell type,and protocol optimization to improve differentiation efficiency is also time-consuming.Thus,these processes can be considerably accelerated if good-quality hPSC lines are selected,and their differentiation propensities into destination cell types are predicted in an early stage.
hPSC quality control
Given that large numbers of iPSC lines are currently and will be generated,the pluripotency of these cells must be determined before they are broadly applied.To confirm whether an iPSC line is fully reprogrammed,a teratoma assay is typically performed to reveal the differentiation capacity of hPSCs into three germ layers based on histological analysis.The pluripotency status can also be determined by detecting the expression of a set of marker genes at the molecular level[20].With the development of high-throughput sequencing techniques,numerous methods have been developed to address this issue.The PluriTest®[21],a rapid test based on microarray and bioinformatics assay,provides quantitative information on hPSC quality.Two summary scores,the pluripotency score and novelty score[22,23],which are generated from global gene expression profiles,can predict whether an hPSC line is pluripotent based on its molecular similarity to other known cell lines and exclude cells that differ substantially from normal hPSC lines.An hPSC line with a high pluripotency score and low novelty score would be regarded as having passed the PluriTest.Another assay,the “deviation scorecard”[1],which combines DNA methylation and gene expression with bioinformatic comparison to an ESC reference,also provides comprehensive information and excludes problematic cell lines that should be avoided for an intended application.However,these methods can only be used to determine whether an hPSC line meets the criteria for pluripotency and do not directly assess the specific differentiation capability of the cells.
Prediction of differentiation potential
As described above,the teratoma assay is the most frequently used method to assess the pluripotency of hPSCs.However,it does not yield quantitative information on lineage differentiation potential[24]nor provide specificity data to support the application-specific selection of the most suitable cell lines[25].Thus,the “TeratoScore”was developed[26]as a quantitative and unified assessment that analyzes RNA sequencing data within heterogeneous hPSC-derived teratomas.This score weighs differences in tissue-specific expression within a teratoma and provides an estimate of the ability of an hPSC line to differentiate[22,23,26]to overcome some of the limitations of histological analysis.
hPSC differentiation is a complex and multiple-step process,the beginning of which involves a particularly heterogeneous status and diverse developmental mixture.During cell fate commitment to a differentiated lineage,genes are regulated by successive transcriptional programs,and thus,the differentiation status can be determined from transcriptional profiles[27].A combination of gene expression profiling and bioinformatics assay is invaluable for predicting the trajectory of cells during differentiation.The “differentiation scale”[27]based on mRNA microarray analysis can indicate the staging of differentiation and show how far the hPSCs have departed from the embryonic pluripotent state.However,this method only measures the overall capability of a cell line towards differentiation into any cell type and cannot clearly distinguish between any direction of differentiation into the three germ layers.In contrast,the “lineage scorecard” assay,which combines simple nondirected differentiation with transcript counting of 500 lineage marker genes,can detect the lineage-specific differentiation propensities of an hPSC line[1].For example,hPSC lines showing high scores for ectoderm and neural differentiation propensities are regarded as well-suited for studying neural function.This prediction has been confirmed in experiments to quantify differentiation efficiencies specific for the ectoderm germ layer.Additionally,other scores calculated based on the expression levels of a set of selected specific gene markers can predict hPSC differentiation potential in similar manners[22,23,28].These qPCR-based assays are more rapid and accessible than high-throughput methods.
According to the hPSC-derived differentiation protocols for various types of cells,embryoid bodies (EBs) are widely used[29]because they mimic many aspects of cell differentiation during early embryogenesis.Because they consist of tissues containing three germ layers[30],EBs can be utilized as a trigger of not onlyin vitrodifferentiation of hPSCs but also in assay predicting differentiation potential.For the latter purpose,EBs are induced to spontaneously differentiate under neutral conditions[1,22]or directed into three germ layer lineages in the presence of specific growth factors[22,28],after which gene expression profiling of EBs is conducted to assess their differentiation potential into the ectoderm,mesoderm,or endoderm lineages.In our study to predict the differentiation potential of iPSCs into melanocytes derived from the ectoderm,an EB-based assay showed that this potential could be predicted by the capability of formation and maintenance of optimal EBs under neutral conditions as well as their expression of germ layer-specific markers such as SALL3 (our unpublished data).Thus,EBs are practical tools for use in prediction assays at early stages of differentiation.In addition to EB-based protocols,monolayer differentiation[23,31]has been adapted and shown to be applicable for evaluating endoderm and mesoderm direction; however,to predict the ectodermal direction,EB-based protocols may be more effective[23].
