Optimizing neoadjuvant radiotherapy for resectable and borderline resectable pancreatic cancer using protons
2019-08-27RomaineCharlesNicholsMichaelRutenberg
Romaine Charles Nichols,Michael Rutenberg
Romaine Charles Nichols,Michael Rutenberg,Department of Radiation Oncology,University of Florida College of Medicine,Jacksonville,FL 32206,United States
Abstract
Key words:Radiation oncology; Pancreatic cancer; Proton therapy
SCOPE OF THE PROBLEM
It is an accepted oncologic principle that local disease control is a necessary condition for curing cancer. In the setting of a pancreatic malignancy,it is also generally accepted that extirpative surgery is a necessary condition for local control. For numerous reasons,however,it is arguable that surgery alone cannot reliably achieve local control in this setting. Historical data suggest that,even in the setting of negative surgical margins and negative lymph nodes,patients undergoing pancreaticoduodenectomy for localized pancreatic cancer experience a 50% to 80% risk of local-regional failure[1,2]. This local-regional failure rate is not unexpected for this procedure given that a portion of the involved organ (the pancreas) is not removed from the patient and given the close proximity of critical structures that cannot be sacrificed (i.e.,the superior mesenteric artery); when negative margins are in fact achieved,they often tend to be close.
Having established that local-regional failure is a significant problem for patients undergoing surgery with negative margins and negative lymph nodes,two contemporary large series from highly respected research institutions suggest that node negativity and margin negativity may be less common than generally appreciated. Investigators from Johns Hopkins University (Baltimore,MD,United States) reviewed the outcomes for 905 patients undergoing pancreaticoduodenectomy from 1995 to 2005 and reported a 79.3% incidence of lymph node positivity and a 41.1% incidence of margin positivity[3]. A similar series from investigators at Memorial Sloan Kettering Cancer Center (New York,NY,United States) reviewing 625 pancreatic resections from 2000 to 2009 reported a 16% incidence of margin positivity along with a 70% incidence of lymph node positivity[4].
ROLE FOR RADIOTHERAPY
Recognizing that local-regional failure is a common occurrence for patients undergoing margin-negative and lymph node-negative resections and simultaneously recognizing that a large share of resections are associated with margin positivity and lymph node positivity,it would appear evident that additional therapies would need to be offered to achieve a high likelihood of local-regional disease control. While systemic drug therapies may reduce the risk of hematogenous dissemination of malignancy,there are no other disease sites where such therapies have been demonstrated to improve local-regional control. Radiotherapy,however,would appear to be the most relevant intervention to address this problem.
POSTOPERATIVE RADIOTHERAPY
Postoperative radiotherapy,one of several radiotherapy options,has the potential to reduce local-regional failures to some degree. This intervention,however,has two important limitations: First,it is difficult to initiate postoperative radiotherapy before 10 to 12 wk have elapsed after major abdominal surgery. This interval allows time for microscopic foci of disease to grow unchecked in a hypoxic tumor bed. To make matters worse,many patients with indications for postoperative radiotherapy do not receive it due to postoperative complications. Secondly,the dose of radiotherapy that can safely be delivered after pancreaticoduodenectomy is generally no higher than 50 Gy with conventional fractionation.
Clinical data suggest that,even when postoperative radiotherapy is delivered,patients experience a nontrivial incidence of local failure. Investigators from Massachusetts General Hospital (Boston,MA,United States) reviewed the medical records of 86 patients undergoing postoperative radiotherapy and demonstrated a 36% local failure rate at 3 years[5]. Data from the RTOG 97-04 trial demonstrated a 23%to 28% local-regional failure rate[6]. Given the difficulty in following these patients,it is likely that these data understate the actual local-regional failure rate. Finally,although valid criticisms of its methodology have been published,the ESPAC trials[7]suggest that postoperative X-ray-based radiotherapy may be associated with a nominal survival decrement[8,9]. In summary,it could be argued,based on radiobiologic principles as well as clinical outcome data,that postoperative radiotherapy is simply “too little and too late” to have a meaningful effect on localregional disease control or survival.
PREOPERATIVE RADIOTHERAPY
From an oncologic perspective,preoperative radiotherapy would make a great deal of sense. In fact,preoperative radiotherapy is the standard intervention for gastrointestinal malignancies in the esophagus and rectum. Preoperative radiotherapy is also viewed as the ideal approach for the treatment of soft-tissue sarcomas. The advantage of preoperative radiotherapy is that it allows for sterilization of high-risk lymphatic sites prior to extirpative surgery. It may also increase the likelihood of a marginnegative resection because tumor shrinkage may occur away from critical structures such as the superior mesenteric artery. Finally,in regards to toxicity,while postoperative radiotherapy radiates normal tissues which will remain in the patient for the rest of his or her life,a large share of the bowel tissue that is irradiated preoperatively will be removed at the time of surgery.
