A comparison of short-term postoperative outcomes including nutritional status between gastrectomy with simultaneous cholecystectomy and gastrectomy only in patients with gastric cancer
2019-07-13YouNaKimJiYeongAnMinGewChoiJunHoLeeTaeSungSohnJaeMoonBaeSungKim
You Na Kim, Ji Yeong An, Min-Gew Choi, Jun Ho Lee, Tae Sung Sohn, Jae Moon Bae, Sung Kim
1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea;
2Department of Surgery,Korea University Anam Hospital, Korea University School of Medicine, Seoul 02841, Korea
Abstract Objective: We aimed to evaluate the effect of simultaneous cholecystectomy on the short-term postoperative outcomes and nutritional status in patients with gastric cancer.Methods: We retrospectively reviewed data from 4,820 patients with gastric cancer who underwent gastrectomy from January 2011 to December 2016. Patients who underwent only gastrectomy (N=4,578) were matched to those who underwent simultaneous cholecystectomy during gastrectomy (N=242) at a 1:1 ratio using propensity score matching analysis. The nutritional status and inflammatory responses preoperatively and postoperatively and postoperative outcomes were compared between the groups.Results: The simultaneous cholecystectomy group showed more intraoperative blood loss and a longer operative time than the gastrectomy only group [150.0 (100.0, 200.0) mL vs. 100.0 (100.0, 200.0) mL, P=0.006; 176.0 (150.0,210.0) min vs. 155.0 (128.0, 188.0) min, P<0.001, respectively]. Intraoperative event rate, postoperative complication rate, and postoperative recovery did not differ between the groups. All parameters including body weight, the hemoglobin level, absolute lymphocyte count, total protein level, albumin level, fasting glucose level,and prognostic nutritional index excluding the cholesterol level were not significantly different between the groups,and their changing patterns were similar. Although the cholesterol level was significantly lower in the simultaneous cholecystectomy group than in the gastrectomy only group at all follow-up points, the mean value of the decreased cholesterol level was within normal range.Conclusions: In gastric cancer patients with gallbladder disease, simultaneous cholecystectomy is safe and not associated with additional nutritional loss.
Keywords: Gastric cancer; cholecystectomy; nutritional status
Introduction
After gastric cancer surgery, most patients experience weight loss and a certain degree of nutritional deficit,which are related to decreased food intake and intestinal malabsorption followed by gastric resection and intestinal bypass (1-3). However, with the increasing incidence of early gastric cancer and its improved survival rate,postoperative quality of life has become more important,and physicians and patients are concerned about postoperative nutritional changes (4).
During the preparation for gastric cancer surgery,various gallbladder diseases, such as gallbladder stones,polyps, adenomyomatosis, and cholecystitis, were sometimes detected by abdominal computed tomography,and simultaneous cholecystectomy is performed selectively at the surgeons’ discretion. When performing simultaneous cholecystectomy with gastrectomy, patients sometimes worry about the additional risks, including perioperative complications and nutritional problems postoperatively.Although the Cholegas study reported that prophylactic cholecystectomy during gastrectomy did not increase the risk of perioperative morbidity, mortality, and medical cost(5). Some authors have reported that prophylactic cholecystectomy increases the risk of perioperative morbidity. However, little information is available on the nutritional status of patients who have undergone simultaneous cholecystectomy during gastrectomy for gastric cancer. Moreover, no reports have determined whether adding prophylactic cholecystectomy to gastrectomy affects the postoperative nutritional status and postoperative outcomes of patients with gastric cancer.Considering that the nutritional status of patients with gastric cancer can affect the prognosis and survival postoperatively (5-11). The effect of adding cholecystectomy on nutritional status and surgical outcomes should be evaluated.
Therefore, this study aimed to evaluate the effect of simultaneous cholecystectomy on the postoperative nutritional status and surgical outcomes of patients with gastric cancer.
Materials and methods
Patients
This study was approved by the Institutional Review Board of Samsung Medical Center, and informed consent was obtained from the patients. Among 7,404 patients with gastric cancer who underwent gastrectomy with lymph node dissection and truncal vagotomy from January 2011 to December 2016, 2,584 patients were excluded from the analysis because of a history of palliative surgery, the presence of co-malignancy, a history of adjuvant chemotherapy, and missing data during the follow-up period. The enrolled patients underwent subtotal distal gastrectomy or total gastrectomy according to the lesion of cancer. Clinicopathological and laboratory data of the remaining 4,820 patients were extracted from a prospectively collected database and analyzed retrospectively. Among the 4,820 patients, simultaneous cholecystectomy with gastrectomy was performed in 242 patients and only gastrectomy was performed in 4,578 patients. The schematic diagram about enrolled patients was showed in Supplementary Figure S1.
