A new type of triptycene-based stationary phases with alkylated benzimidazolium cations for gas chromatographic separations
2019-07-27JunHeMeilingQi
Jun He,Meiling Qi*
Key Laboratory of Cluster Science, Ministry of Education of China, School of Chemistry and Chemical Engineering, Analysis & Testing Center, Beijing Institute of Technology, Beijing 100081, China
Keywords:Triptycene-based materials Benzimidazolium cation Gas chromatography Stationary phase Separation performance
A B S T R A C T Favorable physicochemical properties and unique molecular recognition capability endow triptycenebased materials with good potential as stationary phases for gas chromatography(GC).This work reports a new type of triptycene-based materials functionalized by three benzimidazolium cations with different peripheral alkyl lengths (denoted as TP-3Bim-5C and TP-3Bim-12C) and their GC separation performance. As a result, they shared high resolving performance for the naphthalene isomers but differed for the benzene derivatives with varying polarity. Moreover, their capillary columns exhibited good repeatability and thermal stability.This work presents a facile strategy for tailoring the selectivity of the TP-based stationary phases and demonstrates their promising future for chromatographic analysis.©2019 Chinese Chemical Society and Institute of Materia Medica,Chinese Academy of Medical Sciences.Published by Elsevier B.V. All rights reserved.
Over the past decade, developing new types of selective stationary phases has been the pursuit of researchers to meet the growing needs for gas chromatographic(GC)separations of diverse samples. Some types of them showed advantageous separation features due to their specific chemical structures, such as p-conjugated graphene [1–3], ionic liquids [4–7] and porous materials[8–12].They exhibited high selectivity through different intermolecular interactions.It is encouraging to design a new type of stationary phases with the above features and to explore the effect of structural variations on chromatographic selectivity.
Triptycene (TP) has a three dimensional (3D) rigid structure composed of three benzene rings with three open p-rich cavities[13].Due to their unique structures and high thermal stability and good solubility in common solvents[14],TP-based materials have shown good potential as porous materials for gas separation[15–17] and supramolecular recognition [18–21]. Interestingly, they also showed advantages as stationary phases for GC separations,as reported in our previous work[22,23].In these reports,both of the TP-based stationary phases(PQ,TQPP)showed weak polarity and exhibited high selectivity towards analytes with lower polarity.For polar ones such as phenols and anilines,they tended to peak tailing to some extent.To address these limitations and problems,it is of great value to design new types of TP-based stationary phases with extended selectivity to polar analytes.
In this work,we report two new TP-based materials(denoted as TP-3Bim-5C,TP-3Bim-12C,Scheme 1)as the stationary phases for GC separations. They are integration of the 3D p-rich triptycene with three alkylated benzimidazolium cations paired with the anions of bis(trifluoromethyl)sulfonylimide(NTf2)and differ only in their peripheral alkyl length. As described previously, ionic liquids(ILs)have been employed as GC stationary phases[4–7]and most of them are imidazolium-based ILs with generally[5]chain-like structures. Different from imidazole, benzimidazole (Bim) has a larger p-rich structure and its alkylated cation show high molecular recognition and self-assembly ability [24–26]. Hence,incorporation of alkyl Bim cations onto the triptycene framework may make a difference in their molecular interactions and selectivity.
The synthesis route of the TP-3Bim stationary phases is described in Scheme 1.Briefly,hydroxytriptycene 1 was alkylated with 1,8-dibromooctane to give 2,6,14-tri-(8-bromooctoxy)triptycene 2,which further reacted with alkyl benzimdazole to yield the benzimidazolium ionic liquid 4. Then, the anion exchange of bromide for NTf2anion was carried out to obtain TP-3Bim 5.The above products were characterized and confirmed by1H and13C NMR and Q-TOF-MS. The related information is provided in Supporting information.
The two TP-3Bim stationary phases were individually coated onto capillary columns (5 m 0.25 mm i.d.) by static coating method, and the column coating thickness was about 0.15mm,obtained by the formula df= (dcc)/400, where dfand dcare the coating thickness and the inner diameter (mm) of a capillary column,respectively,c is the concentration of the stationary phase solution (%, w/v). The detailed coating procedure is described in Supporting information.
