Platelet-rich plasma for muscle injuries: A systematic review of the basic science literature
2019-08-22KyleKunzeCharlesHannonJaredFialkoffRachelFrankBrianCole
Kyle N Kunze, Charles P Hannon, Jared D Fialkoff, Rachel M Frank, Brian J Cole
Kyle N Kunze, Charles P Hannon, Jared D Fialkoff, Department of Orthopedic Surgery, Rush University Medical Center, Chicago, IL 60612, United States
Rachel M Frank, Department of Orthopedic Surgery, University of Colorado School of Medicine, Boulder, CO 80309, United States
Brian J Cole, Department of Orthopedics, Rush University Medical Center, Chicago, IL 60612,United States
Abstract
Key words: Platelet rich plasma; Basic science; Muscle; Musculoskeletal; Injury
INTRODUCTION
Over the past several years, there has been increasing interest in biologic agents as both nonoperative treatment modalities and augments to surgical procedures, to potentially accelerate the healing process and expedite return to sport after muscle injury. Platelet-rich plasma (PRP) is a blood product that is rich in platelets and many different cytokines and growth factors, such as transforming growth factor beta (TGFβ1), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor(VEGF), whose influence on the healing of ligaments, muscles, tendons, joints, and soft tissue has been extensively studied[1-5]. In muscle pathology in particular, PRP’s abundance of growth factors and cytokines is purported to expedite healing through the induction of cellular proliferation and migration, increased angiogenesis, and muscle tissue regeneration[6-8]. These notions have led to the clinical use of PRP for both acute and chronic muscle injuries, despite the lack of both basic science and clinical evidence that justifies its use and efficacy[7,9,10]. At best, several clinical studies and trials on the use of PRP for muscle injuries have been published with varying results[3,7-9]. Therefore, it is imperative to clarify which mediators in particular facilitate these potential effects, both to better understand PRP as a biological augment, and to aid in the optimization of PRP preparations for clinical use.
The use of PRP in clinical settings requires controlled clinical trials, with consistent methodology in terms of dosing and preparation. However, what must precede is a rigorous examination of the effects of PRP in vitro and in vivo to fully understand its mechanisms of action and appropriate clinical targets. The purpose of this systematic review is to clarify the effects of PRP on muscular pathologies at the cellular and tissue levels by evaluating the basic science literature. Furthermore, the authors hypothesized that the use of PRP would confer multiple beneficial effects in the healing of muscle injuries in comparison to controls.
MATERIALS AND METHODS
A systematic review of the PubMed/MEDLINE and EMBASE databases was performed in December 2017 using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines with a PRISMA checklist. The search was performed using the following keywords: (platelet rich plasma OR PRP ORautologous conditioned plasma OR ACP) AND (muscle OR myocyte OR skeletal muscle OR muscle injury).
The inclusion criteria for full-text reviews were level III in vivo and in vitro studies examining the effects of PRP on muscles, myocytes and/or myoblasts, that also included a well-defined control group (saline solution, no treatment, or control cell medium). Animal studies that observed the effects of PRP on traumatic and surgically-induced muscle injuries were included. In studies that reported results with additional treatments, only results that compared PRP directly with the control were analyzed. Exclusion criteria included non-English language articles, clinical studies, randomized controlled trials, review articles, articles not published in peerreviewed journals, and studies without a control group. All references within included studies were cross-referenced for potential inclusion. One author independently performed the search and identified studies for inclusion.
Two authors extracted data using a predefined data collection sheet. Collected data included the characteristics and cytology of PRP, growth factor concentration,adhesive protein concentration, clotting factor concentration, fibrinolytic factors,proteases and antiproteases, basic proteins, membrane glycoproteins, dense granule bioactive molecules, pro-inflammatory cytokine concentration, anti-inflammatory cytokine concentration, and other proteins. In addition, study design and methods,study subjects, outcomes measured, and results were recorded. These variables included cell viability, cell proliferation, cell migration, gene expression, mechanical properties, gross appearance, and mechanical or histological examinations. If results were significantly different between the experimental and control cohorts, it was considered to be a positive or negative result based on the relative change observed. If no difference was observed between the experimental and control cohort groups, it was recorded as no change observed. Once data analysis was complete, data were analyzed for trends in outcomes by comparing the PRP treatment with controls.