Evaluation and follow-up
All patients were followed every 3 or 6 months according to our institutional standardized protocol. Clinicopathological characteristics included age, sex, the American Society of Anesthesiologists’ (ASA) score, body mass index (BMI), and tumor stage.
For the nutritional assessment during the first year postoperatively, values of serum hemoglobin, absolute lymphocyte count, protein, albumin, and cholesterol and the prognostic nutritional index (PNI) were evaluated. The PNI was calculated using the following equation:PNI=[10 × serum albumin level (g/dL)] + [0.005 × total lymphocyte count] (12,13). Patients’ nutritional status was evaluated on the preoperative day, postoperative d 5, and postoperative month 3, 6, and 12.
To assess changes in the liver enzyme levels postoperatively, the levels of aspartate aminotransferase(AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin were examined on the preoperative and operative days, postoperative d 3 and 5, and postoperative month 3. To assess the postoperative inflammatory response, the white blood cell (WBC) count and serum C-reactive protein (CRP) level were evaluated on the preoperative and operative days, postoperative d 3 and 5, and postoperative month 3.
Factors associated with the surgical outcomes, such as the operative time, amount of blood loss, extent of resection, reconstruction methods, length of hospital stay postoperatively, intraoperative events, and postoperative complications, were also assessed. Postoperative complications were classified within 30 d postoperatively according to the criteria proposed by Clavien and Dindo(14). Hospital mortality was defined as death during hospitalization or postoperative death due to any cause within 30 d.
Propensity score matching
Because we expected a difference in the number of patients and perioperative characteristics between the gastrectomy only group and simultaneous cholecystectomy during gastrectomy group, propensity score matching analysis was performed to reduce the effects of selection bias and confounding factors in the comparison of nutritional status.Propensity scores were estimated using multivariable logistic regression model as the dependent variables of perioperative nutritional status. As a result, covariates for propensity scores were age, sex, preoperative BMI, ASA physical status score, reconstruction approach, tumor depth, and node and metastasis according to seventh edition of the American Joint Committee on Cancer tumor-node-metastasis classification. Overall, 4,578 patients who underwent only gastrectomy were matched to 242 patients who underwent simultaneous cholecystectomy during gastrectomy using a nearest neighbor matching algorithm with a matching ratio of 1:1. The matched patients were divided into two groups according to cholecystectomy, and no factors were significantly different between these groups. Therefore, all matched factors after propensity score matching were well-balanced in this study.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (Version 23.0; IBM Corp., New York, USA) and R software (Version 3.4.0; R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were compared using the Chi-square test or Fisher’s exact test,and continuous variables were compared using the t-test,Mann-Whitney U-test and repeated measure variation analysis. Risk factors of worse postoperative nutritional status were analyzed with odds ratios (ORs) and 95%confidence interval (95% CI) using binary logistic regression, and a multivariable model was selected using the forward likelihood ratio method. A two-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
Patient characteristics before and after matching are shown in Table 1. Before matching, there was a significant difference in the baseline preoperative and operative characteristics between the patients undergoing only gastrectomy and those undergoing simultaneous cholecystectomy during gastrectomy. Patients in the simultaneous cholecystectomy group were older, more frequently had ASA scores of 2 and 3, and had a higher proportion of advanced gastric cancer than those in the gastrectomy only group. After propensity score matching,the perioperative characteristics of the two groups were not significantly different.
Causes of cholecystectomy
The causes of cholecystectomy were gallbladder stones in 180 patients (74.4%), adenomyomatosis in 29 (12.0%),polyps in 26 (10.7%), mass in 4 (1.7%), and iatrogenic injury in 3 (1.2%). Among the 4 patients with a mass-like lesion in the gallbladder, 2 were diagnosed as having gallbladder cancer, as determined by intraoperative frozen biopsy results, and they underwent radical cholecystectomy.