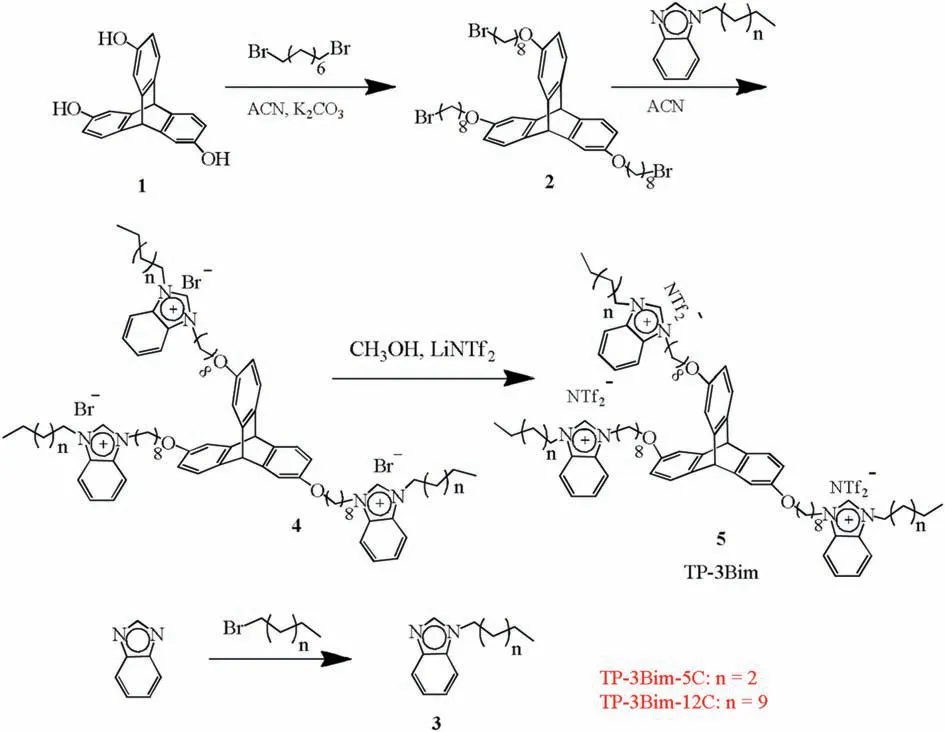
Scheme 1. Synthesis of the TP-3Bim stationary phases.
First, the TP-3Bim-5C and TP-3Bim-12C columns were determined for their column efficiency,polarity and Abraham solvation system constants. Determined by n-dodecane at 120C, the column efficiency was 2570 plates/m for TP-3Bim-5C and 2830 plates/m for TP-3Bim-12C, respectively. Their polarity was measured by McReynolds constants determined by the five probe compounds at 120C, i.e., benzene (X0), 1-buranol (Y0), 2-pentanone (Z0), pyridine (S0) and 1-nitropropane (U0). As shown in Table 1,TP-3Bim-5C and TP-3Bim-12C have the average values of 408 and 235, respectively, suggesting the higher polarity of TP-3Bim-5C than that of TP-3Bim-12C. Their large difference in polarity can be attributed to their different alkyl length at the peripheral positions. As polarity of a stationary phase is closely related with its selectivity, the above difference indicates that these two stationary phases may differ in their selectivity and retention behaviors,which was then investigated in the following work.
Additionally, the Abraham solvation system constants of the two stationary phases were determined by solutes of diverse types and the results are provided in Table 2. Table S1 (Supporting information) provides the solutes used in this work and their solute[6]descriptors.Table 2 indicates that the major interactions of the TP-3Bim stationary phases are dipole-dipole(s)and H-bonding basicity (a) interactions. Besides, as the temperature rises,reduction on the s, a and l values is observed, suggesting that the solute retention would decrease with increasing temperature that usually occurs in GC analysis. On the contrary, the e value becomes slightly larger probably due to the less dense packing of the triptycene units and benzimidazolium moieties in the molecules of the stationary phases. Comparatively, TP-3Bim-12C exhibits noticeably higher l value than TP-3Bim-5C, indicating its stronger dispersion interactions. This result agrees well with the longer alkyl chains of TP-3Bim-12C at the peripheral positions than TP-3Bim-5C.
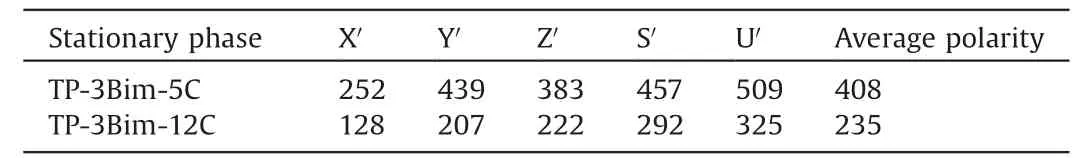
Table 1McReynolds constants of the TP-3Bim-5C and TP-3Bim-12C stationary phases.