RESULTS
Search and literature selection
The search for studies on the use of PRP in muscle pathology yielded 1013 results on PubMed/Medline and 1117 results from EMBASE. Duplicates were excluded, and 1570 studies fit the inclusion criteria for systematic review according to the search parameters described above (Figure 1). The full-text review yielded 23 articles that met the inclusion criteria for muscle pathology. Of the included studies, 15 were in vivo, 6 were in vitro, and 2 had both in vitro and in vivo study arms.
In vitro muscle studies
Of the 6 in vitro muscle studies (Table 1), 4 (66.6%) studied myoblasts[1,11-13], one studied a range of human-derived cells (myocytes, tenocytes, osteocytes and osteoblasts)[7], and one solely studied myocytes[14]. Of the first group, two studied human-derived myoblasts[11,12], while the other two studied murine-derived myoblasts[1,13].
Two studies (33.3%) reported that the platelet concentration of the final PRP preparation was increased compared to control (Table 2)[7,12]. Only one in vitro muscle study reported white blood cell concentration[7]. In this study, 3 different PRP preparations at varying platelet and leukocyte concentrations were created, and cytology was reported. Only one study reported a complete cytology of PRP, which included platelet count, red blood cell count, and white blood cell count.
All six studies analyzed cell proliferation in response to PRP treatment, and all reported significant increases[1,7,11-14](Table 3). In 4 studies (66.6%), the effect of PRP on cell differentiation was examined, and 3 (50%) observed increases[1,7,11,12](Table 3). One study (16.7%) analyzed cell signaling in response to PRP treatment, reporting no effect[1](Table 3). All 6 studies examined the effect of PRP on the regulation of gene expression, and reported significant increases in stem cell markers[7], particularly those indicative of early differentiation[1], cell-cycle mediators[13], myosin heavy chain expression[12], and mediators of cellular migration[14](Table 3). One study reported a dose-dependent increase in cell migration and spreading with PRP treatment[14]. None of the in vitro studies assessed inflammatory mediation, proteoglycan or collagen content (Table 3).
In vivo muscle studies
Of the 15 in vivo studies, 11 (73.3%) reported platelet concentrations in their final PRP preparations[2,3,8,15-22](Table 2). All 11 studies reported platelet concentrations greater than that of whole blood. Five studies (33.3%) reported PRP WBC concentration. Twoof these studies reported an increase in WBC concentration relative to whole blood[15,17]. One study reported an increased RBC count relative to whole blood in their PRP preparation[15]. None of the studies reported a complete cytology of PRP.

Figure 1 PRISMA diagram representing the process of individual study inclusion after application of the study algorithm, and each of the exclusion criteria.
A variety of animal models and muscles were used (Table 4). Studies also performed histologic[3,6,8,9,15,17,18,20-25]and gross anatomical[6,15,17,20,21,23]assessments of the muscles (Table 5). Eight studies reported improved functional and structural variables, reduced fibrosis, and increased muscular regeneration in PRP-treated muscle compared to control muscle[6,15,17,20-23,25]. Several studies reported increased angiogenesis after PRP treatment[17,20,21,23]. Four studies reported increased leukocyte infiltration at the injection site[8,9,18,25]. With regards to changes in collagen deposition,one study reported increased type III collagen, one reported increased type I collagen[25], and two reported no change in collagen content[2,8]. One study reported significant increases in growth factor concentrations (EGF, PDGF-BB, PDGF-AA,HGF, IGF, TGF-β1) compared to control groups, as well as a greater number of VEGF receptors[15](Table 5). In this study, it was found that the growth factors with the most robust increase relative to baseline were PDGF and HGF, and that leukocytes were the main source of VEGF[15].
Combined in vivo and in vitro muscle studies
Of the 2 studies that examined both the in vivo and in vitro uses of PRP, one used human muscle-derived progenitor cells (hMDPCs) from unspecified skeletal muscle from living and post-mortem biopsies for the in vitro component[26], while the other used an established murine myogenic cell line (C2C12)[27](Table 6). Only one study reported platelet count and WBC concentration[24], while neither reported RBC count.For the in vivo component, the gastrocnemius muscle of a mouse[26]and the rotator cuff muscles (supraspinatus and infraspinatus) of a rat[27]were used. The in vitro studies reported the following with regards to PRP treatment relative to controls:Increased cell proliferation[27], increased expression of stem cell markers[26], increased myotube formation and area[27], increased myogenic proliferation and decreased lipid droplets[27], and increased Pax7 and myogenin gene expression[27](Table 7). Furthermore, the in vivo component of one study assessed hMDPC expansion[26](Table 6).