Operative and postoperative outcomes
The outcomes associated with surgery are shown in Table 2. The simultaneous cholecystectomy group showed more intraoperative blood loss than the gastrectomy only group [150.0 (100.0, 200.0) mL vs. 100.0 (100.0, 200.0)mL, P=0.006]. The operative time was significantly shorter in the gastrectomy only group than in the simultaneous cholecystectomy group [155.0 (128.0, 188.0) min vs. 176.0(150.0, 210.0) min, P<0.001]. In patients with no postoperative complications, the duration of the postoperative hospital stay was not different between the two groups (8.0±4.6 d vs. 8.0±2.7 d, P=0.943).
There was no difference in the intraoperative event rate.In the simultaneous cholecystectomy group, there were two intraoperative events: one was common bile duct injury during cholecystectomy, which was treated with choledochojejunostomy; and the other was right hepatic artery injury and ligation during cholecystectomy. In the gastrectomy only group, there was only one intraoperative event: splenic artery injury during lymph node dissection so arterial repair was performed with spleen preservation.Surgical complications were graded by the Clavien-Dindo classification. Grades II-V surgical complications developed in 9.9% of patients in the gastrectomy only group and 12.0% of those in the simultaneous cholecystectomy group. The distribution of the grade of surgical complications was similar between the two groups(Table 3).
Postoperative inflammatory response and changes in liver enzyme levels