Afterwards,their separation capability and retention behaviors were investigated by utilizing a mixture consisting of 13 analytes ranging wide polarity and types, and a number of mixtures of aromatic isomers sharing quite similar physicochemical properties.Fig.1 presents the separations of the mixture of 13 analytes on the two columns and their comparison profile on the retention times. As can be seen, compared to TP-3Bim-5C, TP-3Bim-12C achieved higher resolution for the analytes,especially the pairs of n-butylbenzene/cyclohexanol (peaks 2/3), naphthalene/phenol(peaks 10/11), and p-cresol/p-chloronitrobenzene (peaks 12/13).Moreover, TP-3Bim-12C showed longer retention for aliphatics,alkylbenzenes and halobenzenes. The prolonged retention for these analytes may derive from its stronger dispersion forces and p-p interaction with them.This is also the reason for the reversal elution of n-dodecane/cyclohexanol (peaks 1/3) on the TP-3Bim-12C column. In addition, the H-bonding type analytes, such as aniline, benzyl alcohol, phenol and p-cresol (peaks 8/9/11/12),retained shorter on the TP-3Bim-12C column, which leads to the reversal of dibromobenzene/aniline(peaks 7/8)and naphthalene/phenol (peaks 10/11). This phenomenon originates from the weaker H-bonding and dipole interactions of TP-3Bim-12C with the indicated analytes, as revealed by its McReynolds constants and Abraham system constants.The above results indicate that the selectivity of the TP-3Bim stationary phases can be efficiently tuned by adjusting its substituent units and alkyl chain lengths.
To testify their resolving capability for analytes of high similarity, these two columns were utilized to separate the mixtures of positional isomers with weakly polar to polar nature,i.e., propyl/diisopropylbenzenes, trimethylbenzenes, methyl/[7]dimethylnaphthalenes, bromonitrobenzenes and toluidines. The separation results are illustrated in Fig. 2 and the resolution (R)values for some critical pairs are added in the chromatograms.As can be observed, in general, the TP-3Bim-12C column exhibited good separations of these mixtures despite its slightly lower resolution for the polar ones([8]Figs.2i and j).Interestingly,these two columns showed similar separation results for the middle mixture but noticeably differed for the other ones. Their comparable separation performance for methyl/[9]dimethylnaphthalenes evidenced the dominate contribution of the triptycene framework in the stationary phases to the separations via their strong p-p interactions.On the contrary,they exhibited different performance for the benzene derivatives with varying polarity.Notably,the TP-3Bim-12C column showed advantages in resolving weakly polar isomers ([8]Figs. 2f and g) while the TP-3Bim-5C column achieved higher resolution for the more polar ones ([8]Figs. 2d and e). This finding proved that the peripheral alkyl length did make a difference in modifying the polarity and selectivity of the TP-3Bim stationary phases towards benzene derivatives. Briefly, the above results demonstrate the feasibility of the TP-based stationary
phases for GC separations and the tunability of their selectivity and resolving capability by altering the structural units.Hopefully,the above findings can provide a good reference for building up the family of TP-based stationary phases with tailored selectivity towards analytes of interest.
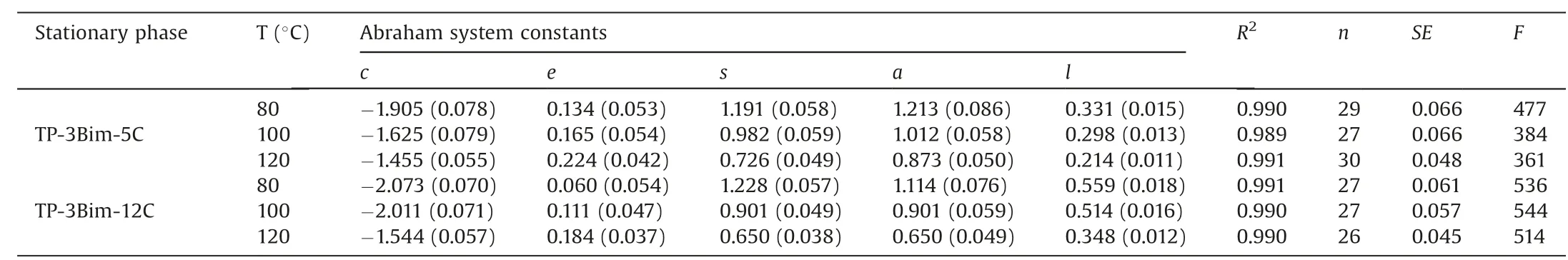
Table 2Abraham system constants (S.D.) of the TP-3Bim stationary phases (b = 0).