Table 1 In vitro PRP and muscle studies – summaries

PRP: Platelet-rich plasma.
DISCUSSION
The principle findings of this systematic review are as follows: (1) In the majority of studies, PRP treatment increased myocyte proliferation, growth factor expression (e.g.,PDGF-A/B and VEGF), leukocyte recruitment, and angiogenesis in muscle models when compared to control groups; (2) PRP preparation techniques remain inconsistent across studies in the basic science literature; and (3) Evidence from in vitro and in vivo basic science studies suggest that PRP has the potential to serve as an efficacious treatment modality that may expedite the healing process for muscular pathologies, based off of the observed effects at the cellular and tissue levels in treatment groups relative to control groups.
The results from the current study suggest that PRP may have differential effects on myoblast proliferation and differentiation, depending on the formulation of PRP at the basic science level. The traditional formulation of leukocyte-poor PRP that is not modulated to remove specific growth factors or platelets was shown to promote myoblast proliferation. Significant increases in myocyte proliferation were observed in all of the in vitro studies included in this review[1,7,11-13,26]. Li et al[26]reported that PRP upregulated the expression of stem cell markers in hMDPCs. Miroshnychenko et al[12]also found that traditional unmodified leukocyte-poor PRP increased proliferation of human skeletal muscle myoblasts. However, the unmodified leukocyte-poor PRP had very little effect on myoblast differentiation. On the other hand, platelet-poor plasma and double spin PRP (aimed to remove platelets) significantly promoted the induction of human myoblasts into the differentiation state. Early cell differentiation was also observed in an additional three in vitro studies[1,11,12]. It is plausible that the increases in both differentiation and proliferation underlie some of the histological findings in the in vivo muscle publications, which demonstrated that PRP treatment accelerated muscular regeneration and reduced fibrosis when compared to controls[6,15,17,20,21,23].
The differential effects of different PRP formulations on myoblast proliferation and differentiation suggest that the growth factors that promote the proliferation of myoblasts may be very different than those that promote myoblast differentiation.Several studies demonstrated PRP-induced quantifiable increases in growth factor expression[15,16], as well as observable histological increases in leukocyte recruitment to the area of injury[8,9,18]. Denapoli et al[15]reported significant increases in VEGF receptors, as well as growth factors including but not limited to PDGF, Flt-1, and EGF in PRP-treated groups when compared to the control group. PRP treatment was also found to increase angiogenesis when compared to the control[17,20,23].
Several studies also demonstrated that PRP formulations that limit the presence of growth factors that are detrimental to muscle regeneration, such as TGF-ß1 and myostatin, had greater influence on myoblast differentiation and healing. Both Denapoli et al[15]and Miroshnychenko et al[12]found that leukocyte-poor formulations of PRP that significantly limited the presence of TGF-B1 had more beneficial effects on muscle healing. These results support the hypothesis that leukocyte-poor PRP may be most effective at mediating skeletal muscle repair through limiting detrimental growth factors and increasing the concentrations of beneficial growth factors that promote angiogenesis, proliferation and differentiation at the site of injury. A significant amount of further research is warranted to determine the optimal growth factor milieu that promotes muscular regeneration and healing. The differential responses of myocytes and myoblasts to different PRP formulations demonstrates theneed for a better basic science understanding of muscular regeneration and healing,and how PRP influences each phase from inflammation to regeneration and fibrosis.

