Colorectal peritoneal metastases: Optimal management review
2019-07-27JuanManuelnchezHidalgoLidiaRodrguezOrtizlvaroArjonanchezSebastiRufiPengelaCasadoAdamAntonioCosanolvarezJavierBriceDelgado
Juan Manuel Sánchez-Hidalgo, Lidia Rodríguez-Ortiz, Álvaro Arjona-Sánchez, Sebastián Rufián-Peña,Ángela Casado-Adam, Antonio Cosano-Álvarez, Javier Briceño-Delgado
Abstract The peritoneum is a common site of dissemination for colorrectal cancer, with a poorer prognosis than other sites of metastases. In the last two decades, it has been considered as a locoregional disease progression and treated as such with curative intention treatments. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is the actual reference treatment for these patients as better survival results have been reached as compared to systemic chemotherapy alone, but its therapeutic efficacy is still under debate. Actual guidelines recommend that the management of colorectal cancer with peritoneal metastases should be led by a multidisciplinary team carried out in experienced centers and consider CRS + HIPEC for selected patients. Accumulative evidence in the last three years suggests that this is a curative treatment that may improve patients disease-free survival, decrease the risk of recurrence, and does not increase the risk of treatment-related mortality. In this review we aim to gather the latest results from referral centers and opinions from experts about the effectiveness and feasibility of CRS + HIPEC for treating peritoneal disease from colorectal malignancies.
Key words: Peritoneal metastases; Colorectal cancer; Cytoreductive surgery;Hyperthermic intraperitoneal chemotherapy; Peritoneal carcinomatosis
INTRODUCTION
We conducted a literature review to provide a comprehensive and updated overview of the actual management of colorectal cancer (CRC) with peritoneal metastases (PM)as the only site of spread. Our specific purpose is to enhance our understanding of the following aspects of this disease: (1) To know the biological pathway for PM, the concept of its locoregional spread, and the associated genetic and molecular factors;(2) To update our knowledge about the prognosis factors of peritoneal disease; (3) To delve into the multidisciplinary approach of peritoneal metastatic CRC, both synchronous and metachronous; (4) To show the recent scientific evidence on clinical trials and meta-analyses on disease-free and overall survival after treatment with cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy(HIPEC); and (5) To show the new and more promising investigation lines and trials for the future management of peritoneal metastatic CRC.
EPIDEMIOLOGY
CRC is the third most common cancer and the second most common cancer-related mortality globally. Patients have a favorable prognosis when diagnosed at an early stage: 70%-80% are eligible for curative-intent surgery, with a 5-year survival of 72%-93% for stages I-II[1]. Approximately 25% of the remaining patients present metastases at the time of diagnosis[2]. Among these individuals, up to 8% have synchronous peritoneal carcinomatosis, and approximately 20% already have liver metastases[3].Recurrent or systemic disease during the follow-up period after curative treatment of the primary tumor will develop in 20%-30% of patients. Half of these recurrences will develop liver metastases[3].
Although it was believed that metachronous PM occur in less than 10% of cases of CRC, being the third most frequent site of recurrence after liver and lung, its prevalence is still not well known. As an example of the underestimation, due to the lack of reliability of traditional imaging and unspecific symptomatology, one study of autopsied patients that did from CRC reported an incidence of 40%-80% unknown metachronous peritoneal carcinomatosis[4]. The peritoneum is the only dissemination location in 4.8% of cases and is more frequent for colon tumors (5.7%) than for rectal tumors (1.7%)[2]. Thus, thorough studies must be performed to exclude another site of metastatic disease because of the high possibility of further spread.
PATHOPHYSIOLOGY OF COLORECTAL PERITONEAL METASTASES
Peritoneal seeding as a dissemination pathway of an invasive cancer is believed to be the final result of the specific expression of oncogenes and binding proteins that allow the detachment of tumor cells to proliferate in the peritoneal environment[5]. The actual deep knowledge of genetics and molecular cancer mechanisms is becoming a strong tool to determine the likelihood for peritoneal spread, to avoid locoregional relapse after the first curative surgery and to assess the real implication on prognosis.
The molecular genetic influence
Many different genetic alterations characterize the two main pathways that have been described for the development of CRC: The conventional adenoma-carcinoma pathway and the serrated pathway. Notably, 80% of sporadic cases of CRC involve chromosomal instability, which includes the molecular targets for most of the novel chemotherapy agents, including K-RAS, B-RAF or pT53. Another group encompasses microsatellite instability (MSI) as a result of inactivation, mutation and/or epigenetic alteration of mismatch repair genes. This mechanism is one of the main causes of hereditary nonpolyposis CRC but also entails 10%-20% of sporadic CRCs. Depending on the proportion and type of microsatellite marker mutation, these tumors are classified into two groups: High (MSI-H) and low MSI or microsatellite stability (MSIL/MSS)[2,6,7].
MSI-H has been reported as a better prognosis condition, with a lower metastatic potential for distant recurrence than MSI-L tumors. However, a large single-center study[8]on outcomes for CRCs with MSI showed that MSI-H recurrences, most of them located on the peritoneum, had a worse survival than MSI-L carcinomas. These studies showed that most of these relapses are not eligible for curative resection and that this type of tumor progression is able to avoid immune mechanisms of protection by several means, which increases its malignant potential. Advanced tumor stages for MSI-H tumors have also been associated with BRAF mutations[9].
The BRAF V600E mutation is observed in 10% of CRCs, and it has been widely related to a worse prognosis. A recent meta-analysis described a more than two times higher risk of mortality in patients carrying this alteration[10]. The mild response to modern chemotherapy is attributed to a frequent acquired resistance to BRAFinhibitor development[11]. This mutation has also been strongly associated with PM[12].However, other studies have reported a more encouraging prognosis when the BRAF V600E mutation is found in early-stage cancers[13]or when the mutation is a non-V600E BRAF mutation[14].
KRAS mutations are present in up to 40% of CRCs sporadic cases, and evidence has shown that codon 12 KRAS mutations encompass a negative prognosis but not codon 13 KRAS mutations[15]. This biomarker has also been reported as a risk factor for a worse prognosis in patients with PM from CRC origin, and its detection has played a major role in patient selection for CRC+HIPEC in recent years[16]. A new oncogenic mechanism that constrains the tumor cell phenotype switch, called the epithelialmesenchymal transition, has been suggested to be an aggressive subtype for the high rates of this mutation detected on carcinomatous nodules in a recent publication[17].