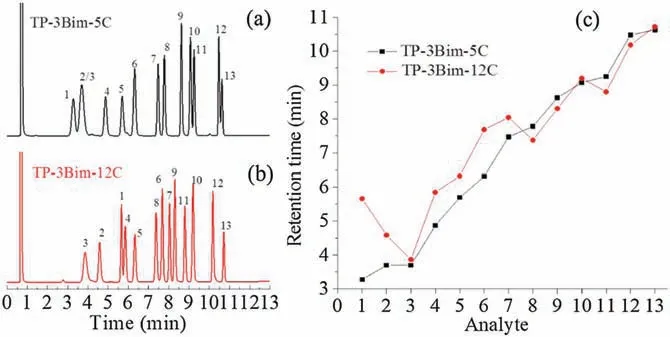
Fig.1. Separation of the mixture of 13 analytes on the (a)TP-3Bim-5C and(b) TP-3Bim-12C capillary columns and(c)comparison profile of the retention times of the analytes on the two columns. Peaks: (1) n-dodecane, (2) n-butylbenzene, (3)cyclohexanol, (4) methyl octanoate, (5) 1-octanol, (6) n-hexylbenzene, (7)p-dibromobenzene, (8) aniline, (9) benzyl alcohol, (10) naphthalene, (11) phenol,(12) p-cresol, (13) p-chloronitrobenzene. Temperature program: 40C–150C at 8C /min, flow rate 0.8 mL/min.
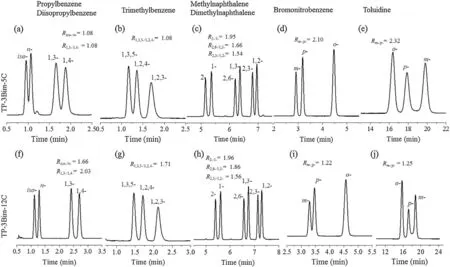
Fig. 2. Separation of the mixtures of aromatic isomers on the TP-3Bim-5C and TP-3Bim-12C columns. (a and f) Trimethylbenzenes, 80C, 0.8 mL/min; (b and g) propyl/diisoprpylbenzenes, 80C (1 min) to 100C at 10C/min, 0.8 mL/min; (c and h) methyl/dimethylnaphthalenes, 80C–150C at 10C/min, 0.8 mL/min; (d and i)bromonitrobenzenes,130C,1.0 mL/min; and (e and j) toluidines, 80C, 0.5 mL/min.
Furthermore, the two TP-3Bim columns were investigated for their repeatability and reproducibility on separation performance for the aromatic isomers described above. The evaluation was made by the relative standard deviation (RSD) of the retention times in terms of run-to-run and day-to-day repeatability and column-to-column reproducibility (n = 4). Table S2 (Supporting information) lists the obtained results for both columns,showing the RSD values of below 0.05%for run-to-run,0.11%–0.28%for dayto-day and 1.8%–3.8% for column-to-column. These results demonstrate their good column repeatability and reproducibility.In addition, their column thermal stability were tested by separating the mixture of methyl/dimethylnaphthalenes after the columns were conditioned from 40[10]C up to each of the indicated temperatures(180C–300C in increments of 30[12]C).The results are provided in Fig. S1 (Supporting information), showing good thermal stability up to 270[13]C.
In summary, the TP-3Bim stationary phases integrate the advantages of 3D p-rich triptycene framework and alkylated benzimidazolium cations and exhibit good separation capability for analytes of high resemblance in structures and properties.Their partial difference in structural moieties brings about large variations in their polarity,selectivity and separation performance.As demonstrated,they share similar resolving performance for the methyl/dimethylnaphthalene isomers but noticeably differ for the benzene derivatives with diverse polarity.This work demonstrates the good potential of the TP-3Bim stationary phases for GC separations and also provides a good example for tuning the selectivity of triptycene-based stationary phases via altering their structural moieties. Importantly, the facile strategy presented in this work may be helpful for developing new types of selective stationary phases in the field of chromatography.
Acknowledgments
The authors are grateful for the financial support by the National Natural Science Foundation of China (No.21575013)and Analysis & Testing Center, Beijing Institute of Technology[14].[3]
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version, at https://doi.org/10.1016/j.cclet.2019.03.008.
杂志排行
Chinese Chemical Letters的其它文章
- Near-infrared small molecular fluorescent dyes for photothermal therapy
- Recent applications of radical cascade reaction in the synthesis of functionalized 1-indenones
- Chemical synthesis and structure determination of venom toxins
- Rhodium(III)-catalyzed intermolecular cyclization of anilines with sulfoxonium ylides toward indoles
- Unexpected activated carbon-catalyzed pyrrolo[1,2-a]quinoxalines synthesis in water
- Ruthenium(II)-cored supramolecular organic framework-mediated recyclable visible light photoreduction of azides to amines and cascade formation of lactams
