Additional laparoscopic gastrectomy after noncurative endoscopic submucosal dissection for early gastric cancer: A single-center experience
2019-08-20YanTaoTianFuHaiMaGuiQiWangYueMingZhangLiZhouDouYiBinXieYuXinZhongYingTaiChenQuanXuDongBingZhao
Yan-Tao Tian, Fu-Hai Ma, Gui-Qi Wang, Yue-Ming Zhang, Li-Zhou Dou, Yi-Bin Xie, Yu-Xin Zhong,Ying-Tai Chen, Quan Xu, Dong-Bing Zhao
Abstract BACKGROUND The necessity of additional gastrectomy for early gastric cancer (EGC) patients who do not meet curative criteria after endoscopic submucosal dissection (ESD)is controversial.AIM To examine the clinicopathologic characteristics of patients who underwent additional laparoscopic gastrectomy after ESD and to determine the appropriate strategy for treating those after noncurative ESD.METHODS We retrospectively studied 45 patients with EGC who underwent additional laparoscopic gastrectomy after noncurative ESD from January 2013 to January 2019 at the Cancer Hospital of the Chinese Academy of Medical Sciences. We analyzed the patients’ clinicopathological data and identified the predictors of residual cancer (RC) and lymph node metastasis (LNM).RESULTS Surgical specimens showed RC in ten (22.2%) patients and LNM in five (11.1%).Institutional review board statement: This study was approved by the Institutional Review Board of the National Cancer Center Hospital.
Key words: Early gastric cancer; Endoscopic submucosal dissection; Laparoscopic gastrectomy; Residual cancer; Lymph node metastasis
INTRODUCTION
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related death in the world[1]. Early gastric cancer (EGC) is defined as a tumor confined to the mucosa or submucosa, regardless of the regional lymph node metastasis (LNM)[2]. The detection rates of EGC have been improved with the increase in cancer surveillance and widespread endoscopic examinations[3]. Endoscopic submucosal dissection (ESD) as a treatment for EGC has been rapidly spreading due to the advantages of this technique including reduced postoperative complications,decreased medical cost, fast recovery, and improved quality of life[4]. As ESD is now performed more frequently, noncurative ESD is also becoming more and more frequent, thus warranting appropriate treatment[3].
For patients who have undergone noncurative ESD, some reports[5-9]recommend additional surgery to prevent residual cancer (RC) or LMN. However, high morbidity,poor quality of life, and medical cost of gastrectomy for these patients cannot be neglected, and it is controversial whether additional gastrectomy is necessary for all patients who do not meet the curative criteria after ESD[10,11]. Therefore, it would be valuable to determine which factors could increase the risk of RC or LNM in patients after noncurative ESD in EGC patients in order to avoid unnecessary surgery.
Laparoscopic gastrectomy (LG) has been accepted as a standard procedure for the treatment of EGC because it is minimally invasive, results in decreased postoperative pain, and has a shorter recovery time than other procedures[12,13]. ESD-induced inflammation causes edema, fibrosis, and intraabdominal adhesions, which might increase the difficulties and the risk of complications during subsequent LG[3,14].However, relatively few data are available on the influence of previous ESD on LG[15-17].
In the present study, we aimed to examine the predictive factors for LNM and RC as well as to explore the appropriate strategy for treating these patients after noncurative ESD. We also aimed to assess the feasibility and safety of LG as additional surgery after ESD.
MATERIALS AND METHODS
In this retrospective cohort study, the clinical data of consecutive EGC patients who underwent additional gastrectomy after ESD at the Cancer Hospital of the Chinese Academy of Medical Sciences, Chinese National Cancer Center between January 2013 and January 2019 were reviewed. The rate of LNM or RC was investigated. The associations between various clinicopathological factors and RC or LNM were examined by univariable and multivariable analyses. This retrospective study was approved by the Institutional Review Board at the Cancer Hospital of the Chinese Academy of Medical Sciences. The need for informed consent was waived due to the retrospective nature of the study, and the data were anonymously analyzed. The datasets in the current study are available from the corresponding author on reasonable request.
Indications and procedures for ESD
Depth of tumor invasion and tumor stage were assessed initially before ESD by endoscopic ultrasonography and contrast-enhanced computed tomography of the abdomen and pelvis. The extended indications for ESD were as follows: (1)Differentiated mucosal cancer without ulceration regardless of lesion size; (2)Differentiated mucosal cancer, with ulceration, < 3 cm in diameter; (3) Differentiated minimally invasive submucosal cancer < 3 cm in diameter; and (4) Undifferentiated mucosal cancer ≤ 2 cm in size.