Table 2 Cytology reporting in all platelet-rich plasma muscle studies
Limitations
This study has limitations for its interpretation. First, several studies reported the cytology of PRP. For comparative purposes, standardization in protocol and PRP composition is required to document the efficacy of PRP across research studies[28].The clinical literature on PRP for orthopedic pathologies has been greatly limited by the lack of impressive reporting of the PRP preparation protocol. Only 10% of clinical studies reported a clear description of the preparation of the PRP utilized, allowing it to be reproduced, which is consistent with the most recent systematic review of PRP protocol standardization that reports a 16% rate of reporting quantitative metrics[28].The results from this study demonstrate that the same shortcomings exist in the basic science literature on the use of PRP for muscle pathology. Given that both Mazzocca et al[7]and Miroshnychenko et al[12]reported differences in findings depending on what PRP preparations they use, it is essential that this important information is documented and standardized. In both clinical and basic science studies on PRP, the authors agree with Chahla et al[28]that the protocol of PRP preparation must be clearly articulated and reproducible in both basic science and clinical studies, so that the results across studies can be compared to ultimately allow us to determine the optimal preparation to treat both chronic and acute muscle injuries. The minimum reporting guidelines established by consensus and published by Murray IR et al[28].should be utilized by all journals as the minimum requirements a study on PRP must meet before publication.
Another limitation of the basic science literature is that the majority of the injuries to animal models are surgically-induced instead of occurring from trauma or wear. It is not uncommon for muscle injuries to present chronically in the clinic, and even acute lesions to muscles may present after a delay. Chronic degeneration and acute injury lead to distinctively different cellular and molecular responses that may respond differently to PRP. In addition, the biologic milieu created by the cellular and molecular responses in both chronic and acute muscle pathology changes over time.Thus, a better understanding of these time-dependent changes in the biologic milieu(e.g., host pH, cytokine and growth factor concentration), and the influence of timing,delivery, and dosing of PRP, needs to be better understood and further studied.
In conclusion, the basic science literature on the use of PRP in muscle pathology demonstrates that PRP confers several potentially beneficial effects on healing in comparison to controls at the basic science level. Future research is needed to determine the optimal cytology, dosing, timing, and delivery method of PRP for both chronic and acute muscle pathology.