Regardless of the detection techniques used,prediction can be achieved many days before the cells exhibit a differentiated phenotype (Table1).For instance,the efficiency of protocols for directing differentiation to cardiac cells can be predicted at as early as day 2[31],and thus can be utilized to optimize differentiation protocols,particularly for patient-specific iPSC lines by using a high-throughput screening procedure.In contrast to the above methods based on EBs or monolayer differentiation protocols,which require a couple of days before evaluation,simpler methods with limited specific markers can be used for earlier prediction using undifferentiated hPSCs.The expression level of SALL3 mRNA was used as a diagnostic marker to predict the differentiation tendency of both iPSCs and ESCs into ectodermal cells[32].hPSCs expressing the highest levels of SALL3 mRNA tend to differentiate into the most ectodermal system,while cells expressing the lowest levels of SALL3 mRNA tend to differentiate into the most mesodermal or endodermal cell types.Specifically,three genes,FGF-1,RHOU,andTYMP,were selected as predictors of hepatic differentiation,with low prediction scores linked to low hepatic differentiation[33].
Collectively,the lineage-specific differentiation potential of hPSC lines can be predicted in an early stage using multiple assays,including the teratoma assay,different scorecards calculated by high-throughput sequencing data collected from EBs or monolayer-based differentiated hPSCs,or specific maker expression in undifferentiated hPSCs.
Evaluation of malignancy potential
The safety of using hPSCs in clinical application is one of the greatest concerns for both clinicians and patients[34].Currently,tumorigenicity tests are well-designed,and animal transplantation studies have been used to detect the malignancy potential of hPSC-derived cell products[35,36].Although residual undifferentiated cells are present among the differentiated cell population,these cells can be ablated by various techniques[37,38].Additionally,evaluation of hPSCs rather than their derivatives can provide safety-related information.As described above,the teratoma assay is commonly used to measure the pluripotency and differentiation potential of hPSCs[22,39].It is also feasible to predict the malignancy potential by detecting the immaturity of teratomas and formation of carcinoma or sarcoma in tumors derived from hPSC lines[40].Furthermore,a combination of histologic examination and“TeratoScore”,which involves computational quantification of gene expression data derived from teratoma tissue,can provide much greater detail for evaluating whether a cell line has the malignant potential[22,23].It is also important to examine chromosomal abnormalities by karyotype analysis[40,41]and assess mutations in cancer-related genes[40].
LIMITATIONS AND CHALLENGES
Collectively,multiple screening tools are available for hPSC selection with different features and prediction capabilities,and researchers can choose one or more methods according to the intended applications (Table1 and Figure1).However,concordance between two different prediction methods is low[22,23],and even with the same prediction assay,distinct results may be obtained under different differentiation conditions.The lack of concordance of results makes it very difficult for researches to determine the most appropriate method for their purposes.Moreover,each prediction method has limitations,thus largely restricting its wide applications.Highthroughput analysis methods such as microarray or RNA-sequencing are informative but generate excessive data,some of which are non-functional.Additionally,identifying objective genes is time-and labor-intensive as well as costly[33].The platforms used in these assays are not available to most laboratories and require customized downstream analysis,which also restricts their applications.Although teratoma formation reflects thein vivodifferentiation capability of hPSCs and gene expression analysis can provide more definitive and quantitative information when combined with histological assessment,these methods are also very time-consuming to be feasible for validating a large number of hPSC lines[1].
Because of these limitations,the methods described above are not sufficient to meet the needs of researchers in routine work.Additionally,hPSC characteristics are not stable during long-term culture,which can be affected by several factors.Further,hPSCs can vary when they are cultured using different commercial products or show different features in different laboratories,even under the same culture conditions.Therefore,a simpler and quicker detection assay with high specificity and sensitivity that can be used to monitor the status of hPSCs at any time is needed.Although most current methods are useful for lineage-direction prediction,fewer targeted markers that are more specific for a given cell type rather than all cell types are urgently needed.Moreover,EBs are ideal candidates for replacing the teratoma assay,and they should be useful for predicting differentiation potential when they are combined with modified detection methods such molecular probes,which could detect targeted markers directly.