ESD was performed by one experienced gastrointestinal endoscopist in our hospital. An incision line were made at about 5 mm lateral to the margin of the cancerous lesion using a needle. Hypertonic saline mixed with epinephrine (1:10000)and sodium hyaluronate were injected into the submucosal layer to lift the lesion. A circumferential mucosal incision surrounding the marking dots was performed. The submucosa beneath the target lesion was dissected and the entire lesion was completely removed with a surgical electronic knife.
Histopathological evaluation
After being fixed in 10% formalin, resected specimens were sectioned perpendicularly at 2-mm intervals. The histological evaluation was based on the World Health Organization classification of gastric cancer. Gross types were categorized into elevated, flat, or depressed ty
pe. Well or moderately differentiated tubular adenocarcinoma and papillary adenocarcinoma were classified as differentiated adenocarcinoma type, while poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma were classified as undifferentiated type.Tumor involvement in the lateral or vertical resection margin, tumor size,lymphovascular invasion, neural invasion, and the depth of tumor invasion were evaluated. The depth of tumor invasion was measured and quantified and was classified as M (mucosal invasion), SM1 (submucosal invasion < 500 μm of the lower margin of the muscularis mucosae), and SM2 (tumor invasion into submucosa > 500 μm from the muscularis mucosa).
Criteria for noncurative resection of ESD
The lesions that were considered not to meet the noncurative criteria for ESD were defined as lesions that met at least one of the following criteria based on histopathologic findings of the ESD specimens: (1) Positive horizontal margin; (2)Positive vertical margin; (3) Presence of lymphovascular involvement; (4) SM2 or deeper invasion; (5) Differentiated mucosal cancer with ulceration and size ≥ 30 mm;(6) Differentiated SM1 cancer ≥ 30 mm; and (7) undifferentiated cancer accompanied by submucosal invasion, size > 20 mm, or ulceration.
Statistical analysis
Univariate analyses by the χ2test or Fisher’s exact test were performed to explore the clinicopathological differences between the RC and non-RC groups, and between the LNM and non-LNM groups. Furthermore, multivariate logistic regression analysis was used to identify independent risk factors for RC and LNM, including those factors with P < 0.3 in univariate analysis. A P-value < 0.05 was considered significant. All analyses were performed with SPSS for Windows version 22.0.
RESULTS
Demographics and clinicopathological characteristics of the patients
A total of 640 ESDs were performed, and 45 (7.0%) noncurative ESDs were found during the study period. The demographics and clinicopathological characteristics of the patients who received additional gastrectomy because of noncurative ESD are summarized in Table 1. The reasons for additional gastrectomy consisted of positive horizontal margin (7 cases), positive vertical margin (29 cases), SM2 (31 cases),lymphovascular invasion (19 cases), and undifferentiated type (14 cases). And two cases were suspected recurrence on esophagogastroduodenoscopy at the 3-month follow-up after ESD. Of the 45 patients, 34 (75.6%) were male and 11 (24.4%) were female. The mean age was 58.2 ± 9.3 years. The median interval between ESD and additional gastrectomy was 47 ± 26 d. The final depth of tumor invasion was M in 9 patients, SM1 in 5, SM2 in 26, muscularis propria in 2, and subserosa in 3.
Associations between clinicopathological characteristics and RC
RC was found in 10 (22.2%) of the 45 patients. The patients who did and did not have RC were compared in terms of their clinicopathological characteristics, as shown in Table 2. Univariate analyses determined that horizontal margin (P = 0.034) and neural invasion (P = 0.007) were significant factors for RC. In contrast, tumor location,macroscopic type, tumor size, histological differentiation, Lauren type, vertical margin, depth of invasion, and lymphovascular invasion did not show significant correlations. Multivariate analysis showed that horizontal margin [ (odds ratio OR) =13.393, 95% confidence interval (CI): 1.435-125, P = 0.023] and neural invasion (OR =18.495, 95%CI: 1.585-215, P = 0.020) were associated with a higher incidence of RC within specimens after surgery (Table 3).