Peritoneal seeding theory
Peritoneal implants are believed to be the consequences of primary abdominal tumor cell detachment or malignant cell dissemination during surgical manipulation of the tumor when the margins of resection are very close and for lymphatic or blood vessel transection. These cells attach to the peritoneum as a result of the molecular interaction between cancer cells and host elements, and invade the subperitoneal layer,where angiogenesis promotes their growth. Furthermore, numerous metachronous peritoneal implants developed along the surgical planes that were opened during the first surgery, becoming trapped by fibrin as part of the healing process, which can be a difficult location to reach by systemic chemotherapy[5].
CLINICAL PRESENTATION
Risk factors for developing peritoneal metastases from colorectal cancer
Population-based studies agree on a set of risk factors for developing metachronous PM for CRC, which includes the stage at diagnosis (incidence for pT4 stage,established as an independent risk factor, has been reported to be 17%-50%; and for pT3 stage of 5%-10%)[18,19], intraabdominal colon location, principally right-side colon cancer, infiltrative and ulcero-infiltrative carcinomas, mucinous adenocarcinoma,younger patients than 70-75 years, emergency procedures because of obstructive or perforated cancer at diagnosis, lymph node metastases and nonradical oncological resection during the first surgery[20-22](Table 1). By knowing which patients are more likely to develop peritoneal spread of the disease, several prophylactic or early detection strategies have been designed, as listed below.
Complicated disease presentation: Recommendation for management
Clinical presentation of intestinal obstruction and/or perforation involves a poor prognosis, independent of stage. Even uncommon, if this situation occurs in the context of a synchronous PM scenario, with the main aim of offering the best prognosis possible to these patients, the expert global recommendations include the following: (1) To perform the minimal surgical action needed to resolve the emergency situation. Primary resection should only be performed in perforated tumors.Obstructed patients should be treated by creating derivative stomas. Non-obstructed tumors should not be resected but treated by stomas or stents, although colonic stenting should be avoided in patient candidates for antiangiogenic agents because of higher rates of perforation reported[1]; (2) Always provide adequate biopsies of the primary tumor and/or peritoneal implants; (3) Describe the extension of the peritoneal disease using the peritoneal cancer index (PCI) score. If limited peritoneal disease is found, there is still a high recommendation to not perform surgical resection in the emergency context because it does not add a better free-disease survival and could hinder a better combined treatment modality[23,24].
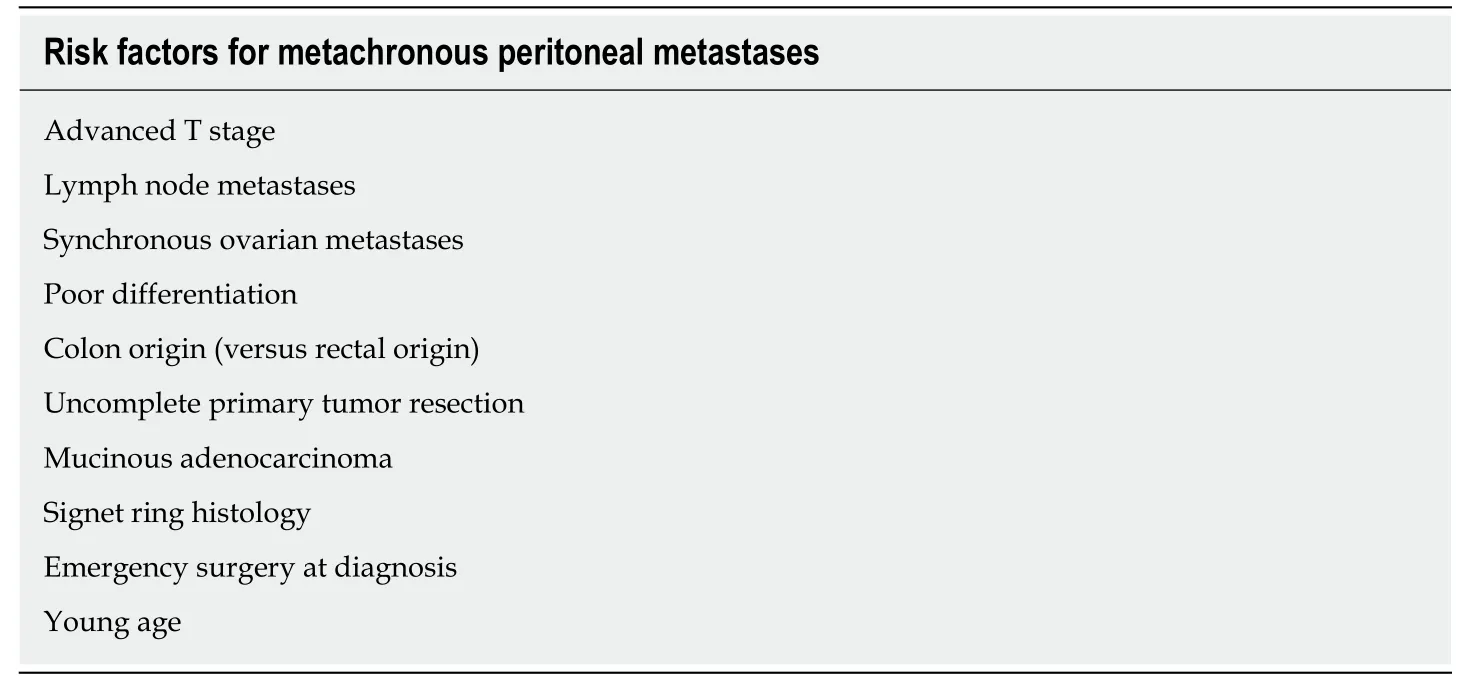
Table 1 Risk factors for metachronous peritoneal metastases
THE NATURAL EVOLUTION OF COLORECTAL PERITONEAL DISEASE
PM is a negative prognostic factor in patients with metastatic CRC. Patients with isolated nonperitoneal sites (including liver and lungs) had significantly better overall survival than that of patients with isolated peritoneal metastatic CRC. A recent large cohort study[25]showed that the combination of peritoneal involvement with two nonperitoneal sites had a similar survival compared with peritoneal metastasis alone.In fact, given the poor prognosis of PM itself, in the eighth edition of the tumor-nodemetastasis classification published in 2017, CRC with peritoneal metastasis is categorized as M1c, with or without other organ involvement, separately from M1a(one organ metastases) and M1b (≥ two organ metastases).