The WBC count (Figure 1A) and serum CRP levels (Figure 1B) showed similar changing patterns with no significant difference at each follow-up point. Changes in liver function-related markers, such as AST, ALT, ALP,and total bilirubin levels, are shown in Figure 2. AST and ALT levels increased more in the simultaneous cholecystectomy group than in the gastrectomy only group immediately postoperatively, but they decreased and stabilized on postoperative d 5. At 3 months postoperatively, the liver enzyme parameters were not different between the two groups. The total bilirubin level was significantly higher on postoperative d 3 in the simultaneous cholecystectomy group than in the gastrectomy only group (P=0.035), but it normalized on postoperative d 5. To confirm the normalization of all biochemical parameters postoperatively, data at 3 months postoperatively were assessed, and there was no difference between the two groups.
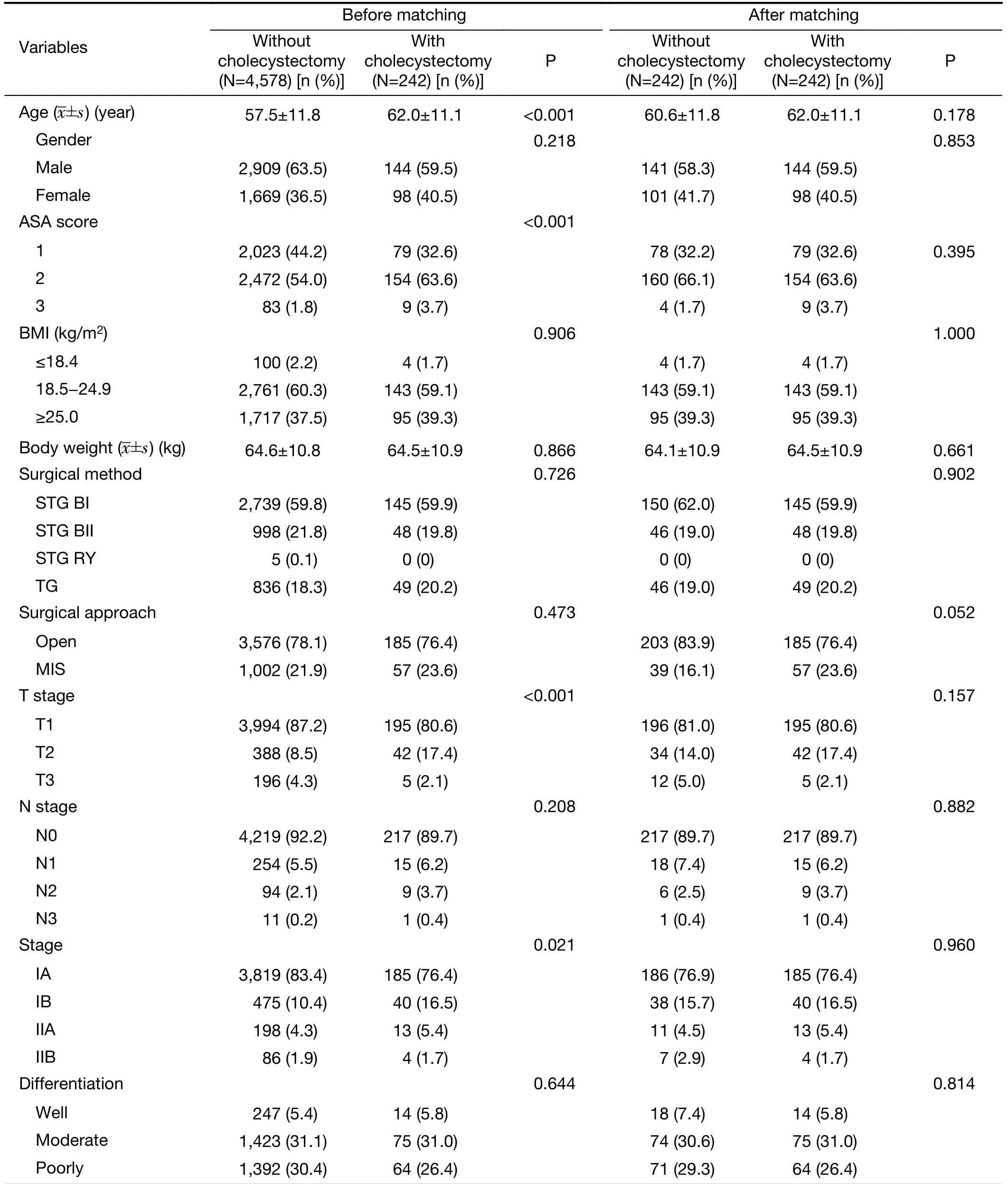
Table 1 Baseline clinicopathological characteristics before and after matching
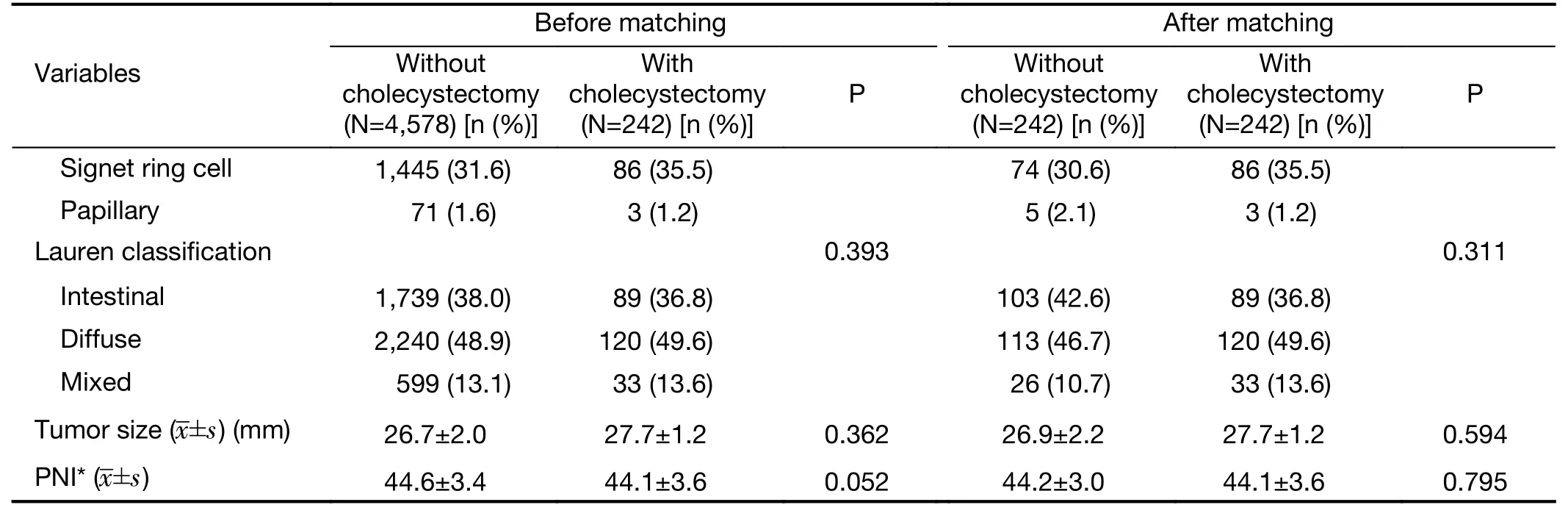
Table 1 (continued)
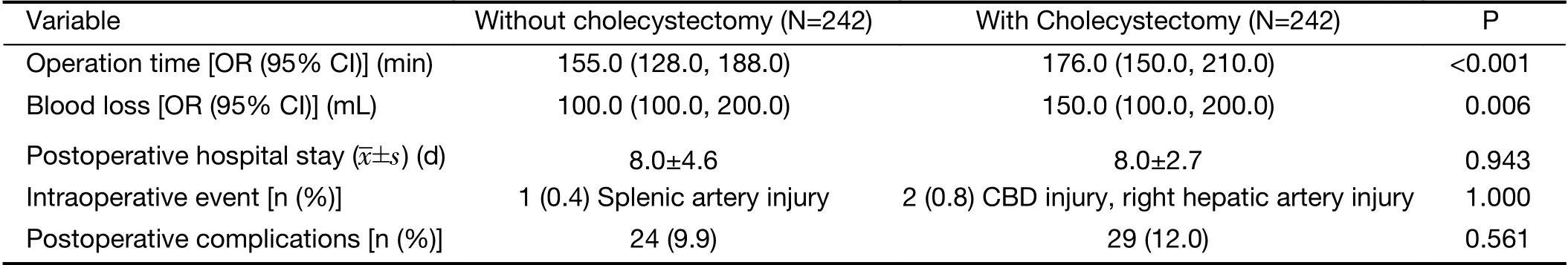
Table 2 Comparison of perioperative outcomes
Changes in postoperative nutritional status
There was no significant difference in the preoperative nutritional status, including PNI, between the two groups(Table 1). The average postoperative changes in body weight; values of hemoglobin, absolute lymphocyte count,total protein, albumin, cholesterol, and fasting glucose; and