Associations between clinicopathological characteristics and LNM
LNM was detected in 5 (11.1%) out of 45 cases. Relationships between clinicopathological characteristics and LNM are summarized in Table 4. Undifferentiated type was the only significant factor for LNM (P = 0.027). Macroscopic type and depth of tumor invasion had weak relationships. Multivariate analysis revealed that undifferentiated type (OR = 12.000, 95%CI: 1.197-120, P = 0.035) was associated with a higher incidence of LNM within specimens after surgery. All five patients showed tumor depth of more than SM1 in the specimen from the initial endoscopic resection. Of the five patients with LNM, four previously exhibited undifferentiated type post-ESD treatment.
Operative data and postoperative outcomes
Details of the intraoperative course and postoperative course are shown in Table 5.The type of LG was determined based on the tumor location. Proximal gastrectomy was performed in 15 (33.3%) cases and distal gastrectomy in 23 (51.1%). Total gastrectomy was performed in five (11.1%) cases and partial gastrectomy in two(4.4%). The mean number of harvested lymph nodes was 29.7 ± 13.7. The mean operative time and mean estimated blood loss were 180 ± 47 min and 107 ± 69 mL,respectively. The time to first flatus was 3.4 ± 0.8 d, the time to recommencement of oral intake was 5.3 ± 1.4 d, and the length of hospital stay was 9.9 ± 2.9 d.Postoperative complications occurred five (11.1%) patients. Two patients developed leakage from the anastomotic site, and one each developed wound infection,hemorrhage, and abdominal infection. These complications were conservatively treated and consequently improved. None of these patients died.
DISCUSSION
