Hepatocellular carcinoma: Mechanisms of progression and immunotherapy
2019-07-12YuJiangQiuJuHanJianZhang
Yu Jiang, Qiu-Ju Han, Jian Zhang
Abstract Liver cancer is one of the most common malignancies, and various pathogenic factors can lead to its occurrence and development. Among all primary liver cancers, hepatocellular carcinoma (HCC) is the most common. With extensive studies, an increasing number of molecular mechanisms that promote HCC are being discovered. Surgical resection is still the most effective treatment for patients with early HCC. However, early detection and treatment are difficult for most HCC patients, and the postoperative recurrence rate is high, resulting in poor clinical prognosis of HCC. Although immunotherapy takes longer than conventional chemotherapy to produce therapeutic effects, it persists for longer.In recent years, the emergence of many new immunotherapies, such as immune checkpoint blockade and chimeric antigen receptor T cell therapies, has given new hope for the treatment of HCC.
Key words: Hepatocellular carcinoma; Mechanisms; Immunotherapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide in 2018,accounting for approximately 841,000 new cases and 782,000 deaths annually. Primary liver cancer includes HCC and intrahepatic cholangiocarcinoma as well as other rare types, with HCC accounting for 75%-85% of cases[1,2]. The main risk factors for HCC are chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV),aflatoxin-contaminated foodstuffs, heavy alcohol intake and type 2 diabetes[2]. Studies on the mechanisms of HCC processes have confirmed that the inactivation of multiple tumor suppressor genes (such as p53), abnormal activation of oncogenes (K-ras, etc.,)and multiple signaling pathways (PI3K, MAPK, JAK/STAT, NF-κB, Wnt/β-catenin,etc), abnormal regulation of epigenetic events (such as microRNAs), and even exosomes that deliver a large number of protumorigenic molecules are all involved in HCC development and progression[3]. The liver also acts as a special immune organ.In addition to the above carcinogenic factors, the immunological microenvironment in the liver is associated with HCC occurrence and development. In recent years, the interaction between various immune cells and tumor cells has attracted extensive attention. Many molecular mechanisms associated with the biological characteristics of tumor cells during hepatocarcinogenesis also have important effects on the immune system. Although improvements have been made in surgery, radiofrequency ablation and chemotherapy for HCC, the prognosis of HCC patients remains unsatisfactory due to the high rates of recurrence and metastasis. The emergence of many new immunotherapies, such as immune checkpoint blockade and chimeric antigen receptor (CAR) T-cell therapies, has given new hope for the treatment of HCC. Here, we review the molecular mechanisms that influence HCC progression and the latest advancements in immunotherapy by combining the latest research progress and results from our laboratory.
MOLECULAR MECHANISMS UNDERLYING HCC
The promotive effect of HBV on HCC
Chronic hepatitis caused by HBV infection is one of the main causes of HCC.Numerous studies have confirmed that HBV can activate a variety of signals to promote viral replication and inflammation progression and to accelerate hepatocarcinogenesis[4-7].
HBx and HCC:We also found that HBV further promoted viral replication by activating signal transducer and activator of transcription 3 (STAT3) signaling in HBV+HCC, while blocking STAT3 can inhibit HBV replication and proliferation and angiogenesis in HBV+HCC[8]. The HBV genome encodes four proteins, including the envelope protein (S/Pre-S), the core protein (C/pre-C), the polymerase (P), and the X protein (HBx). Among them, the multifunctional HBx protein has attracted substantial attention. HBx can block p53-mediated apoptosis and activate numerous signal transduction cascades (STAT, NF-κB, AP-1, etc.,) associated with cell proliferation and survival, promoting HCC occurrence and development[9-13]. HBx mutations in HCC patients haven been shown to be important for the development of HCC[14-16].