PNI are shown in Figure 3. All parameters, except for the cholesterol level, were not significantly different between the two groups, and their changing patterns were similar.The cholesterol level was significantly lower in the simultaneous cholecystectomy group than in the gastrectomy only group at all follow-up points. However,the mean value of the decreased cholesterol level was within normal range.
Risk factor analysis for nutritional deficiency
As shown in Table 4, risk factor analysis for nutritional deficiency at 12 months postoperatively in 484 patients enrolled through matching was done using a binary logistic regression model. The reference point of nutritional deficiency was determined by the preoperative mean value of PNI in all patients, and a mean PNI<43 was defined as nutritional deficiency. Only the preoperative PNI value was identified as an independent risk factor for postoperative nutritional deficiency; therefore, a lower preoperative PNI was a risk factor for nutritional deficiency at 12 months postoperatively. Simultaneous cholecystectomy during gastrectomy was not associated with postoperative nutritional deficiency, even after adjusting other confounding factors.
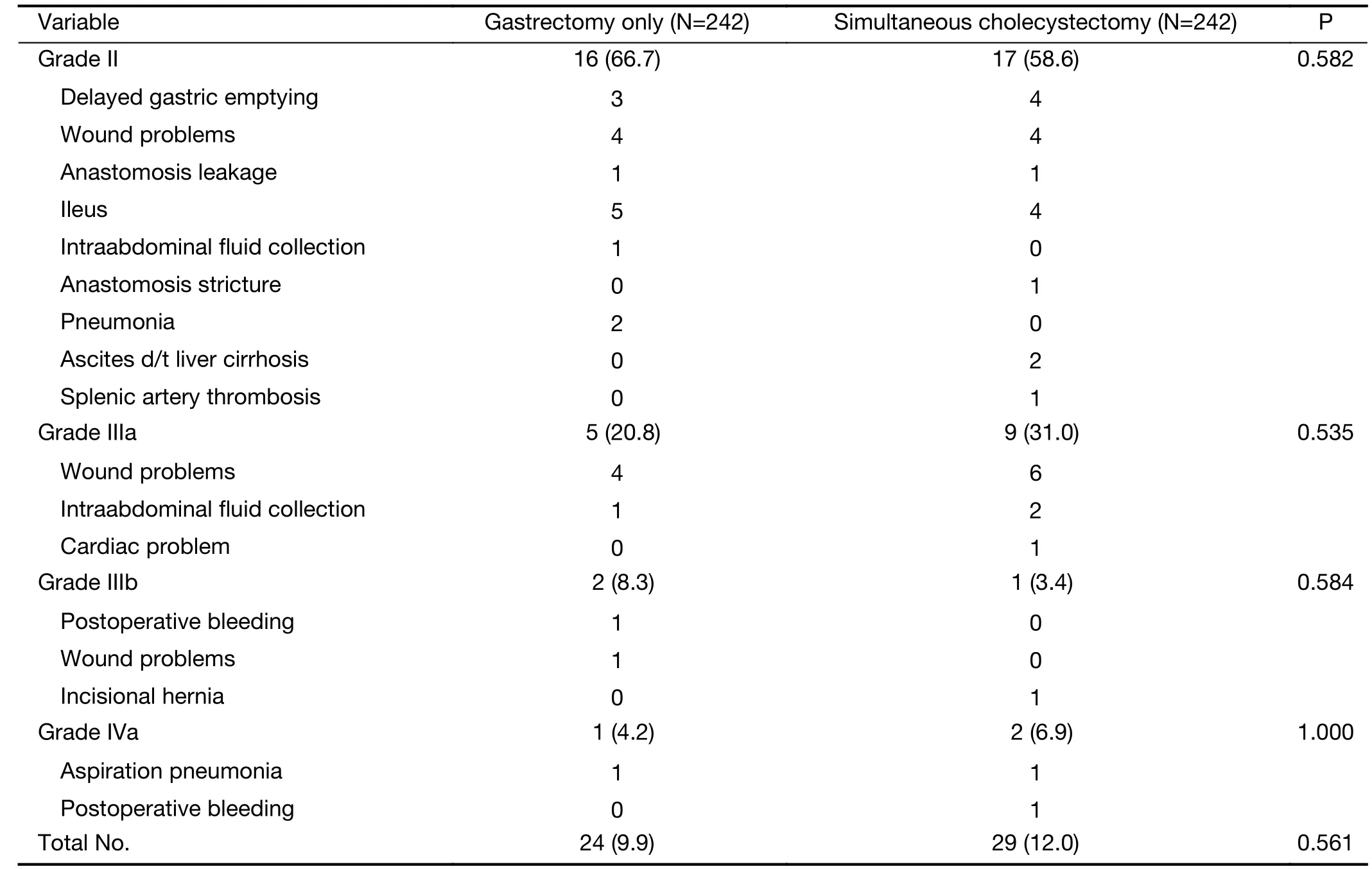
Table 3 Postoperative Clavien-Dindo grade II-IV complications
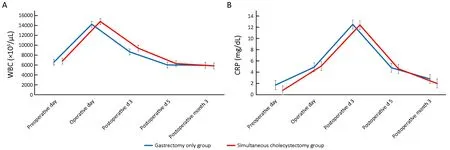
Figure 1 Postoperative inflammatory response. (A) WBC count; (B) CRP level. WBC, white blood cell; CRP, C-reactive protein.
Discussion