Historically, as peritoneal carcinomatosis was considered a terminal stage of disease, patients used to receive only supportive treatment or palliative chemotherapy. Generally, survival did not reach 6 mo, and patients were extremely symptomatic because of abdominal distension, intestinal obstruction and tumoral cachexia for constitutional syndrome[26]. Palliative surgery was not a better option, since it reached a high perioperative mortality and morbidity (over 12% and 22%, respectively)[27].Currently, the best survival reported for only systemic modern chemotherapy and supportive care for PM from colorectal origin is 15.2-23.4 mo[28,29]. These poor results prompted the need to find a more effective approach for this stage of disease.
CYTOREDUCTIVE SURGERY AND HYPERTHERMIC INTRAPERITONEAL CHEMOTHERAPY
Why should this treatment be considered?
In the 1980s, according to Sugarbaker et al[30]publications, peritoneal carcinomatosis ceased to be considered a systemic metastatic disease and therefore a terminal condition. Currently, peritoneal carcinomatosis is referred to as PM, and it is determined to be a locoregional spread that is eligible for a curative intent approach based on optimal CRS plus HIPEC[25,28,31-34]. This treatment entails a major, expensive and complex surgery that requires an optimal selection of the patients, with an adequate performance status and an accurate preoperative extension study, and the key for the best survival outcomes is to ensure a complete cytoreduction with no residual tumor remaining. Therefore, a presurgical study is paramount to optimizing the indications of the patients who would benefit the most from this treatment.
Patients selection
The performance status of the patient is a fundamental aspect considering the morbidity of CRC+HIPEC. Eastern Cooperative Oncology Group or World Health Organization indices > 2 and serious comorbidities (severe cardiopulmonary or renal failure) are considered major contraindications[35]. Age is a factor to be considered globally, but there is no cut-off to contraindicate CRS plus HIPEC.
Preoperative scores: Tools for prognosis and surgical indication
Peritoneal carcinomatosis index:PCI is the most accepted score for both evaluating the tumor burden and estimating the patient prognosis, suggesting this score as a helpful tool for surgical indication as well (Figure 1). Faron et al[36]showed a significant relationship between two factors: 5-year survival was noticeably higher (53%) for PCI < 10, up to 23% for PCI between 10-20 and only 12% for PCI > 20. Currently, the majority of guidelines accept a PCI > 20 as a contraindication for CRS plus HIPEC[37].
Peritoneal surface disease severity score:The peritoneal surface disease severity score (PSDSS) is another commonly used and validated preoperative severity score.Some experts consider a PSDSS > IV (10 points) a contraindication for CRS due to its ominous outcomes. No additional benefit has been shown for PSDSS over PCI[38].
Completeness of the cytoreduction score:Completeness of the cytoreduction score is another useful tool widely used to assess prognosis. A complete resection of macroscopic tumors is a necessary requirement for the long-term benefit of CRS; thus,incomplete resections or debulking have shown survival improvement[32,39]. In other words, CRS must not be performed if less than a CC1 (< 2.5 mm) cytoreduction cannot be assured (for peritoneal disease, that small amount of residual tumor is expected to be eradicated by HIPEC)[40].
Resectability
For every patient diagnosed with CRC, abdomen and chest computed tomography(CT) and complete colonoscopy (or a CT colonography if the former cannot be done[41]) with biopsies for histopathological study must be performed. Positron emission tomography (PET) is not routinely recommended by the experts, although PET is considered a helpful tool for a more truthful evaluation of the tumor extension in cases of extra-abdominal disease suspicion and for obtaining additional information on equivocal lesions. Special attention should be paid to the detection of radiological signs of peritoneal metastasis on the abdominal CT scan to contemplate the best treatment approach for the patient. These signs include ascites, mesenteric effacement, peritoneal nodules or masses, luminar narrowing, peritoneal thickening and enhancement (Figure 2).
Assessment of a preoperative PCI with imaging would allow a preliminary evaluation of complete tumor resectability that can be useful to avoid unnecessary surgeries. However, a nonnegligible rate of inaccuracy between radiological PCI and surgical PCI has been observed, mainly because of the underestimation of tumor burden and operator dependence[42]. Implant sizes less than 5 mm and locations are the main factors for missing disease[43]. Magnetic resonance imaging has been reported to have a higher sensitivity than CT scan, especially for implants located on the small bowel (quadrants 9 to 12) and for unexperienced radiologists[42,44]. Combining both techniques can also increase the precision of preoperatory estimation[45].
Sugarbaker et al[46]proposed a series of radiologic features that could predict, if two or more are present, unresectability, suboptimal surgical resection or complex resections due to the tumor burden. PET/CT has demonstrated good sensitivity and specificity for the detection of peritoneal disease[47]. However, its accuracy can be altered in cases of small implants, mucinous and gastric tumors, as well as under some inflammatory conditions (e.g., inflammatory bowel disease or abscesses).
Consideration of synchronous lymph node infiltration
Lymph node infiltration is widely recognized as a poor prognosis factor for recurrence in the setting of the primary tumor. Publications on this theme also note that lymph node metastases present a more aggressive tumor biology at the time of CRS+HIPEC and have a dismal effect on survival[48,49].
Consideration of synchronous liver metastases
Concurrent liver and PM were initially considered a nonresectable condition due to the poor prognosis. However, the more recent consideration of liver implants with a poorer metastatic potential than peritoneal lesions and its excellent response (up to 60%) to modern systemic chemotherapy have changed the minds of surgeons concerning its approach[a].
In the last decade, several publications have suggested the feasibility and shown the survival improvement of liver metastases resection without adding morbidity[50,51].There is no consensus on the number of liver metastases that limits the indication for CRS plus HIPEC as long as complete resection can be fulfilled[52]. Elias developed a nomogram to estimate the prognosis of patients according to the number of liver metastases and the PCI[53].