HBV and microRNAs:A large number of recent studies have shown that microRNAs play important roles in the occurrence and development of HBV-related HCC.Through the analysis of microRNA profiles, the expression levels of various micro-RNAs, such as miR-150 and miR-342-3p, were found to be changed in HBV-related HCC[17]. The analysis of a large number of clinical samples showed that microRNAs such as miR-375, miR-25, and let-7f are specific for HBV and have potential clinical value for the prediction and diagnosis of HBV+HCC[18-20]. Further studies have demonstrated that HBV promotes HCC by intervening with Wnt, MAPK, Notch and other signaling pathways through different microRNAs[17,21-24](Figure 1). However,miR-122 expression is inhibited in HBV+HCC, which suggests that this microRNA likely plays an inhibitory role in HCC progression[25]. Mao et al[26]found that the tolerance of HBV+HCC patients to sorafenib was significantly higher than that of non-HBV-infected HCC patients, which was related to activated Mcl-1-mediated inhibitory effects on miR-193b, and restoration of miR-193b expression could increase the sensitivity of HBV+HCC to sorafenib. These phenomena indicate that the role of microRNAs in the progression of HBV-related HCC is complex and not simply promotive or suppressive.
HBV and immune tolerance:In addition to the above mechanisms, HBV-induced suppressive effects on innate and adaptive immune cells promote the evolution from inflammation to tumorigenesis. In patients with chronic HBV hepatitis, the activation and function of natural killer (NK) cells are significantly inhibited, and these impaired NK cells cannot effectively clear HBV, which further accelerates the progression of hepatitis to HCC[27,28]. The TGF-β-miR-34a-CCL22 signal induces regulatory T (Treg)cell infiltration and promotes the metastasis of HBV+HCC[29], and the imbalance between helper T (Th)-17 and Treg cells is a risk factor for patients with HBV infection progressing to HCC[30]. In the course of chronic HBV infection, the expression of programmed death-1 (PD-1), cytotoxic T lymphocyte antigen-4 (CTLA-4), CD244 and other inhibitory receptors on virus-specific CD8+T cells is increased, which mediates T cell depletion[27,31]. Recently, Zong et al[32]showed that in HBsAg-transgenic mice, the expression of TIGIT, a promising immune checkpoint in tumor immunotherapy,main-tained the tolerance of CD8+T cells to HBV, and disrupting this tolerance by TIGIT blockade or deficiency could induce chronic hepatitis in HBsAg-transgenic mice and eventually lead to HCC development, suggesting that immune checkpoint therapy in HBV carriers might increase the risk of chronic hepatitis and liver cancer.
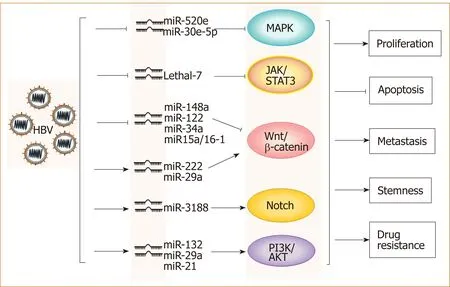
Figure 1 Hepatitis B virus promotes hepatocellular carcinoma by intervening various signal pathwaysthrough different microRNAs. Lines ending with arrows or bars indicate promotion or inhibitory effects, respectively.HBV: Hepatitis B virus.
To date, the role of HBV in HCC progression has been widely studied, providing new ideas for the effective prevention and treatment of HCC.
STAT3 and related signaling pathways
As an important member of the STAT family, STAT3 is constitutively activated in many tumor and immune cells in the tumor microenvironment. The abnormal activation of STAT3 signaling is closely related to the occurrence, proliferation, drug resistance and stemness of various tumors, including HCC. STAT3 also plays an important role in the regulation of the complex network formed in the tumor microenvironment[33-36].
STAT3 and microRNAs: STAT3 deficiency prevents hepatocarcinogenesis in the thioacetamide-induced liver injury model[37]. Constitutive activation of STAT3 is observed in HCC cells and tissues, and STAT3 decoy oligodeoxynucleotides (STAT3-decoy ODN) can specifically block the activation of STAT3 signaling in HCC,resulting in the inhibition of proliferation and apoptosis in tumor cells[38]. MicroRNAs such as miR-589-5p and miR-500a-3p maintain the drug resistance and stemness of HCC by activating STAT3 signaling[39-41]. However, some microRNAs have been found to inhibit tumor development by interfering with STAT3. MiR-345 upregulation has been shown to inhibit epithelial-mesenchymal transition (EMT) in HCC by targeting interferon regulatory factor 1 (IRF1)-mediated mTOR/STAT3/AKT signaling[42].Furthermore, miR-451 may function as a potential suppressor of tumor angiogenesis in HCC by targeting IL-6R/STAT3/VEGF signaling, indicating a promising therapeutic strategy for HCC[43]. Although different microRNAs have different effects on HCC, STAT3 plays a key role in the regulation of microRNA signaling during hepatocarcinogenesis.