Table 3 In vitro platelet-rich plasma and muscle studies – variables reported

Table 4 In vivo platelet-rich plasma and muscle studies - summaries

Hammond et al[6], 2009 Femoral/renal veins or intracardiac punctures on five adult male Sprague-Dawley rats (20 mL blood/each). PRP separated from whole blood (Symphony II Platelet Concentration System, DePuy). PPP used for control.Remaining PRP subjected to high frequency ultrasound(10 s). 100 µL used for injections Not reported 72 adult male Sprague-Dawley rats had strain of tibialis anterior induced with superimposed lengthening contraction onto maximal isometric contraction using either a single repetition or multiple repetitions.Outcomes measured at days 3, 5, 7, 14, 21 Maximal isometric contraction and torque,Isometric torque,histology, and gene and protein expression of MyoD and myogenin,PDGF and IGF-1 concentrations in PRP and PPP PRP had higher concentrations of PDGF and IGF-1. In single repetition group, PRP resulted in increased force only at day 3. No difference in return to function. For multiple repetitions, PRP improved force at multiple time points and faster return to function Li et al[17], 2016 PRP isolated from three rats and mixed in citrate-phosphatedextrose isolated as above. Centrifuged at 160 × g/20 min.Supernatant transferred,centrifuged 400 × g/15 min. Pellet resuspended with remaining plasma to yield PRP PRP - Plt count equal to 6.44 ± 0.64 × 106/µL,WBC 22.37 ± 2.25 ×103/µL 16 male Fisher rats injured with cardiotoxin injection into tibialis anterior. Four treatments: (1) Control,(2) 50 µL PRP, (3) 50 µL PRP neutralized with 280 ng/µL TGF-β1 antibody, (4) 50 µL PRP neutralized with 1400 ng/µL TGF-β1 antibody. Outcomes at 7, 14 d Assessed muscle regeneration and collagen deposition with histology. IMHC for CD31, Alpha-SMA, Pax-7, CD68,transglutaminase-2,dystrophin to determine differentiation and mechanism for repair PRP accelerated muscle regeneration (increased regenerating myofibers),increased angiogenesis(increased MVD-CD31,MVD-α-SMA) TGF-β1 neutralization of PRP reduced collagen deposition, PRP reduced macrophages and inflammatory response Martins et al[19], 2016 Whole blood centrifuged 180 × g/10 min. Supernatant transferred and centrifuged at 1000 ×g/10 min. Pellet resuspended and activated with 10%calcium gluconate PRP - Plt count equal to 4904/µL Gastrocnemius Muscle contusion model studying the effect of PRP and reactive oxygen species over a 7-d treatment course Reactive species byproducts (TBARS,DCFHRS), mitochondria function (MTT assay),antioxidant enzyme activities (GSH, CAT,SOD) and myeloperoxidase