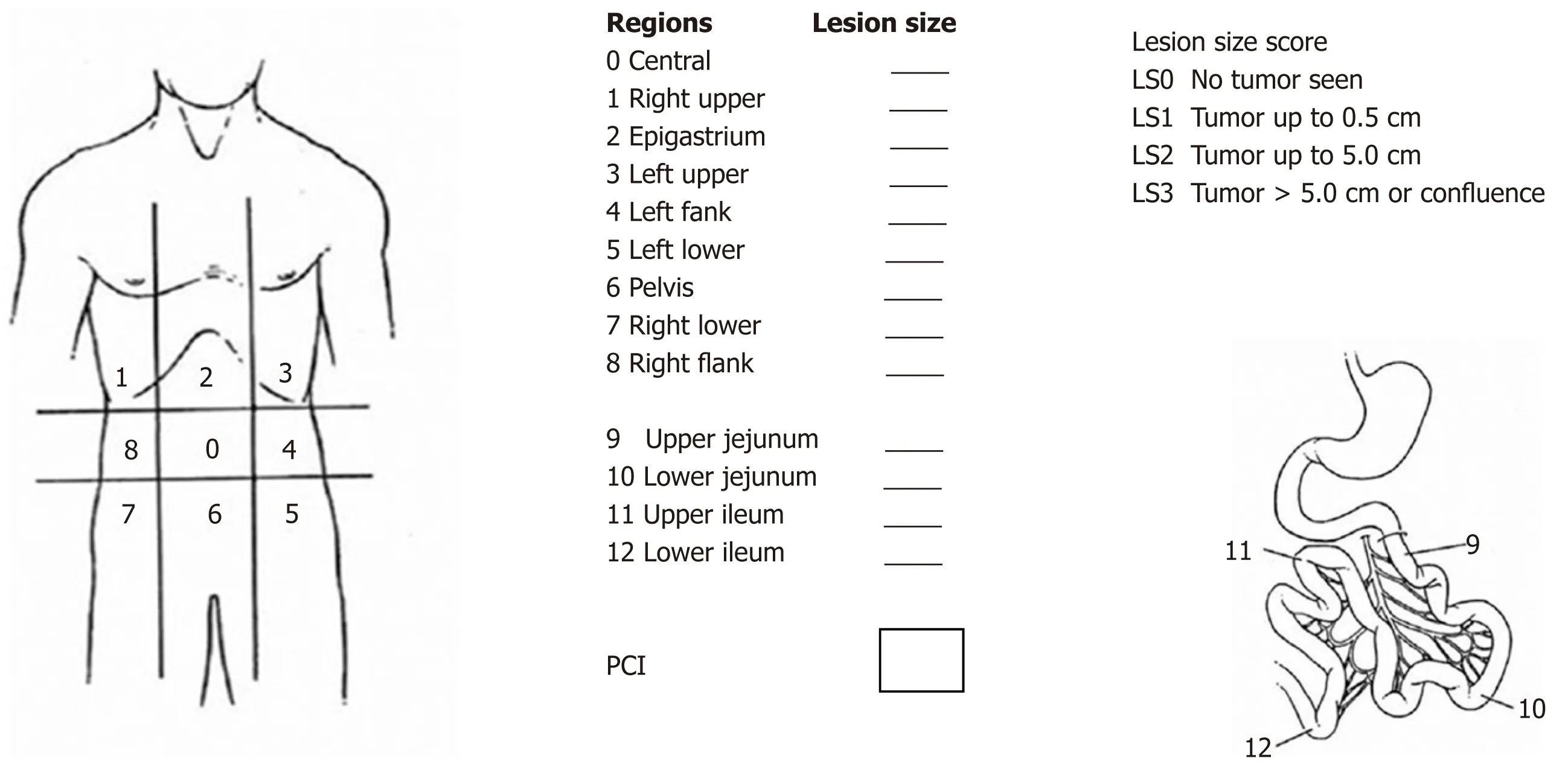
Figure 1 Peritoneal carcinomatosis index described by Sugarbaker P.
Although the presence and amount of liver disease are no longer a contraindication for CRS plus HIPEC, a recent meta-analysis on the survival benefit of these patients found a negative or no significant impact on survival in most of the studies compared to patients with PM alone[54]. However, ablation or resection of liver metastases has proven to offer better survival compared to palliative treatment[55]. Therefore, multidisciplinary consensus and individual evaluation must be considered for every particular case.
Absolute exclusion criteria for CRS plus HIPEC
Absolute exclusion criteria for CRS plus HIPEC: Bulky and/or diffuse peritoneal metastasis (Figure 3); Unresectable extra-abdominal metastases; Vast small bowel serosa or small bowel mesentery involvement; Multi-segmentary malignant bowel obstruction or non-affected length of small bowel < 150 cm; Massive affectation of the hepatic hilus; The presence of unresectable liver metastases or the requirement of a major hepatectomy, which could lead to insufficient hepatic function.
THE ROLE OF SYSTEMIC CHEMOTHERAPY
The effectiveness of neoadjuvant and adjuvant systemic chemotherapy for patients with CRS and PM has long been controversial among different publications.
Neoadjuvant chemotherapy
With the present experience, no survival benefits can be attributed to the administration of neoadjuvant chemotherapy for PM from CRC origin, without extra-abdominal disease. A recent systematic review found no strong evidence for its efficacy regarding overall survival[56]. Neoadjuvancy has only shown survival improvement in univariate analysis in some publications, and even in two papers[57,58]multivariate analysis suggested a worse median survival when neoadjuvant chemotherapy was used. A prospective study reported the first experience using modern systemic chemotherapy with and without biological agents[59]and showed no effects on unresectable disease, considering a minimal study sample and a high percentage of unfavorable histology.
Notably, there are wide and non-standardized chemotherapy regimens used for the different teams and no randomized controlled trial has been performed in this context, which hinders the potential implication of this therapy. Similarly, there is no reliable data concerning the safety of the surgery following neoadjuvant chemotherapy with biological agents, such as bevacizumab, a vascular endothelial growth factor inhibitor that has been suggested as a risk factor for anastomotic leaks due to its implication in tissue regeneration. There are few publications on this issue, initially brought to light by Eveno et al[60], whose retrospective analysis showed a statistically significant increase in major morbidity (mainly because of intraabdominal abscesses)when bevacizumab was included in the neoadjuvant treatment. Subsequently, Ceelen et al[61]published their experience using bevacizumab neoadjuvant regimens, and they not only found worsening of the postoperative morbidity but also reported a beneficial effect on overall survival. Other recent publications describe no major postoperative complications related to the use of bevacizumab in the neoadjuvant protocol[62,63], but there are no further studies specifically on CRC.