TLR4 and STAT3:The relationship between inflammation and tumors has been well established[44]. Approximately 70% of the blood supply to the liver comes from the outflow of intestinal veins, and the presence of the hepato-intestinal axis makes the liver the first line of defense against enterogenous antigens. Pathogen-associated molecular patterns (PAMPs) derived from the intestinal microbiota play a regulatory role in liver diseases by activating Toll-like receptors (TLRs). In a liver injury-cancer model induced by a combination of diethylnitrosamine (DEN) and the hepatotoxin carbon tetrachloride (CCl4), TLR4-/-mice showed a significant decrease in tumor number and volume formation compared to wild-type mice. Moreover, intestinal microbiota-derived lipopolysaccharides (LPS) can activate TLR4 signaling in hepatocytes to promote inflammation-induced hepatocarcinogenesis[45]. We also found that TLR4 was constitutively expressed in HCC, and further study demonstrated that TLR4 promoted HCC occurrence and progression depending on the activation of the Cox-2/PGE2/STAT3 axis and was associated with multiple drug resistance[46].Significantly, sorafenib can inhibit HCC by blocking TLR4/STAT3/SUMO1 activation[47].
STAT3 and the tumor microenvironment:With advancements in research, the occurrence and development of tumors is thought to arise due to not only the deterioration and proliferation of tumor cells, but also the immunosuppressive tumor microenvironment. As a key transcription factor, STAT3 is constitutively activated in both tumor cells and immune cells in the microenvironment (Figure 2). We found that blocking STAT3 in HCC cells could effectively disrupt tumor-induced immune tolerance and induce an antitumor reaction in tumor-bearing mice, which might be related to the downregulation of transforming growth factor-beta (TGF-β) and interleukin(IL)-10 and the upregulation of type I interferons (IFNs)[48,49]. Alternatively,STAT3 could directly regulate miR-146a expression to upregulate the expression of TGF-β, IL-17 and VEGF and downregulate the expression of type I IFNs, mediating the inhibitory effect of NK cells on HCC[50]. More importantly, STAT3 could inhibit the Th1 immune response and promote the formation of an immunosuppressive microenvironment[51,52]. Studies have shown that tumor-associated macrophages (TAMs)promote HCC progression by secreting cytokines such as IL-8 and IL-6 to activate STAT3 in HCC[53,54]. HCC-associated fibroblasts induce the activation of STAT3 pathways in neutrophils and dendritic cells (DCs), and these STAT3-over-activated neutrophils and DCs display protumorigenic roles[55,56]. Blocking STAT3 activation in immune cells such as TAMs and DCs can inhibit HCC progression[56,57]. Meanwhile,blocking STAT3 not only inhibits HCC proliferation but also upregulates NKG2D ligand expression, such as ULBPs and MICA/B, in HCC cells to increase the sensitivity of HCC to NK cell-mediated cytolysis and enhance the anti-HCC activity of NK cells[48,49].
In summary, STAT3 signaling can interact with multiple pathways to promote HCC. STAT3 plays an important role in both the maintenance of HCC malignancy and the suppression of the immune microenvironment, which makes STAT3 an ideal target for HCC treatment. The development of STAT3 inhibitors used for clinical application is an attractive research topic. STAT3 inhibitors, including AZD9150 and TTI-101, have entered the clinical trial phase for HCC treatment (ClinicalTrials.gov identifier: NCT03195699 and NCT01839604, respectively). Several drugs, which were not initially applied for tumor treatment, have also been found to exert anticancer effects by blocking STAT3[58-60]. Thus, the identification of new STAT3-targeted inhibitors is still an important direction for drug development.