The rate of RC in our series (22.2%) was similar to those in the previous reports (5.2%-28.6%)[3,4,18-24]. LNM was detected in 5 (11.1%) out of 45 cases. The majority of these cases harbored neither RC nor LNM, indicating that additional surgery may be unnecessary. Therefore, it is important to identify which patients will benefit the most from additional gastrectomy after noncurative ESD for EGC. However, the studies of predictive factors for RC and LNM in additional surgery gastrectomy specimens after ESD have been very limited. Our study revealed that positive horizontal and neural invasion were independent risk factors for RC. Undifferentiated type was an independent risk factor for LNM.
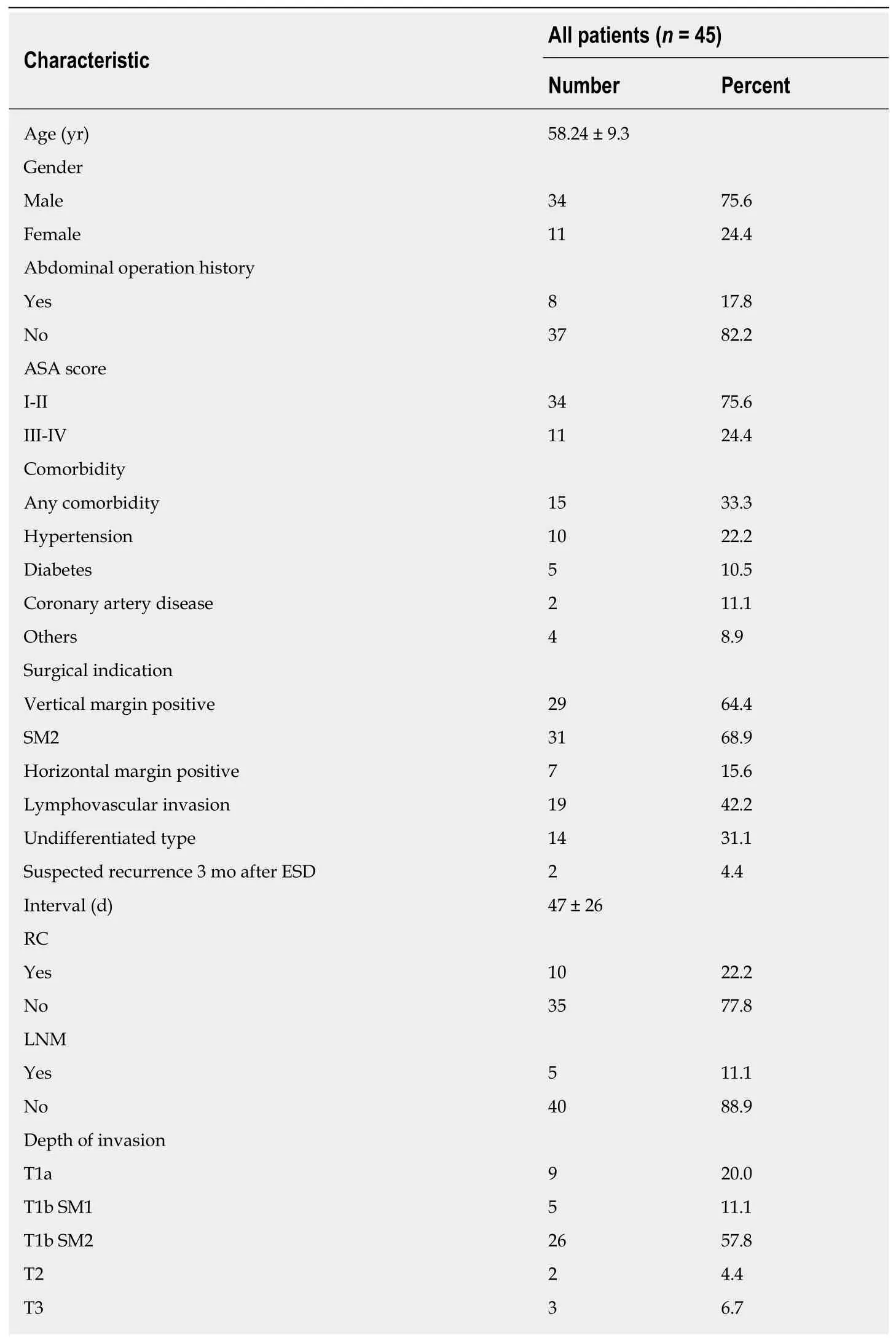
Table 1 Demographic characteristics of the patients
Regarding RC, positive vertical margin and positive horizontal margin were independent predictors in some previous studies[18], while many authors also reported only positive horizontal margin as a risk factor for RC, as found in our study[4,21,22].Hyuk et al thought that the possibility of the tumor cells in the corresponding area opposite an involved resection margin being completely removed by the cautery effect was much lower in the horizontal rather than in the vertical direction[4,5]. The feasibility of secondary ESD for local control in positive horizontal margin cases has been reported; however, the management of these patients is debated[25]. If there is an additional noncurative factor combined with the positive horizontal margin,additional surgery should be considered. Neural invasion is a way of cancer spreading and is related to advanced stage, higher risk of recurrence, and poor longterm survival in gastric cancer[26,27]. In the stomach, the nerve plexuses are concentrated in the Meissner’s plexus in the submucosa and Auerbach’s plexus between the circular and longitudinal fibers of the muscularis propria[28]. Thus, neural invasion is observed more frequently in advanced gastric cancer. Interestingly, neural invasion has not been established as a predictor of RC after noncurative ESD, while our study confirmed that neural invasion was an independent risk factor for RC.Although the number of cases was limited, it is a reminder that RC might be detected for those patients with neural invasion and additional gastrectomy may be needed.