Figure 2 Radiological computed tomography signs for peritoneal disease. Wide white arrow: Omental cake; Thin white arrow: Peritoneal thickening; White arrow-head: Malignant ascites; Black arrow-head: Peritoneal nodules.
Adjuvant chemotherapy
The same systematic review[56]suggests the positive effect of adjuvant chemotherapy on overall survival, despite the heterogeneity of the studies. The most recent publications concur on reporting improvement in median survival with modern versus standard chemotherapy protocols[64], and these authors also agree on the actual approach of M1c as a curable stage for CRC[65]. The clinical value of biological therapies remains uncertain. The concept of blocking angiogenesis was a new promising tool for metastatic CRC and has gained popularity in recent years. Previous publications described no increase in survival using these agents after resectable or unresectable disease[64,66]. Conversely, a recent meta-analysis[67]on the different types of anti-VEGF antibody combination therapies has shown a significant improvement in progression-free survival, overall survival and response rate.
The number of adjuvant chemotherapy cycles did not demonstrate a clear relationship with survival in previous publications[56]. A recent study[68]encompassing six phase III trials evaluating the noninferiority of 3 versus 6 months administration of adjuvant modern chemotherapies with either FOLFOX (fluorouracil, leucovorin, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin) was conducted. Among 12384 patients with stage III colon cancer, the 6-month duration of FOLFOX therapy increased the rate of disease-free survival, particularly among patients with high-risk cancers (T4, N2, or both). However, efficacy was maintained with the 3-month duration for low-risk patients and for the CAPOX regimen, which suggests that this protocol could be evaluated to prevent adverse effects, such as persistent neurotoxicity associated with oxaliplatin.
However, the most important independent factor for a better survival is the radical resection of the tumor with curative intent[69]; thus, the effectiveness of any treatment highly depends on the extent of the tumor and the completeness of cytoreduction,attempting to avoid a delay of adjuvant treatment due to surgical complications.
OUTCOMES OF CYTOREDUCTIVE SURGERY PLUS
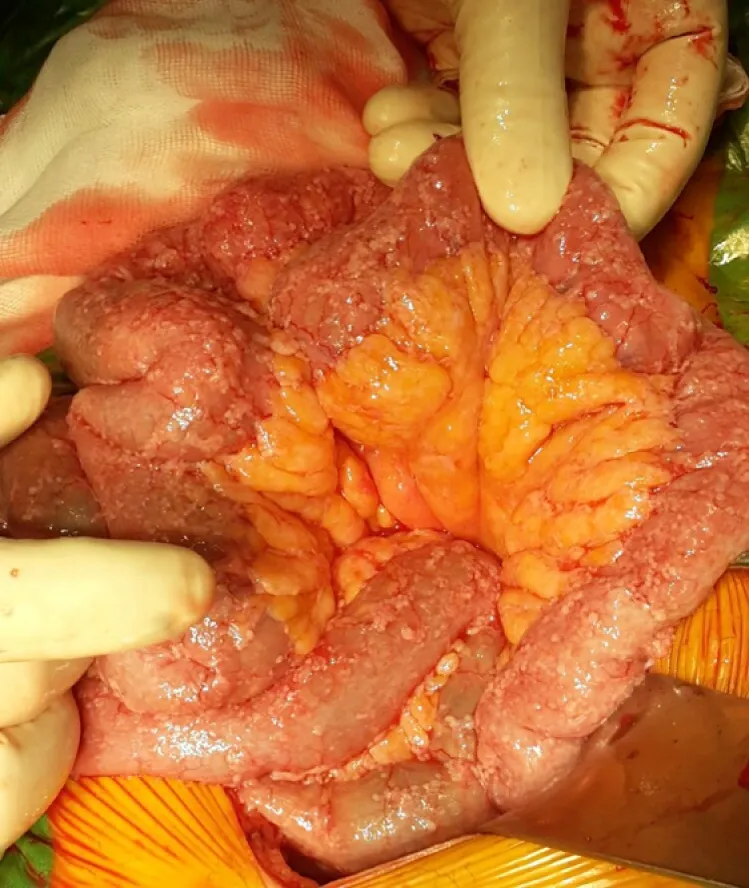
Figure 3 Diffuse miliary carcinomatosis on the small bowel as an example of contraindication for complete cytoreductive surgery.
HYPERTHERMIC INTRAPERITONEAL CHEMOTHERAPY
Morbidity and mortality
A large meta-analysis[65]found that intention-to-cure treatments improved overall survival in patients with CRC + PM better than palliative strategies. The risks reported have progressively decreased (recent studies report 1%-5% mortality rates at centers of excellence). The morbidity associated with curative treatments was higher,but it did not increase the risk of treatment-related mortality or caused an early termination of the treatment. Additionally, the reported morbidity rates are similar to those of other major abdominal procedures: Increased treatment-related complications, longer hospital stays and higher rates of short-term readmission[33,70,71](Table 2).
A consideration that should be highlighted is that experience and learning curve play an important role in the morbidity and mortality outcomes, so it is strongly recommended that patients should be treated in experienced centers, mentored by specialized institutions for peritoneal diseases, such as PSOGI[70]. Regarding the laparoscopic approach, HIPEC delivery by the laparoscopic approach has already been accepted as a safe and feasible procedure that is performed for different indications. However, there is a lack of knowledge about the oncological quality of laparoscopy CRS for CRC with peritoneal implants. Some groups have published their early experiences in this field; all of these reports suggested the careful selection for thin patients with a PCI < 10 to ensure a complete resection[72-74].
Survival outcomes
In recent years, CRS plus HIPEC plus systemic chemotherapy (both neoadjuvant and after surgery) comprise the multidisciplinary treatment performed in most of the referral centers. Overall survival results with this management can reach up to 62 mo if optimal cytoreduction is achieved (this means, following the cytoreduction completeness score: CCO, no tumor nodules left or CC1, implants ≤ 2.5 mm in maximum dimension)[25]. Increasing evidence from large multicenter cohort studies and some randomized controlled trials suggests that, in selected patients, CRS plus HIPEC as a combined management definitely provides improved overall and diseasefree survival compared to that conferred by systemic chemotherapy alone, and possibly a major part of this benefit has to be attributed to cytoreduction[25,28,31,34,65,75](Table 3).