Homeobox genes
Homeobox genes were first discovered in the fruit fly Drosophila, which are divided into many subfamilies (Hox, PAX, NKX, etc.,) on the basis of the level of similarity among them[61]. Homologous homeobox genes have been found in mammals. Most homeobox genes are involved in regulating the expression of genes related to embryonic development and cell differentiation. Mutations in these genes can lead to abnormal organ development in eukaryotes[61-64]. In addition, in recent years, a variety of homeobox genes have been found to be involved in the occurrence and development of tumors[64,65], and different homeobox genes have been shown to play different roles in the progression of cancer such as HCC.
Hox and HCC:The Hox gene, an important member of the homeobox family, is abnormally expressed in multiple malignant solid tumors[65]. The role of Hox has also been studied in HCC over the years. Among HOX genes, HOXA13 has been reported to be the most deregulated in HCC. HOXA13 overexpression in HCC cell lines results in increased colony formation and migration but reduced sensitivity to sorafenib[66-68].Knockdown of endogenous HOXA7 results in decreased proliferation of HCC cells by inhibiting cyclin E1/cyclin-dependent kinase-2[69]. HOXB7 can promote EMT and stemness formation by upregulating the expression of c-Myc and Slug in HCC[70].
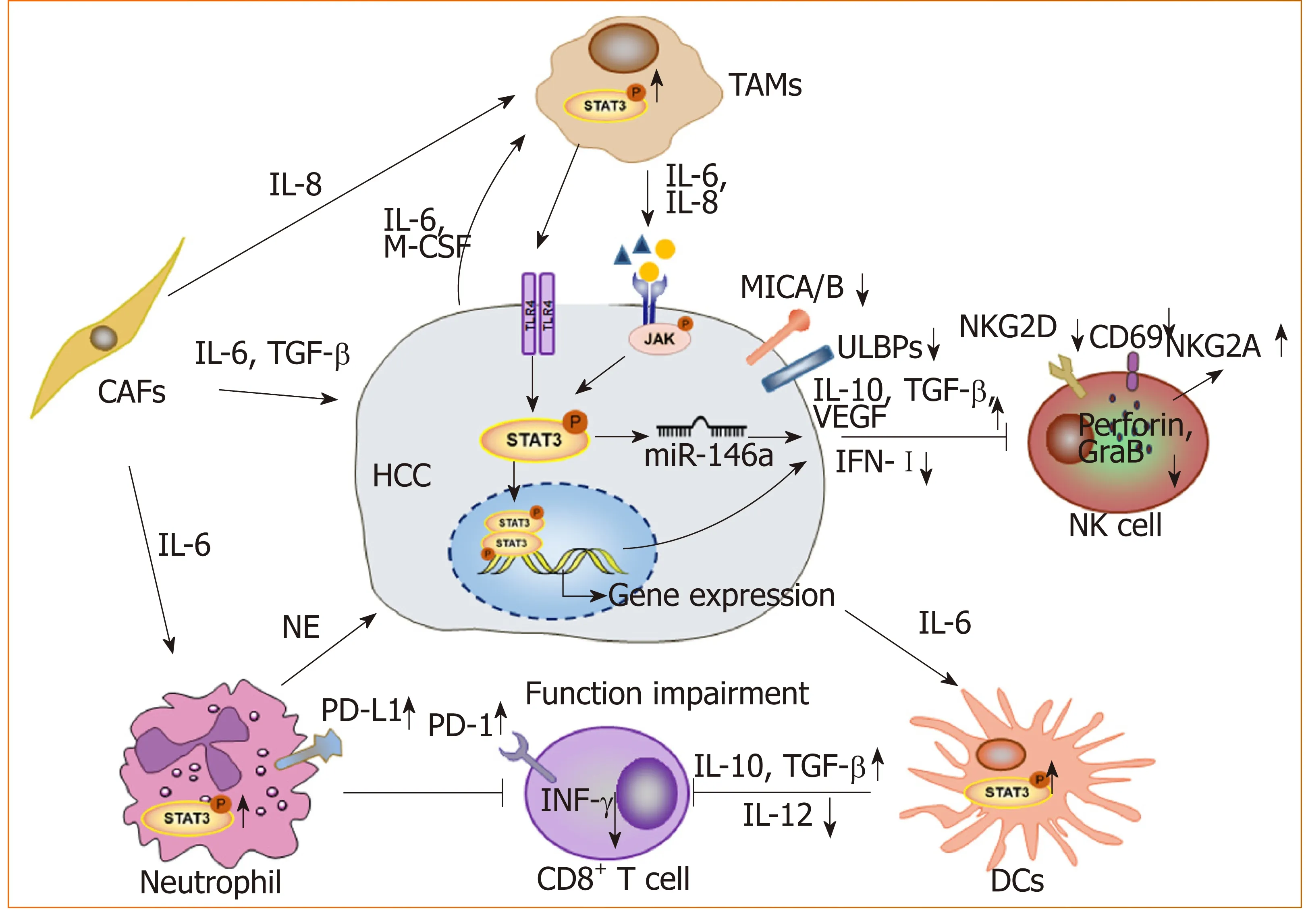
Figure 2 STAT3 signaling contributes to form an immunosuppressive microenvironment in hepatocellular carcinoma. Long lines ending with arrows or bars indicate activating or inhibitory effects, respectively. Short arrows pointing up or down indicate up-regulated or down-regulated, respectively. TAMs: Tumor-associated macrophages;CAFs: Cancer-associated fibroblasts; NK cell: Natural killer cell; DCs: Dendritic cells; NE: Neutrophil elastase; HCC:Hepatocellular carcinoma.
HMBOX1 and HCC: Homeobox containing 1 (HMBOX1), a novel human homeobox gene, was first isolated from the human pancreatic cDNA library. HMBOX1 belongs to the HNF homeobox class of the homeobox family[63,71]. The expression of HMBOX1 is reported to be up- or downregulated in some tumors[72-75]. Our previous study revealed that the expression level