To our knowledge, previously, no report has determined whether adding cholecystectomy affects postoperative nutrition in patients with gastric cancer; our study showed that there was no relationship between adding cholecystectomy during gastrectomy and postoperative nutritional status in patients with gastric cancer. Although the cholesterol level was lower in the simultaneous cholecystectomy group than in the gastrectomy only group,their levels were within normal range.
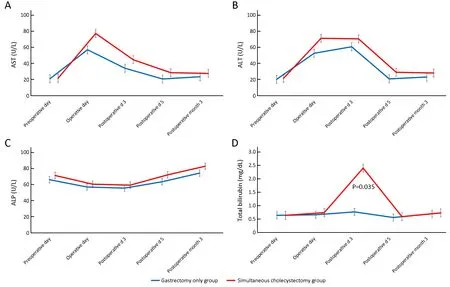
Figure 2 Changes in liver function-related markers. (A) AST level; (B) ALT level; (C) ALP level; (D) Total bilirubin level (P=0.035). AST,aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
Prophylactic cholecystectomy during gastric cancer surgery has been a controversial issue. Rapid weight loss followed by gastric resection and decreased gallbladder motility and biliary stasis by vagotomy can increase the risk of gallbladder stone formation and acute cholecystitis(15-17). Therefore, several studies have argued that prophylactic cholecystectomy should be added to gastrectomy because it would be more beneficial considering postoperative adhesion (16-19). However,some surgeons worry that concomitant cholecystectomy could increase the occurrence of intraoperative or postoperative morbidity (20-22). Considering that only 6%of patients with asymptomatic gallbladder stones subsequently undergo cholecystectomy because of symptomatic cholecystitis after gastrectomy (16,17,20),prophylactic cholecystectomy seems to be overtreatment in these patients.