A recent review of current guidelines about the management of patients with colorectal PM shows no definitive agreement about the role of CRS plus HIPEC, but most of them recommend this approach as a standard therapy for selected patients,reaching a consensus in 71% with a level of evidence 1b[35]. The treatment approach for these patients should always be assessed by a multidisciplinary team discussion,which includes at least a surgeon (ideally, an oncologist surgeon), an oncologist and a radiologist expert[52]. Considering these concepts, the latest National Comprehensive Cancer Network (NCCN) guidelines[32]recommend that CRS plus HIPEC for colorectal PM be considered only at experienced centers for selected patients with limited PM for whom complete cytoreduction is deemed to be achieved.
The role of HIPEC
The lack of consensus about the role of HIPEC may be due to several reasons: The marked heterogeneity of protocols, drugs, carrier solutions and methods of HIPEC administration (open, semi-open, closed techniques) and the discrepancy concerning patient eligibility and lack of randomized trials in the era of modern chemotherapy and targeted therapy.

Table 2 Patient and operative factors associated with cytoreductive and hyperthermic intraperitoneal chemotherapy morbidity (modified from Newton et al[70])
The preliminary results of the PRODIGE 7 trial[76], presented at the American Society of Clinical Oncology (ASCO) meeting in 2018, questioned the widespread conviction of the beneficial effects of HIPEC. After complete cytoreduction of M1c CRC, 265 patients were randomized to standard treatment plus HIPEC with oxaliplatin or standard treatment alone. No significant difference in overall survival was found, with a median of 41.7 months in the HIPEC arm vs 41.2 mo in the non-HIPEC arm [Hazard ratio (HR) = 1.00, 95% confidence interval (CI): 0.73-1.37] and no significant difference in relapse-free survival (13.1 vs 11.1 mo, HR = 0.90, 95%CI: 0.69-1.90). However, a trend toward better disease-free survival was found on the Kaplan-Meier curves for the first 18 months after surgery, and a subgroup analysis for patients with a PCI between 11 and 15 showed significantly better overall and recurrence-free survival for the HIPEC group.
Regarding morbidity, the study reported a higher late, grade 3-5 morbidity (up to 60 d after surgery) in the HIPEC arm (24.1% vs 13.6%, P = 0.03). The unexpected results have encouraged the scientific community to continue searching for the role of HIPEC in PM, as its advantageous effects have been extensively reported in the biomedical literature for CRC and recently proven for other origins[77]. To our knowledge, high quality and complete cytoreduction has been confirmed once again as a pivotal pillar of treatment for peritoneal dissemination of CRC. Efforts are now focused on electing patients who would benefit the most from HIPEC because this trial remarks high PCI as an already known impaired factor.
Another goal is to ascertain the real morbidity (as most of the publications only report the 30-day morbidity-mortality and have widely been compared, similar to other major abdominal surgeries[78]) and reduce the side-effects of HIPEC[79]. This may be achieved by either minimizing drug doses (which has been one critic of the PRODIGE 7, considering previous experimental studies)[80], establishing the benefits of hyperthermia alone and combined with the chemotherapy agents, or trying different drugs or delivery systems. Additionally, the final results are published; to date, only one multicenter randomized trial studying the effects of HIPEC vs standard treatment for patients with established PM of CRC origin (NCT02179489). Therefore, the search is ongoing, and further trials are needed to determine what HIPEC can offer.
REITERATIVE CYTOREDUCTIVE PROCEDURES
Approximately 70-80% of the patients undergoing CRS plus HIPEC will develop recurrence disease, despite the curative intention of this approach. Half of these recurrences will be confined to the peritoneal cavity[81]. This reality has led to the study of the feasibility and safety of reiterative CRS and even HIPEC procedures in recent years. The morbidity and mortality of these surgeries are similar to those of the first procedure in high volume centers[82]. Furthermore, this active approach to recurrent abdominal disease has reported a median survival from 39 to 42.9 mo, a clearly better long-term survival compared to that obtained with systemic treatment alone[82-84]
Keeping in mind that HIPEC therapy has not been proven to be an independent risk factor itself for postoperative complications[71]and that the morbidity reported for CRS in experienced centers is similar to that in other major surgeries, the combined CRS plus HIPEC approach seems an acceptable strategy for this poor stage of disease,as studies continue to show further evidence. Moreover, traditional adjuvant systemic chemotherapy can still be performed following surgery (as it would be the only treatment if surgery could not be performed or it would be rejected). Issues such as the adjunctive contribution of intraperitoneal chemotherapy to CRS and optimal chemotherapy regimens still require further study.