PRP reduces oxidative damage and MPO enzyme, increases antioxidants Ozaki et al[21], 2016 4mL blood from cardiac puncture combined with 0.2 mL 10% sodium citrate. Centrifuged 200× g/15 min. Top two fractions isolated and centrifuged, at 500 ×g/10 min PRP- Plt count equal to 4999 × 103/µL Thirty-five male Wistar rats in 5 groups (n=7):control (C), control lesion (CL), lesion treated with low-level laser therapy (LLt),lesion treated with PRP(LP), and lesion treated with both techniques(LLtP). Muscle injury by stretching gastrocnemius muscle.PRP (100 μL) injected into distal third of tibia to be applied to gastrocnemius muscle belly Histology for morphology,inflammatory infiltrate,oxidative stress using Raman scattering spectroscopy, collagen content CL group had increased macrophages and oxidative stress. LP group had decreased inflammation, increased tissue organization, and increased presence of regeneration cells Pinheiro et al[24], 2016 Intracardiac puncture -3 mL blood/each rat centrifuged 1200 × g/15 min to yield three layers. Isolated PRP/RBC layer,centrifuged 1min. PRP(0.2 mL) separated and activated with calcium gluconate (0.01 mL) to yield PRP gel Cytology not provided Ultrasound study following PRP therapy in a gastrocnemius muscle injury model Pennation angle, Muscle thickness, Mean pixel intensity, claudication scores No significant difference found Quarteiro et al[8], 2015 Four blood samples (8 mL/rat) from five rats mixed with anticoagulant Samples centrifuged and plasma separated. Plasma centrifuged and supernatant removed leaving PRP (1mL)PRP - Plt count equal to 1019 ± 182.25 × 103/µL Gastrocnemius muscle injury model Histologic assessment No difference in collagen content at 21 days. Inflammatory process observed in groups treated with PRP
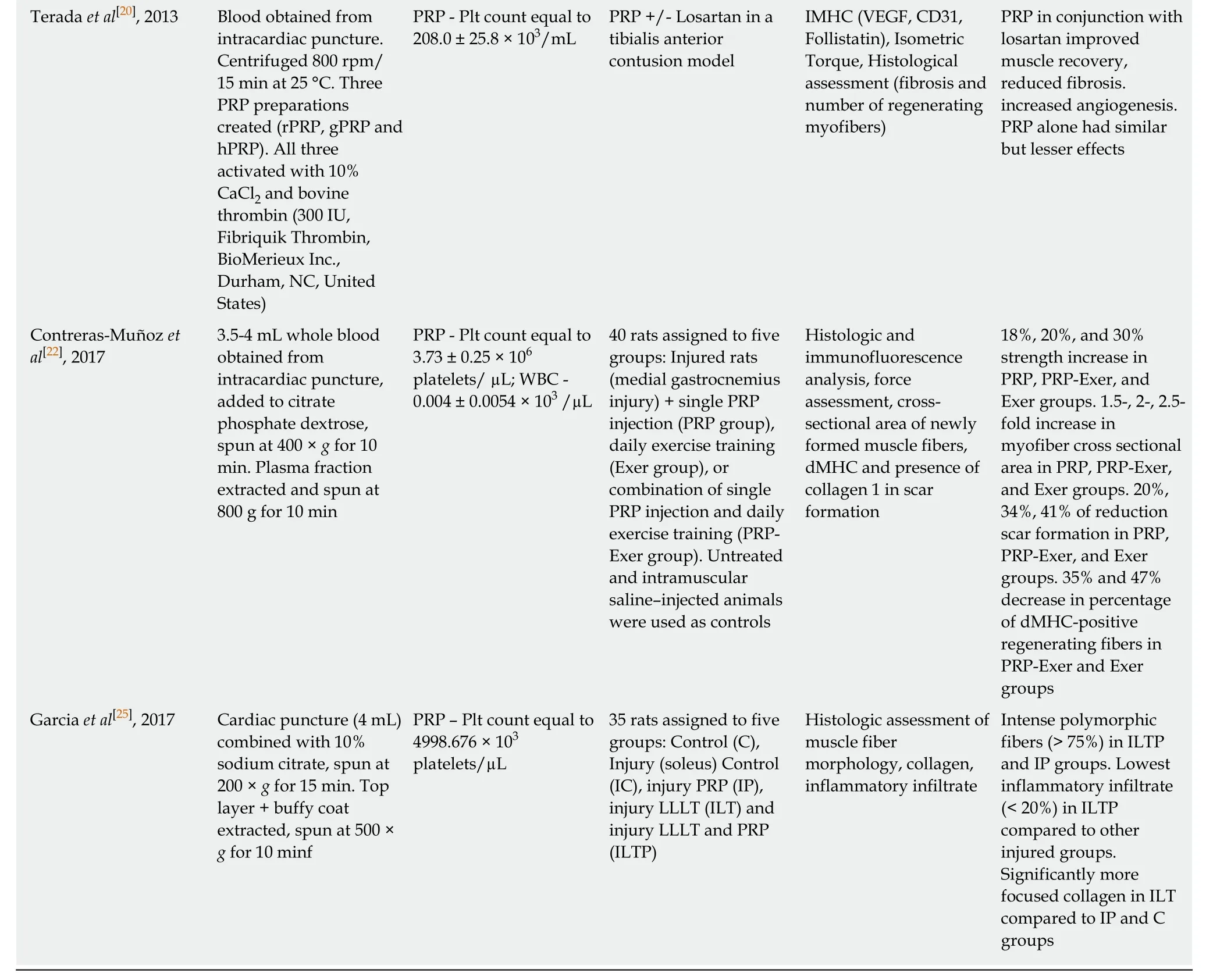
Terada et al[20], 2013 Blood obtained from intracardiac puncture.Centrifuged 800 rpm/15 min at 25 °C. Three PRP preparations created (rPRP, gPRP and hPRP). All three activated with 10%CaCl2 and bovine thrombin (300 IU,Fibriquik Thrombin,BioMerieux Inc.,Durham, NC, United States)PRP - Plt count equal to 208.0 ± 25.8 × 103/mL PRP +/- Losartan in a tibialis anterior contusion model IMHC (VEGF, CD31,Follistatin), Isometric Torque, Histological assessment (fibrosis and number of regenerating myofibers)PRP in conjunction with losartan improved muscle recovery,reduced fibrosis.increased angiogenesis.PRP alone had similar but lesser effects Contreras-Muñoz et al[22], 2017 3.5-4 mL whole blood obtained from intracardiac puncture,added to citrate phosphate dextrose,spun at 400 × g for 10 min. Plasma fraction extracted and spun at 800 g for 10 min PRP - Plt count equal to 3.73 ± 0.25 × 106 platelets/ µL; WBC -0.004 ± 0.0054 × 103 /µL 40 rats