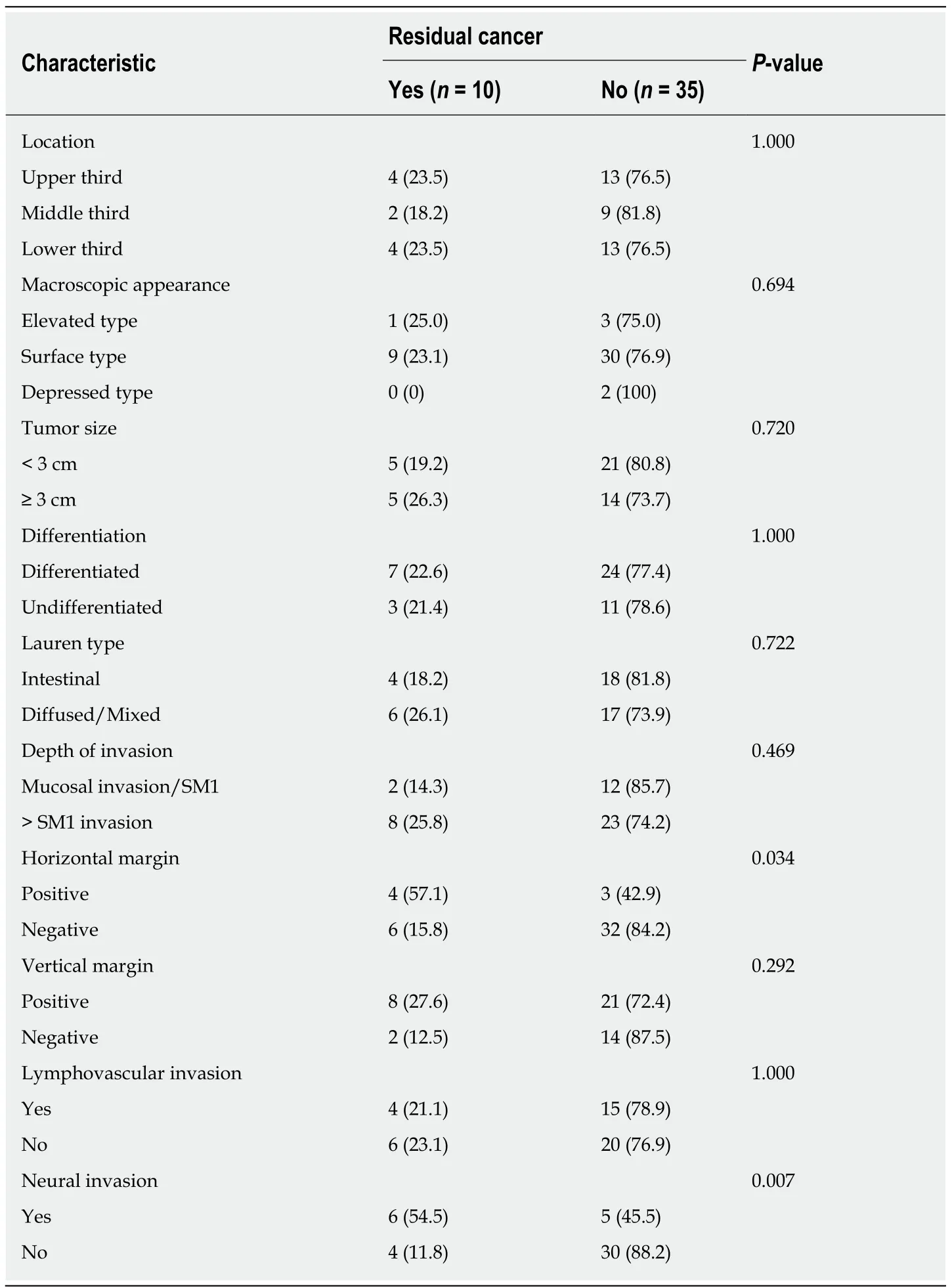
Table 2 Characteristics of cases with and without residual cancer, n (%)
In previous studies of patients who underwent additional surgery following noncurative ESD of EGC, the LNM rates ranged from 5.1% to 9.8%[4,18,19,21,23,24,29,30], which are similar to the present finding of 11.1%. Previous reports have indicated that lymphovascular invasion, SM2 invasion, lesion size > 3 cm, and positive vertical margin were associated with a greater risk of LNM in patients with EGC[31-33].Lymphovascular invasion has been proven to be an independent risk factor for LNM in those patients who underwent noncurative ESD[18,21,34,35]. However, lymphovascular invasion was not correlated with LNM in the present study and two patients without lymphovascular invasion were found to have LMN. Previous studies have demonstrated that the rate of LNM was higher in patients with differentiated EGC with undifferentiated components than in those with EGC without undifferentiated components[4,36]. Lee et al[37]reported that the rate of LNM increased with the increase in undifferentiated components in differentiated type mucosal cancers. Κim et al[38]and Abdelfatah et al[39]demonstrated that undifferentiated histology was an important risk factor for LNM. In the present series, undifferentiated histology was a major risk factor for LNM. SM2 invasion was another factor reported to be associated with a greater risk for LNM in patients with EGC[30,40]. This was thought to be due to the presence of larger diameter lymphatic vessels in the deeper third of the lamina propria, and the progressive increase in diameter as these vessels go deeper into the submucosal layer, where the lymphatic network is richer[39]. In our study, tumors in five lymph node-positive patients showed invasion deeper than SM1 in the surgical pathology specimen. Therefore, cases with submucosal invasion deeper than SM1 require additional gastrectomy and lymphadenectomy.