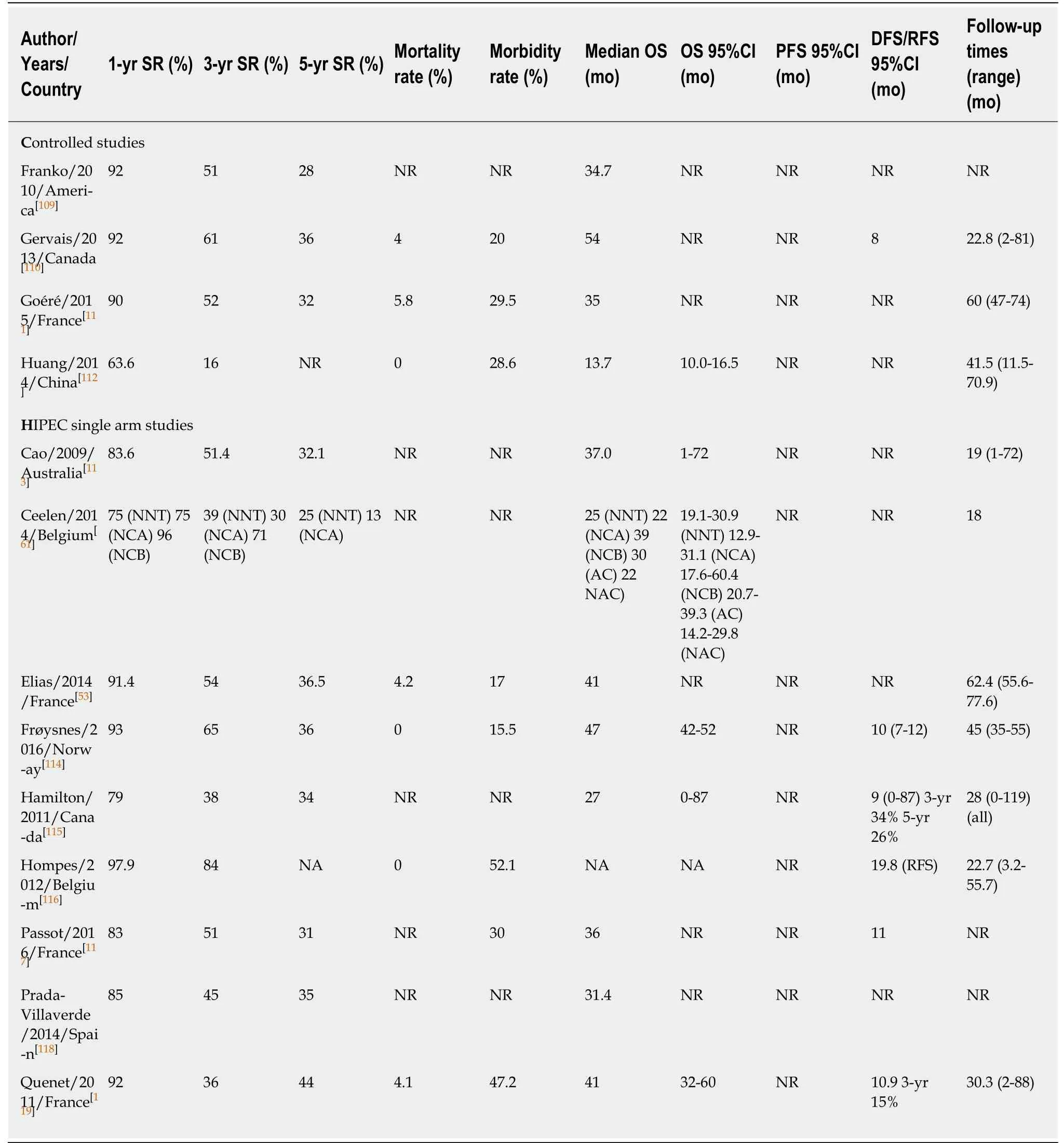
Table 3 Survival of patients with peritoneal metastases from colorectal cancer treated by cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy
IMPORTANCE AND RECOMMENDATION FOR THE FOLLOW-UP
At present, there are no shared guidelines for the ideal duration and intervals for CRC patient follow-up. The NCCN and European Society of Medical Oncology guidelines recommend abdomen and chest CT surveillance every 6 to 12 mo for 3 to 5 years. The American Society of Clinical Oncology (ASCO) and American Cancer Society recommend CT every 12 mo for 5 years.
Ultrasonography provides no additional advantages for the early detection of CRC recurrence[85]. Colonoscopy is recommended at approximately 1 year after the resection (or at 3-6 mo if not performed preoperatively because of emergency surgery), then at the third year, and every 5 years thereafter. Additionally, 18FDGPET/CT was proposed as a complementary tool for prompt detection of asymptomatic recurrence, but consecutive studies have shown additional costs with no particular benefit for resectable disease and, hence, no improvement of overall survival[86].Currently, it is only advisable for patients with tumor marker elevation without other evidence of disease or for those in whom recurrences are suspected with normal serum marker levels[87].
Several studies have focused on the role of an intensive follow-up after curative surgery for CRC, combining imaging resources plus CEA level screening. The global results report an improved rate of recurrence detection that could be treated by intentionally curative iterative surgery[88]. However, these early findings of metachronous disease do not seem to correspond to better results on overall survival,independent of the stage at diagnosis[89-92]. No advantage has been observed in the CEA and CT combination, and any strategy has proven a better survival advantage over a symptom-based approach[93]. Peculiarly, these findings are not consistent with the early treatment of recurrences found in rectal cancer, below the peritoneal reflection, which seem to benefit from a more intensive follow-up in pursuit of better survival[90].
Despite the limitations and bias reported in these studies, the optimal follow-up regimen for CRC patients of any stage remains controversial, and care should be taken concerning unnecessary radiation exposure of the patients and the costeffectiveness of overly intense schedules. Notwithstanding, as radical surgery is the only actual curative treatment for colon cancer, intensive surveillance can be justified in high-risk patients, while quality evidence is achieved.
CURRENT AND FUTURE LINES OF RESEARCH
Prophylactic approach to peritoneal metastases from CRC
As mentioned before, knowing the risk factors for peritoneal spread (Table 1) allows proactive strategies for patients with high risk of developing PM to be value to set the benefit of a radical resection of located disease over the morbidity added to a patient without objective tumor spread (for example, in cases of pT4 colon cancer, which can develop peritoneal recurrence at rates of 30%-40%)
Second look surgery and prophylactic HIPEC
Second-look evaluation has always gone together with a close follow-up by CT of the chest, abdomen and pelvis, colonoscopies and CEA level surveillance. Although combined with adjuvant chemotherapy treatment, the results of the different followup protocols and guidelines are still heterogeneous, and the optimal management remains controversial.
Some studies have found encouraging survival outcomes for proactive strategies,such as the second-look approach[94,95], prophylactic resection of target organs for peritoneal implants during the first surgery (omentectomy, appendectomy, hepatic round ligament resection and bilateral adnexectomy)[96]or prophylactic HIPEC administration in locally advanced tumors without peritoneal carcinomatosis[97-99].
Several phase III trials are currently ongoing for the evaluation of the influence of prophylactic HIPEC in high-risk patients[100](Table 4). For example, the ProphyloCHIP trial led by Elias has already reported some preliminary data showing no morbidity increased on patients who received second-look surgery, but no advantages in 3-year disease-free survival or overall survival have been found over classical follow-up[101].The initial results from the COLOPEC trial, presented at ASCO 2019, do not show adjuvant HIPEC to improved 18 mo over adjuvant systemic chemotherapy on PMfree survival for high-risk patients[102].
The role of laparoscopy in the staging and second-look for peritoneal disease has been shown as a useful and feasible tool, even in previously laparotomized patients for restaging. Some experienced centers even use laparoscopy as a routine step for all the elective cytoreductive surgeries to prevent patients from an unnecessary xyphopubic incision. Recent publications highlight the relevance of wide small bowel involvement over a proper estimation of the PCI to reject CRS[103], which could enhance the value of the laparoscopic approach in this context. Although the risk factors for peritoneal recurrence are well known and firmly confirmed by numerous publications, there is currently no strong evidence of the benefit of proactive strategies versus adequate surveillance for high-risk patients. We still await the results of the process phase III trials to shed light on the optimal management.