assigned to five groups: Injured rats(medial gastrocnemius injury) + single PRP injection (PRP group),daily exercise training(Exer group), or combination of single PRP injection and daily exercise training (PRPExer group). Untreated and intramuscular saline–injected animals were used as controls Histologic and immunofluorescence analysis, force assessment, crosssectional area of newly formed muscle fibers,dMHC and presence of collagen 1 in scar formation 18%, 20%, and 30%strength increase in PRP, PRP-Exer, and Exer groups. 1.5-, 2-, 2.5-fold increase in myofiber cross sectional area in PRP, PRP-Exer,and Exer groups. 20%,34%, 41% of reduction scar formation in PRP,PRP-Exer, and Exer groups. 35% and 47%decrease in percentage of dMHC-positive regenerating fibers in PRP-Exer and Exer groups Garcia et al[25], 2017 Cardiac puncture (4 mL)combined with 10%sodium citrate, spun at 200 × g for 15 min. Top layer + buffy coat extracted, spun at 500 ×g for 10 minf PRP – Plt count equal to 4998.676 × 103 platelets/µL 35 rats assigned to five groups: Control (C),Injury (soleus) Control(IC), injury PRP (IP),injury LLLT (ILT) and injury LLLT and PRP(ILTP)Histologic assessment of muscle fiber morphology, collagen,inflammatory infiltrate Intense polymorphic fibers (> 75%) in ILTP and IP groups. Lowest inflammatory infiltrate(< 20%) in ILTP compared to other injured groups.Significantly more focused collagen in ILT compared to IP and C groups
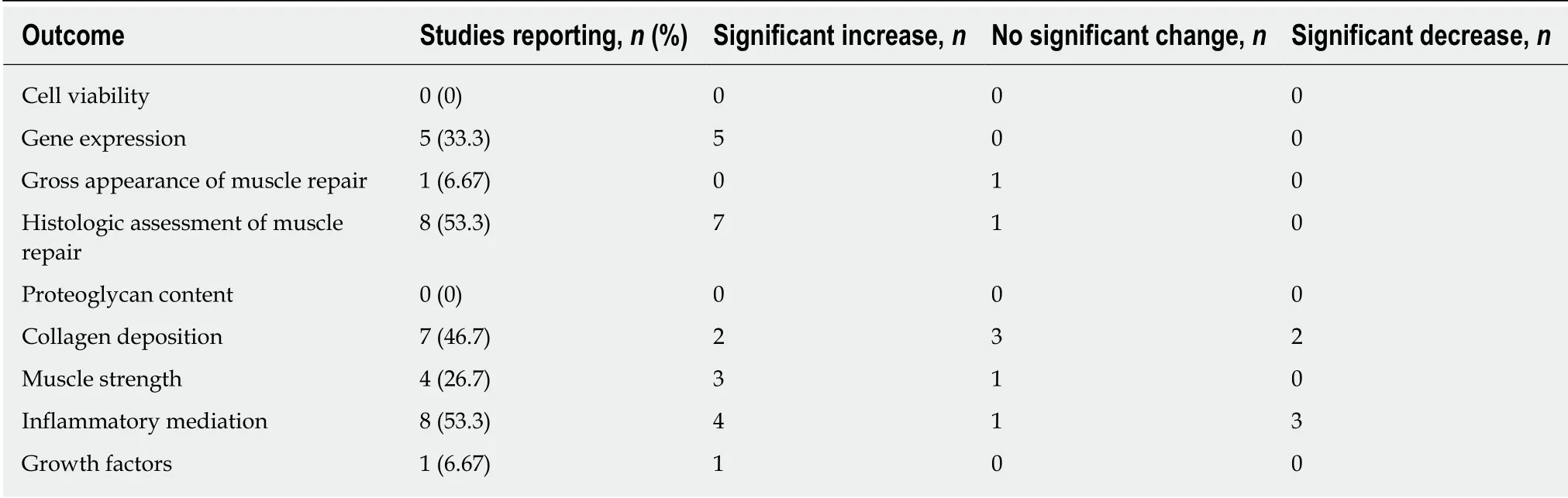
Table 5 Variables reported - in vivo muscle studies

Table 6 In vivo and in vitro muscle studies

Table 7 Variables reported – combined in vivo and in vitro muscle studies
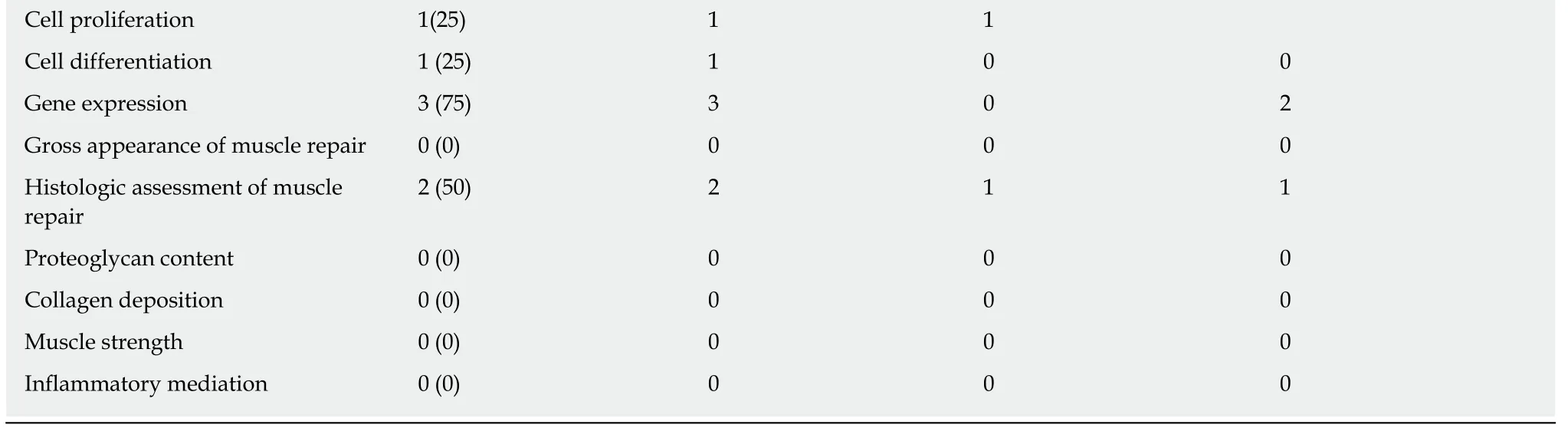
Cell proliferation 1(25) 1 1 Cell differentiation 1 (25) 1 0 0 Gene expression 3 (75) 3 0 2 Gross appearance of muscle repair 0 (0) 0 0 0 Histologic assessment of muscle repair 2 (50) 2 1 1 Proteoglycan content 0 (0) 0 0 0 Collagen deposition 0 (0) 0 0 0 Muscle strength 0 (0) 0 0 0 Inflammatory mediation 0 (0) 0 0 0
ARTICLE HIGHLIGHTS
Research background
Platelet-rich plasma (PRP) is a biological adjunct derived from autologous blood, which is thought to aid the healing of various bone, ligament, cartilage, and muscle injuries. PRP is composed of various cytokines, growth factors, and concentrations of leukocytes and platelets.PRP is often used clinically to expedite healing as a non-operative treatment or operative adjunct. However, studies have reported mixed effects of PRP, and clinicians continue to employ this adjunct despite little understanding of its mechanism of action.
Research motivation