Exceptionally,the potential prediction is not a prerequisite when iPSCs from patients with monogenic disease are utilized for disease modeling because the differentiation capability is probably interfered by gene mutation[42].However,iPSC quality control is still necessary.When using these patient-derived iPSCs for cell therapy,it is also critical to predict their differentiation potential after some special strategies such as gene editing,which can revert their defective capability.
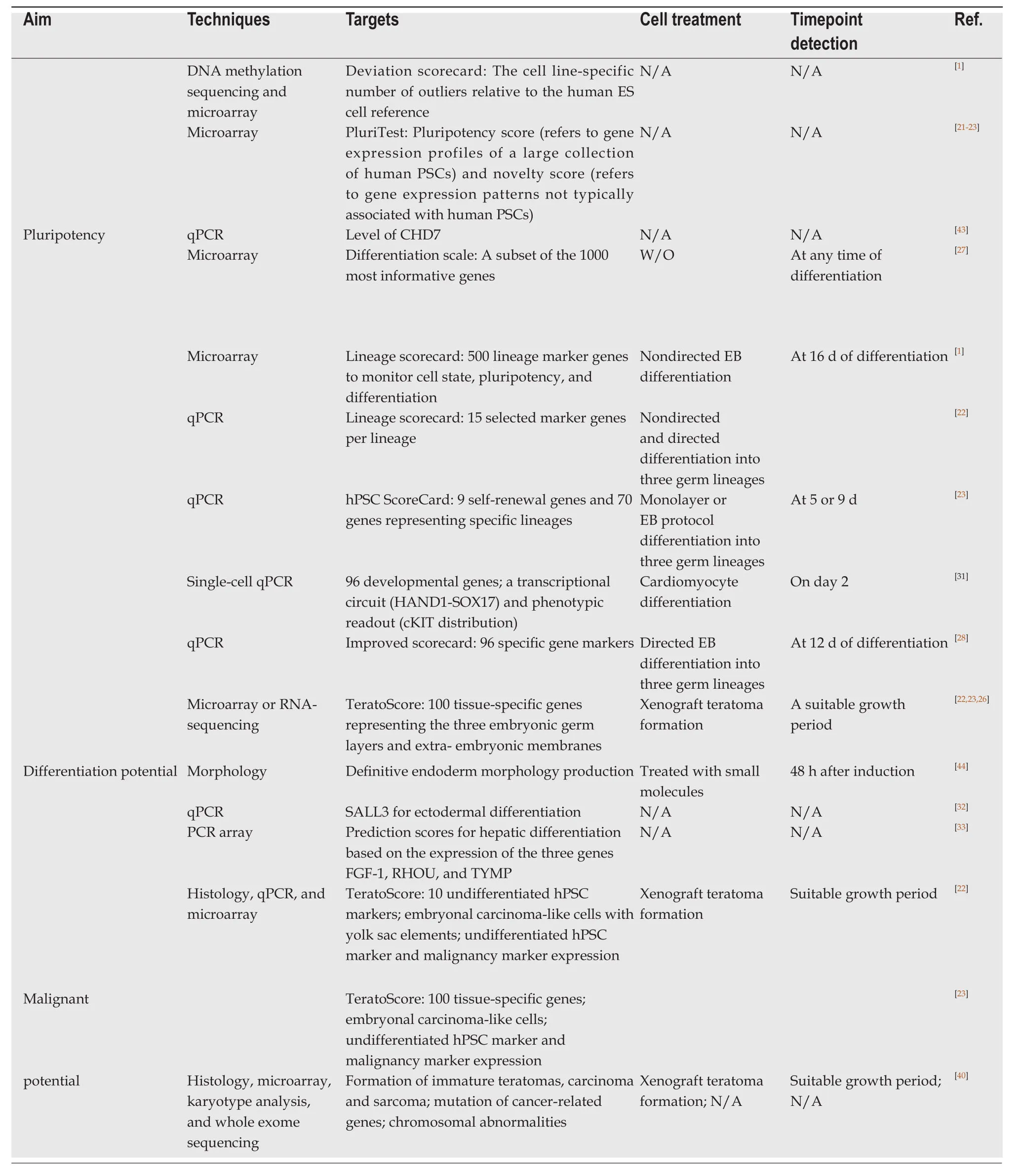
Table1 Methods for evaluating human pluripotent stem cell lines for pluripotency,differentiation potential,and malignant potential