Table 4 Studies on the ClinicalTrials.gov registry investigating the role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on high risk patients for preventing peritoneal metastases from colorectal origin
Pressurized intraperitoneal aerosol chemotherapy
Better survival results have been tried to achieve by boosting the cytotoxic effect of chemotherapy agents of HIPEC. A novel example of this effort is the development of a new drug delivery system known as pressurized intraperitoneal aerosol chemotherapy (PIPAC). In the context of experimental trials, this procedure is being used for patients with peritoneal region as only place of metastases from different cancer origins, but without indication for cytoreductive plus HIPEC surgery as no complete resection can be performed.
This laparoscopic and iterative procedure nebulizes the cytotoxic agents into the expanded peritoneal cavity and maintains a steady pressure with the aim of increasing drug penetration into the tissues with a more homogeneous distribution than with liquid chemotherapy. Theoretically, as the pressure decreases venous blood outflow, drugs would spend more time in contact with the tissues, so higher drug concentrations could be reached with lower doses (therefore, this would minimize systemic toxicity).
In recent years, its feasibility and safety have been extensively reported, and its efficacy for a histological response and survival benefits has been described by several teams[104]. Referring to CRC, there is still little experience but encouraging results published[105], and currently, 4 prospective clinical trials are ongoing evaluating the oncological efficacy of PIPAC for selected patients who currently would be considered for palliative care (Table 5). Pursuing an optimal macroscopic cytoreduction for the best guarantee of prognosis, some trials have proposed the use of intraoperative imaging techniques that can guide the detection of malignant lesions using tumortargeted fluorescence traces[106].
Some other trials have attempted to rescue high PCI patients for an eventual CRS surgery by using a combination of intraperitoneal plus systemic chemotherapy regimens. The aim of this bidirectional chemotherapy is to reach peritoneal implants not only from the peritoneal cavity but also from subperitoneal blood vessels[107,108].
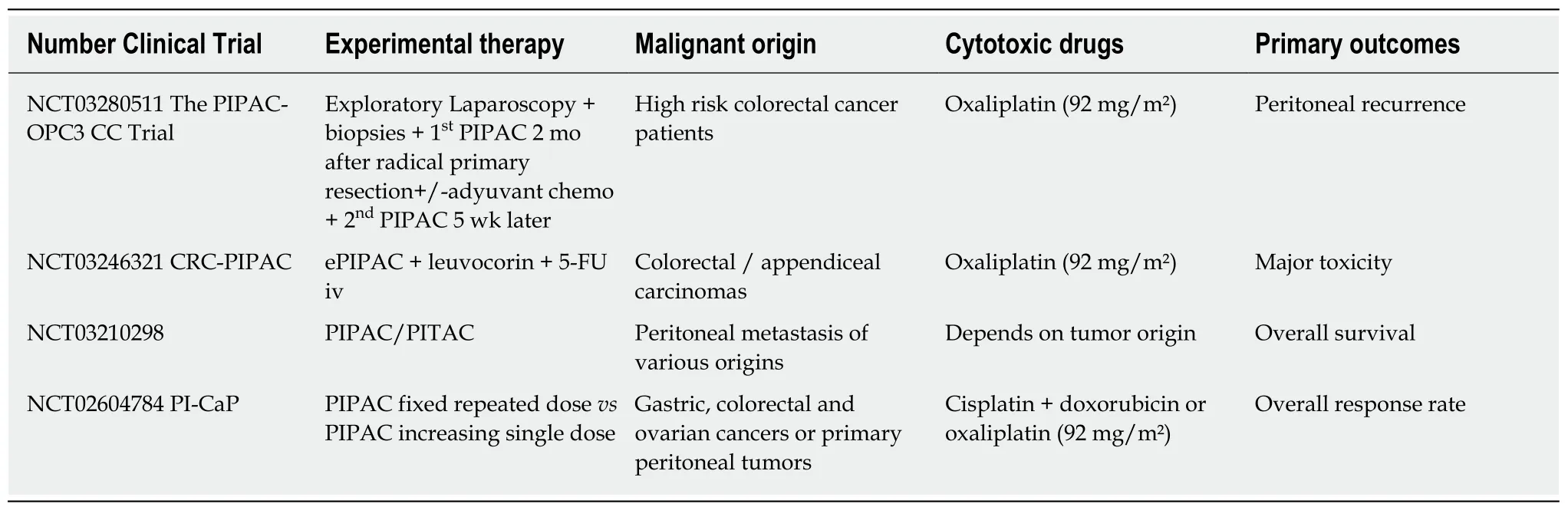
Table 5 Studies on the ClinicalTrials.gov registry investigating the role of pressurized intraperitoneal aerosol chemotherapy
CONCLUSION
CRS plus HIPEC combined with systemic modern chemotherapy is feasible for the management for PM of CR origin most widely accepted by experts, as accumulative evidence suggests that it improves recurrences as well as overall and peritoneal disease-free survival. Because of the lack of randomized clinical trials and the conflicting data on clinical efficacy, this approach remains controversial. Optimization of preoperative imaging assessment of tumor burden and molecular biology categorization are two promising approaches for better individualized treatment. In referral centers, the morbidity and mortality associated with this procedure do not seem to be higher than other major abdominal surgeries. Iterative CRS for local recurrences has proven to improve overall survival without adding a significant morbidity. It is paramount that patients with peritoneal carcinomatosis continue to be referred to experienced centers that can offer a multidisciplinary, tailored evaluation and high-quality surgery. As complete CRS has confidently proven to improve patient survival, future strategy targets are focused on assessing the real role of modern systemic chemotherapy and HIPEC for the treatment and prevention of PM in highrisk patients.
杂志排行
World Journal of Gastroenterology的其它文章
- Stricter national standards are required for credentialing of endoscopic-retrograde-cholangiopan-creatography in the United States
- Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders
- lmmune suppression in chronic hepatitis B infection associated liver disease: A review
- Device-assisted enteroscopy: A review of available techniques and upcoming new technologies
- ldentifying high-risk individuals for gastric cancer surveillance from western and eastern perspectives: Lessons to learn and possibility to develop an integrated approach for daily practice
- ls the treatment outcome of hepatocellular carcinoma inferior in elderly patients?