The main topics of the current study are (1) The various mechanisms of PRP action at the molecular and tissue levels for muscle injuries; and (2) Reporting patterns of PRP preparations in these studies. The current study seeks to clarify the underlying mechanisms of action of PRP, in terms of its ability to induce cellular changes and changes at the histologic and tissue levels,which are not well-described.
Research objectives
The main objective of the current study is to clarify the effects of PRP at the cellular and tissue levels through synthesizing its mechanisms of action from available basic science studies on muscle injuries. A secondary objective that was realized was that it is important to understand PRP preparations across multiple studies to allow for the standardization of study protocols and better comparisons.
Research methods
A systematic review of basic science studies from the PubMed/MEDLINE and EMBASE databases was conducted, as these studies would allow for the best understanding of the mechanism of action of PRP at the cellular and tissue levels. Using a custom pre-determined spreadsheet of a wide variety of growth factors, cytokines, and other molecular markers, each study was analyzed, and these variables were subsequently extracted. The PRP preparation methods were also extracted.
Research results
A total of 23 articles were identified. PRP conferred multiple beneficial effects on muscles both in vitro and in vivo through the upregulation of genes beneficial to healing and muscle regeneration,increasing cellular proliferation and differentiation, and producing superior tissue quality and biomechanical properties in comparison to placebo. However, this study also identified the lack of PRP cytology reporting among these studies, of which only one study reported a full cytology.
Research conclusions
PRP confers multiple beneficial effects at the basic science level in models of muscle injury compared to placebo through changes at the cellular level, which include gene expression,growth factor and cytokine concentrations, increased angiogenesis, and cellular differentiation and proliferation. PRP also mediates increased muscle regeneration at the gross level, and superior histologic quality when compared to placebo in a few studies. There was significant variability in both PRP preparation and reporting among the included studies.
Research perspectives
This study highlights the importance of understanding processes at the basic science level in order to provide better insight into clinical practice. Future research is needed to determine the optimal cytology, dosing, timing, and delivery method of PRP for muscle injuries. Higher level randomized studies will need to be performed in order to determine these factors. Furthermore,it will be essential for future studies to use standardized protocols, such that outcomes and practices with PRP become reproducible.