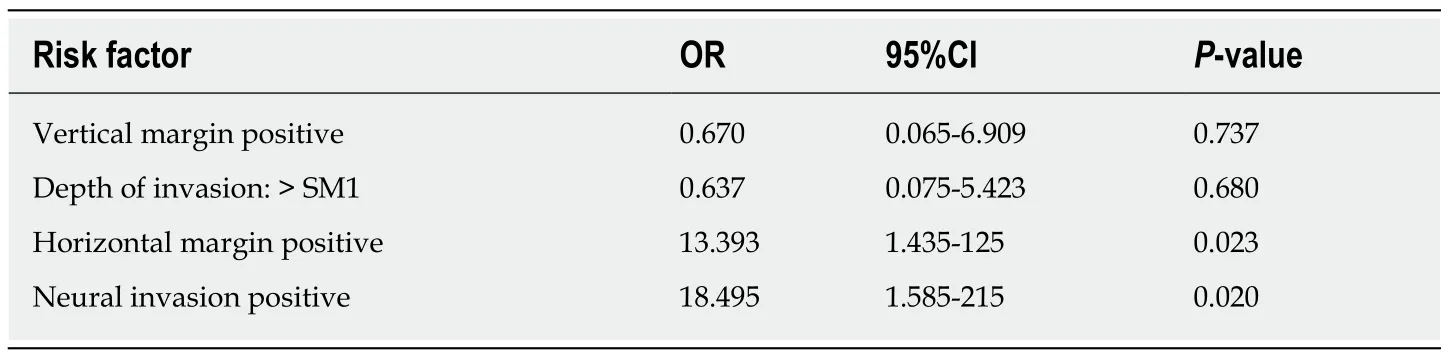
Table 3 Multivariate analysis of the risk factors for residual cancer
ESD in EGC causes an artificial gastric ulceration, local inflammation, subsequent fibrosis, and even adhesions in the outer gastric wall, which has a negative intraprocedural impact on additional LG in patients who have undergone noncurative ESD[14]. Previous studies have demonstrated that ESD is not associated with postoperative complications during or after an additional LG in patients who underwent noncurative ESD[15-17]. Our study found that LG can achieve good shortterm surgical outcomes for gastric cancer after noncurative ESD.
This study had several limitations. First, it was a retrospective study conducted in a single center and the sample size was relatively small. Such limitations may lead to issues of selection bias and heterogeneous patient group. Second, we did not report long-term outcomes of patients with noncurative ESD because the mean follow-up period was too short.
In conclusion, gastrectomy is necessary not only for patients who have a positive margin in ESD, but also for cases with neural invasion, undifferentiated type, and submucosal invasion more than 500 µm due to the risk of RC or LMN. In terms of short-term surgical outcomes, LG is a safe, minimally invasive, and feasible procedure for additional surgery after noncurative ESD. However, further studies are needed to apply these results to clinical practice.
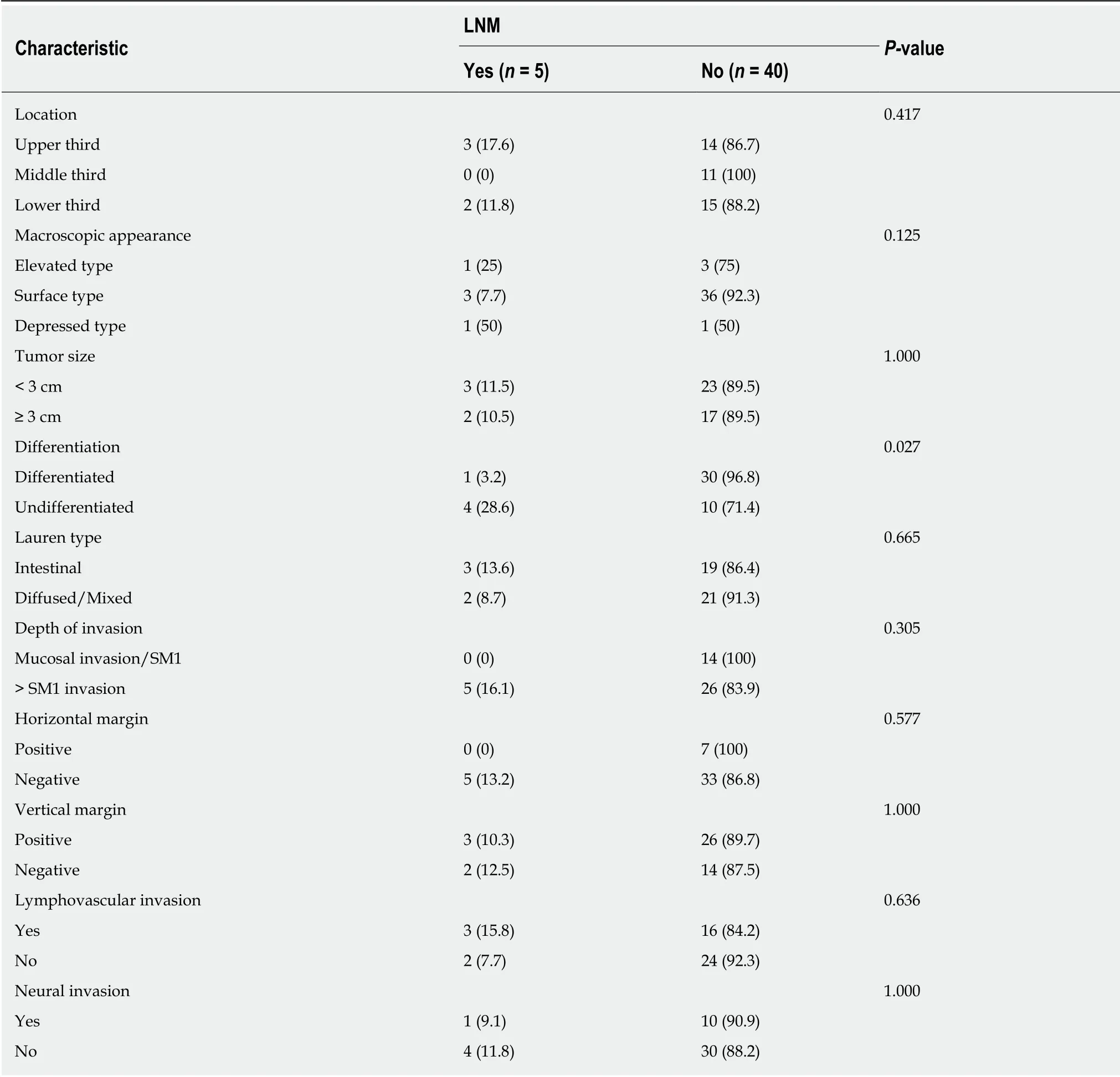