of HMBOX1 in liver cancer was lower than that in adjacent noncancerous tissues[74]. Further study showed that HMBOX1 expression was negatively correlated with the differentiation and clinical stage of HCC, and HMBOX1 overexpression could inhibit HCC by promoting autophagy, inhibiting the cancer stem cell phenotype and increasing tumor cell sensitivity to NK cell-mediated cytolysis. The underlying mechanisms may be related to changes in the expression of Fas and programmed cell death ligand 1 (PD-L1) in HCC cells mediated by HMBOX1 overexpression[76]. Additionally, HMBOX1 expression in hepatocytes has been shown to prevent inflammation and liver injury by reducing macrophage infiltration and acti-vation, thereby blocking inflammation-related tumor development[77].
Other homeobox genes and HCC: Prospero-related homeobox 1 (PROX1) is closely related to proliferation, differentiation and prognosis in HCC. PROX1 can upregulate IL-8 expression and activate NF-κB and β-catenin signals to promote HCC angiogenesis and sorafenib resistance[78-80]. NK3 homeobox 1 (NKX3.1) was initially found to play an important role in the regulation of prostate development and tumorigenesis[81]. Recently, NKX3.1 expression was shown to be decreased in HCC tissues,and NKX3.1 overexpression induced G1/S phase arrest in HCC cells through upregulation of FOXO1[82]. HLXB9 is highly expressed in poorly differentiated HCC samples[83].
From the above, different homeobox genes exhibit different functions via related mechanisms to promote or inhibit HCC progression (Table 1). Because the homeobox family has a large number of genes, its role in HCC and other tumors remains to be further explored.
Wnt signaling
As a highly conserved signaling pathway during biological evolution, the Wnt signaling pathway plays an important role in a variety of physiological and pathological processes[84]. With advancements in research, the regulatory role of the Wnt signaling pathway in cancer progression and the emergence of stemness has attracted widespread attention. According to the different mechanisms of signal tran-sduction,the Wnt pathway is generally divided into the canonical Wnt/β-catenin and noncanonical β-catenin-independent pathways.