While preoperative radiotherapy covering the primary tumor and at-risk lymph nodes makes sense oncologically,many surgeons are reluctant to employ this intervention given a concern that preoperative radiotherapy runs the risk of complicating what is already a complicated and time-consuming operation. Given the fact that the median operative time for a pancreaticoduodenectomy exceeds 6 ½ h[10],is understandable that a surgeon would be concerned about any intervention that could potentially lengthen the operation time.
PROTON RADIOTHERAPY
It is reasonable to be concerned that conventional X-ray based radiotherapy or intensity modulated X-ray therapy (IMRT),which involves delivering radiotherapy to the entire cylinder of the upper abdomen,might increase the risk of surgical complications. Proton radiotherapy,however,by virtue of improvements in dose distribution,would theoretically be associated with a lower risk of surgical complication while offering the oncologic benefit of neoadjuvant therapy (Figure1).
An early study using dosimetric data comparing proton therapy and IMRT demonstrated statistically significant dose reductions to the small bowel,stomach,and right kidney[11]. Subsequent clinical data demonstrated,an absence of grade 3 toxicities for 20 patients treated with aggressive proton therapy for unresectable disease,and marginally resectable disease in the postoperative setting. Only three(15%) patients experienced grade 2 gastrointestinal toxicity in this series[12].
SURGERY AFTER PROTON THERAPY
Of particular interest from a surgical perspective,investigators at the University of Florida (Jacksonville,FL,United States) reported on a series of 5 patients who received high-dose proton radiotherapy as definitive treatment for unresectable disease who were ultimately able to undergo pancreatectomy[13]. Because these patients were felt unlikely to become surgical candidates,they all received full-dose radiotherapy to a dose of 59.4 Gy RBE over 33 fractions with concomitant capecitabine chemotherapy. The median duration of surgery for these patients was 419 minutes with a range of 290 min to 484 min. The median estimated blood loss was 850 mL. The median intensive care unit stay was 1 d. The median hospital stay was 10 d. These metrics were comparable to published data for patients undergoing pancreatectomy without prior radiotherapy[14-16]. Based on these data,we feel comfortable arguing that patients who might receive preoperative radiotherapy with protons to a lower dose(i.e.,50 Gy RBE) for resectable or borderline resectable disease are not at risk of unexpected surgical complications attributable to the proton radiotherapy.
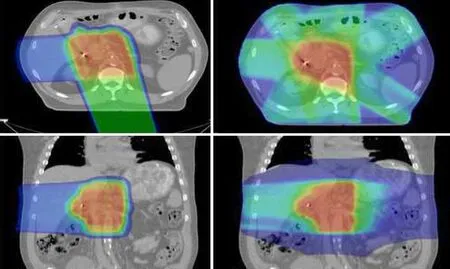
Figure1 Images on the left side demonstrate a typical dose distribution for a patient receiving proton therapy for pancreatic cancer. Images on the right side show corresponding dose distributions for the same patient treated with intensity-modulated radiotherapy (IMRT). It is evident that protons are associated with significantly less bowel and gastric exposure compared with the IMRT plan.
LIMITATIONS OF STEREOTACTIC BODY RADIOTHERAPY
Stereotactic body radiotherapy (SBRT) has been advocated at various institutions as one form of neoadjuvant radiotherapy for patients with resectable and borderline resectable disease. This modality involves the delivery of 5 fractions of high-dose focused radiotherapy to the site of gross disease a few days prior to surgery. The putative advantage of this form of radiotherapy is that it does not delay surgery or chemotherapy. The oncologic disadvantage of this modality is that it can address only a small target containing gross disease and is unable to safely deliver meaningful dose to regional lymph nodes at high risk of harboring microscopic disease. This theoretical shortcoming of SBRT has recently been demonstrated by in the literature[17,18]where it is reported that patients undergoing pancreatectomy after SBRT demonstrate a high risk of disease recurrence in regional lymphatics that would have been irradiated by a conventionally fractionated course of preoperative radiotherapy with X-rays or protons. As such,we would argue that SBRT should not be considered an oncologically appropriate intervention in the setting of resectable or borderline resectable disease.
CONCLUSION
Patients with resectable and borderline resectable pancreatic cancer are at a high risk of suffering postoperative local-regional failure. Preoperative radiotherapy directed to gross disease and regional lymphatic beds at high risk of harboring microscopic disease appears to be an oncologically rational intervention to reduce this risk. When proton-based radiotherapy addressing gross disease as well as high-risk regional lymphatic beds is delivered prior to surgery,it does not appear to increase the risk of surgical complications or the duration of surgery. Because of this,we would argue that proton-based preoperative radiotherapy should be considered for patients with resectable and borderline resectable disease.
ACKNOWLEDGEMENTS
The authors would like to thank Jessica Kirwan,MA,and Christopher Stich for their editorial assistance and manuscript preparation.