Table 4 Characteristics of patients with and without lymph node metastasis in surgical specimens, n (%)
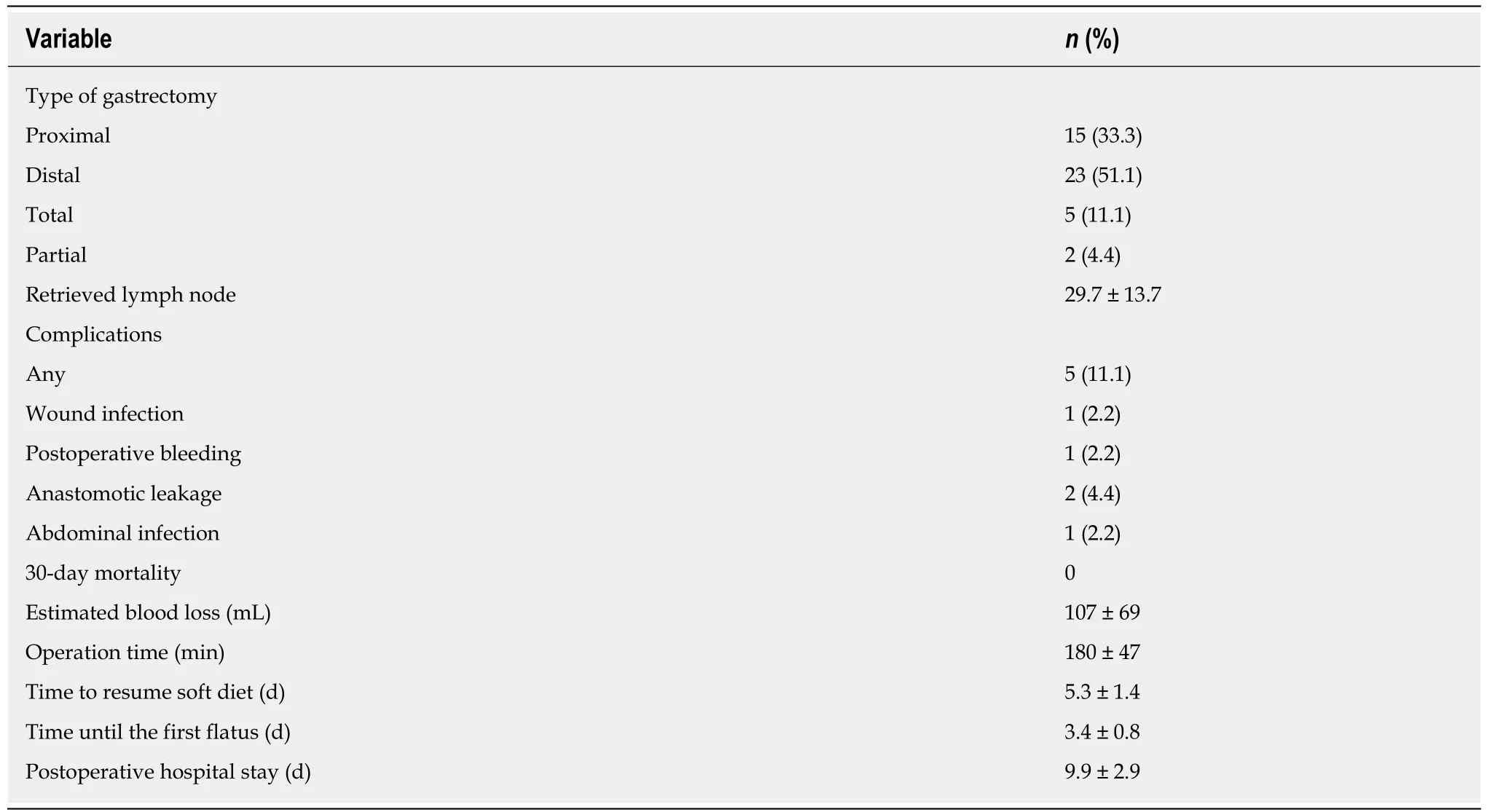
Table 5 Operative data and postoperative outcomes
ARTICLE HIGHLIGHTS
Research background
Endoscopic submucosal dissection (ESD) as a treatment for early gastric cancer (EGC) has been rapidly spreading. As ESD is now performed more frequently, noncurative resection after ESD is also becoming more frequent. It is controversial whether additional gastrectomy is necessary for all patients who do not meet the curative criteria after ESD.
Research motivation
It would be valuable to determine which factors could increase the risk of residual cancer (RC) or lymph node metastasis (LNM) in patients after noncurative ESD of EGC in order to avoid unnecessary surgery.
Research objectives
The objectives of this study were to identify the predictive factors for LNM and RC as well as to explore the appropriate strategy for treating those after non-curative ESD. We also aimed to assess the feasibility and safety of LG as additional surgery after ESD.
Research methods
We analyzed the patients’ clinicopathological data and identified the predictors of RC and LNM.
Research results
Surgical specimens showed RC in ten patients and LNM in five. Multivariate analysis revealed that positive horizontal margin and neural invasion were independent risk factors for RC.Undifferentiated type was an independent risk factor for LNM. Tumors in all patients with LNM showed submucosal invasion more than 500 μm. Postoperative complications after additional laparoscopic gastrectomy occurred in five patients, and no deaths occurred among patients with complications.
Research conclusions
Our study revealed that positive horizontal and neural invasion are independent risk factors for RC. Undifferentiated type is an independent risk factor for LNM. Laparoscopic gastrectomy is a safe, minimally invasive, and feasible procedure for additional surgery after noncurative ESD.Gastrectomy is necessary not only for patients who have a positive margin in ESD, but also for cases with neural invasion, undifferentiated type, and submucosal invasion more than 500 μm due to the risk of RC or LMN. Laparoscopic gastrectomy is a safe, minimally invasive, and feasible procedure for additional surgery after noncurative ESD.
Research perspectives
A study of larger sample size is needed. Long-term outcomes of patients with noncurative ESD need to be investigated in a prospective multicenter trial.
杂志排行
World Journal of Gastroenterology的其它文章
- Hepatocellular carcinoma and metabolic syndrome: The times are changing and so should we
- Healthy axis: Towards an integrated view of the gut-brain health
- Post-endoscopic retrograde cholangiopancreatography pancreatitis:A systematic review for prevention and treatment
- Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer
- Liquid biopsy for non-invasive assessment of liver injury in hepatitis B patients
- LncRNA MEG3 acts a biomarker and regulates cell functions by targeting ADAR1 in colorectal cancer