Wnt signaling and HCC:Aberrant activation of the Wnt/β-catenin signaling pathway has been observed in HCC patients, and various molecules, such as the protein components of HBV and HCV, as well as hypoxia-induced factor (commonly known as HIF), can activate the Wnt/β-catenin pathway in HCC[5,85-88]. The activation of Wnt/β-catenin signaling is closely related to the occurrence and development of HCC, the formation of stemness and drug resistance[79,89-91](Figure 3). In addition, Wnt signals regulate HCC progression by interacting with Hippo and Notch signaling pathways[92-94]. In addition to mutations in CTNNB1, AXIN1 and other related genes[95-98], epigenetic regulation is involved in the aberrant activation of the Wnt signaling pathway. For instance, the long noncoding RNA lncTCF7 promotes stemness and dissemination in HCC by activating Wnt signaling[99]. In HBV-related HCC,HBx silences secreted frizzled-related proteins (SFRPs) by mediating DNA methylation to activate Wnt signaling[100]. Many microRNAs regulate the activation of Wnt signaling at the posttranscriptional level to affect HCC progression. For example,miR-542-3p can target the frizzled 7/Wnt signaling pathway to inhibit HCC[101], while Octamer 4/miR-1246 promotes stemness by inhibiting AXIN2 and GSK3 and thereby activating Wnt/β-catenin signaling in HCC[102].
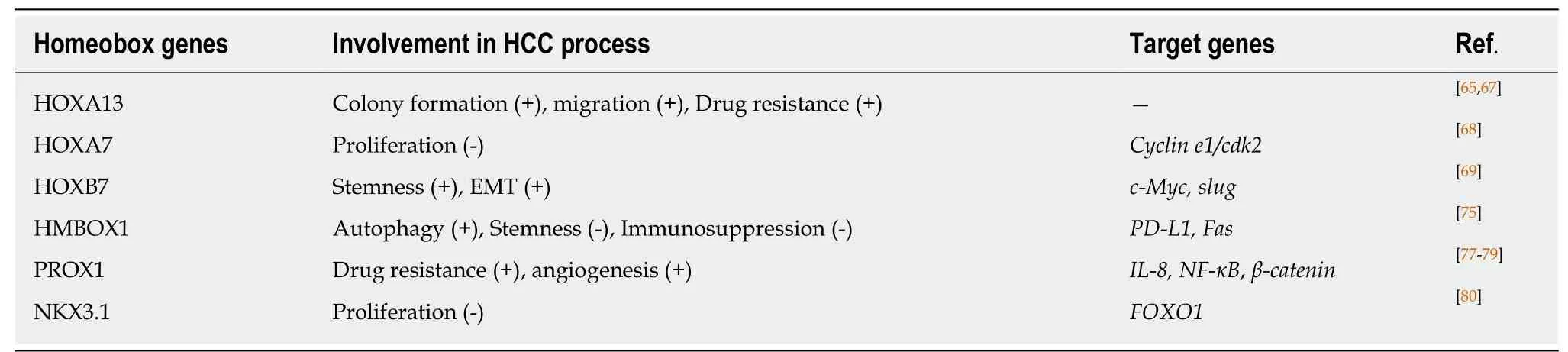
Table 1 Homeobox genes show different roles in the progression of hepatocellular carcinoma
Wnt signaling and the tumor microenvironment:In addition to the effect on tumor cells themselves, Wnt signaling has recently been found to play an important role in the formation of a tumor immunosuppressive microenvironment. β-catenin activation in DCs can inhibits the process of antigen cross presentation at CD8+T cells[103,104]and participates in the differentiation and activation of Treg cells[105]. Wnt/β-catenin activation in TAMs facilitates M2 polarization, which promotes HCC[106]. TAMs also activate β-catenin by secreting CCL17 to promote EMT in HCC[107]. Although many studies have demonstrated that Wnt activation plays an immunosuppressive role, the mechanism of Wnt activation in the tumor microenvironment remains to be further explored. A better understanding of the role of Wnt signaling in HCC progression is thus essential for the prevention and treatment of HCC.