In the present study, we did not aim to argue whether prophylactic cholecystectomy is necessary during gastric cancer surgery. Instead, we wanted to confirm the perioperative surgical safety and to assess the nutritional outcome after gastrectomy with cholecystectomy in gastric cancer patients with gallbladder lesions. Our study showed that there was no significant difference in intraoperative and postoperative complications between the simultaneous cholecystectomy group and gastrectomy only group using propensity score matching. This finding was in line with that of a recent prospective, multicenter Cholegas study,which showed that prophylactic cholecystectomy did not increase postoperative complications (5). Although the operative time was longer and blood loss was higher in the simultaneous cholecystectomy group than in the gastrectomy only group, the duration of hospital stay and postoperative inflammatory response were similar between the two groups. The rapidly increased levels of liver enzymes in the cholecystectomy group were normalized by postoperative d 5. These results mean that additional cholecystectomy did not delay postoperative recovery following gastric cancer surgery.

Figure 3 Changes in biochemical markers related to postoperative nutritional state. (A) Weight; (B) Value of hemoglobin; (C) Value of absolute lymphocyte count; (D) Value of protein; (E) Value of albumin; (F) Value of cholesterol; (G) Value of fasting glucose; (H) PNI.PNI, prognostic nutritional index.
Nutrition is another important issue of gastric cancer surgery. Most patients with gastric cancer experience postoperative weight loss and nutritional deficiency after gastrectomy (1,3). Because the postoperative nutritional status is related to quality of life and survival, the significance of nutritional aspects is becoming increasingly important in patients with gastric cancer who undergo gastrectomy (6,9-11). Therefore, physicians and patients make efforts to decrease nutritional loss postoperatively. If additional cholecystectomy is associated with further nutritional loss or weight loss, the argument for performing prophylactic cholecystectomy in patients with asymptomatic gallbladder stones would be weak.
Although most patients with cholecystectomy are diagnosed as having benign diseases, 2 patients were diagnosed as having gallbladder cancer based on frozen biopsy results, and 1 patient underwent radical cholecystectomy. In patients with a mass-like lesion in the gallbladder, cholecystectomy is essential, and tissue confirmation by frozen biopsy should be performed intraoperatively.
The limitation of this study was that there were no data on the incidence of gallbladder stones and subsequent cholecystectomy in the gastrectomy only group. Moreover,the incidence of perioperative events and complications in those who underwent subsequent cholecystectomy was not evaluated. Because of severe adhesion and intraperitoneal anatomic change after primary gastric cancer surgery,subsequent cholecystectomy would be expected to be more difficult than simultaneous cholecystectomy. Several previous studies have reported a few intraoperative events and postoperative complications and a longer operative time in cases of subsequent cholecystectomy after gastrectomy (18,19). Although propensity score matching was used to overcome the limitation of the retrospective study design, we could not deliver concrete results that could be achieved by conducting a prospective, randomized controlled trial.
Conclusions
Simultaneous cholecystectomy during gastric cancer surgery showed similar short-term surgical outcomes to gastrectomy only, and patients’ postoperative nutritional status was similar with both procedures. In gastric cancer patients with gallbladder disease, simultaneous cholecystectomy is safe and is not associated with additional nutritional loss.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Cancer IgG, a potential prognostic marker, promotes colorectal cancer progression
- Correlation of radiotherapy with prognosis of elderly patients with hormone receptor-positive breast cancer according to immunohistochemical subtyping
- Impact of crizotinib on long-term survival of ALK-positive advanced non-small-cell lung cancer: A Chinese multicenter cohort study
- Burden of colorectal cancer in China, 1990-2017: Findings from the Global Burden of Disease Study 2017
- Hexokinase II promotes the Warburg effect by phosphorylating alpha subunit of pyruvate dehydrogenase
- Mutant p53 increases exosome-mediated transfer of miR-21-3p and miR-769-3p to promote pulmonary metastasis