Role of exosomes in HCC
Exosomes can be released by all cell types, including cancer cells and immune cells,and play important roles in intercellular communication[108]. As carriers and transporters, exosomes deliver a variety of biological molecules, including proteins, lipids,and nucleic acids. Exosomes have been shown to play important roles in most cancerassociated processes.
Exosomes promote HCC: We have reported that exosomes present in the sera of chronic hepatitis B patients contain both HBV-derived nucleic acids and HBV proteins and can transfer HBV to hepatocytes in an active manner. Moreover, exosomes mediate the transmission of HBV into NK cells, resulting in the impairment of NK cell functions[109]. This may contribute to the progression of chronic HBV infection to HCC.Exosomes derived from metastatic HCC cell lines carry a large number of protumorigenic RNAs and proteins, such as MET protooncogenes and S100 family members. These exosomes promote metastasis by triggering the PI3K/AKT and MAPK signaling pathways in hepatocytes and increasing the secretion of active matrix metalloproteinase (MMP) 2 and MMP-9[110].
Exosomes and chemoresistance:HCC is highly resistant to chemotherapy. Qu et al[111]found that exosomes derived from HCC cells can induce sorafenib resistance by activating the HGF/c-Met/Akt signaling pathway. Moreover, exosomes derived from highly invasive HCC cells have greater efficacy than exosomes derived from less invasive cells[111]. Exosomal miR-32-5p can induce multidrug resistance in HCC by activating the PI3K/Akt pathway[112]. Therefore, HCC cell-derived exosomes might be an important target for reversing chemoresistance.
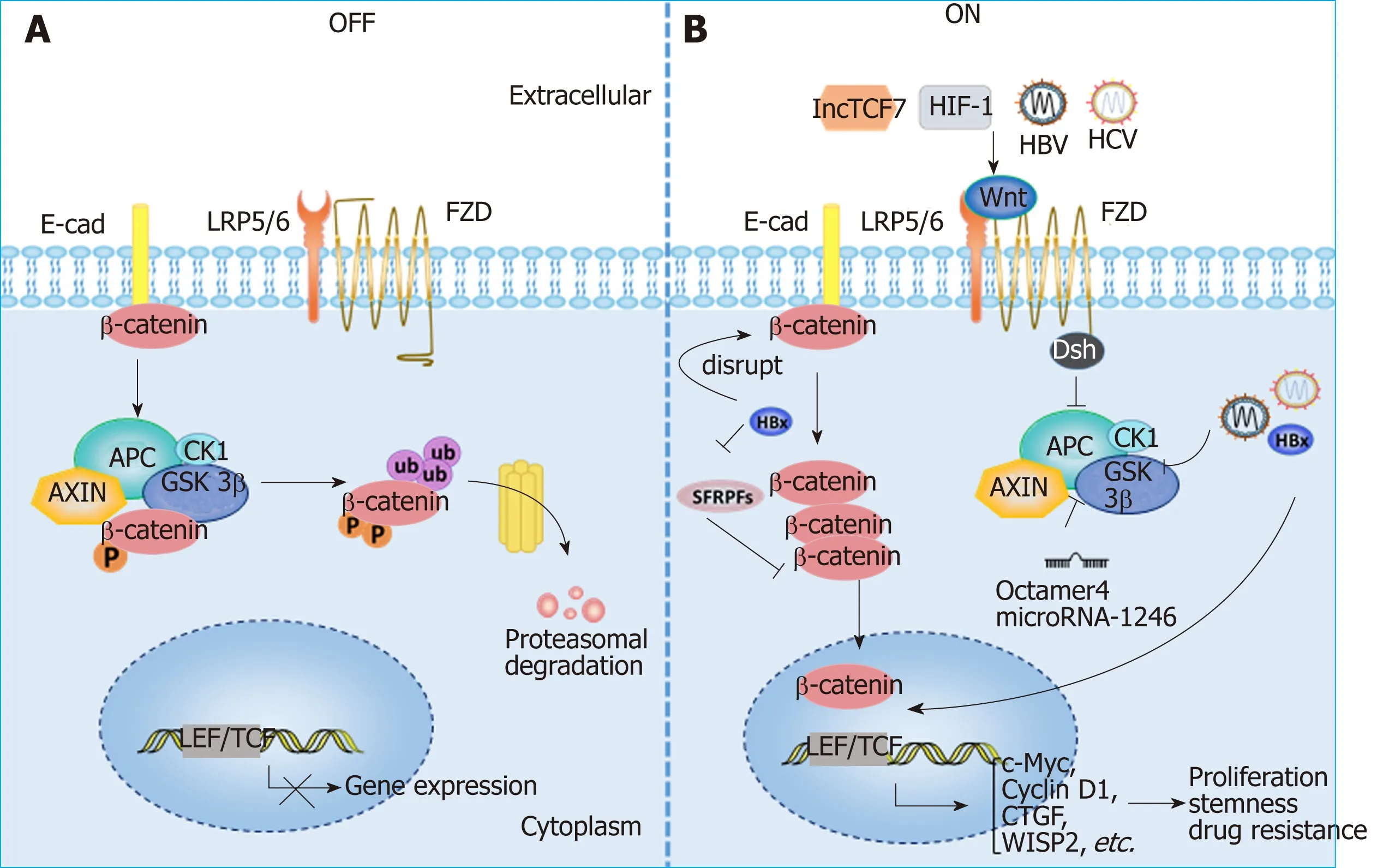
Figure 3 Aberrant activation of the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. A: Wnt signaling is inactive in the absence of Wnt ligands(OFF); B: Wnt signaling can be activated by various molecules in HCC (ON). HBV and HCV can active Wnt/β-catenin signaling by activating TCF or inhibiting GSK3β;HBx can silence SFRPs to activate Wnt signaling; LncTCF7 triggers Wnt7a and TCF7 expression to activate Wnt signaling. Lines ending with arrows or bars indicate activating or inhibitory effects, respectively. HIF1α: Hypoxia-inducible factor 1α; LEF: Lymphoid enhancer-binding factor; LRP: Low-density lipoprotein receptor-related protein; TCF: T cell factor; FZD: Frizzled; E-cad: E-cadherin; SFRPs: Secreted frizzled-related proteins; CTGF: Connective tissue growth factor; WISP2: Wnt1 inducible signaling pathway protein 2.
Exosomes and the tumor microenvironment:As carriers, exosomes play an important role in cell-cell interactions in the tumor microenvironment. Anticancer drugs induce the release of exosomes containing heat shock proteins (HSPs) from HCC cells,which elicit effective NK cell-mediated antitumor responses[113]. However, 14-3-3ζ proteins delivered by exosomes can be transmitted from HCC cells to tumorinfiltrating T cells, impairing the functions, proliferation and activation of T cells[114].Additionally, HCC-derived exosomes containing lncRNA TUC339 can be taken up by macrophages to play important roles in macrophage activation and regulation of M1/M2 polarization[115]. Additionally, highly metastatic HCC cell-secreted exosomal miR-1247-3p directly targets B4GALT3, leading to the activation of β1-integrin-NF-κB signaling in fibroblasts, which converts normal fibroblasts to cancer-associated fibroblasts. Activated cancer-associated fibroblasts further promote cancer progression. Clinical data also show that high serum exosomal miR-1247-3p levels are correlated with lung metastasis in HCC patients[116].
Therefore, exosomes play important roles in the development of HCC (Figure 4).Nevertheless, more studies on how exosomes mediate HCC progression are still needed to promote the clinical utilization of exosomes.
HCC IMMUNOTHERAPY
Clinical treatment of HCC includes liver transplantation, surgical resection,chemotherapy, radiotherapy, interventional therapy and immunotherapy. Liver transplantation is the only treatment option for HCC patients with unresectable tumors or cirrhosis[117]. The 5-year survival rates can reach 60%-70% in patients with HCC after liver transplantation[118]. However, the number of patients that need liver transplantation exceeds the number of available donor organs[119]. Therefore, HCC patients must be selected very carefully in terms of tumor size and number of tumor nodules[120]. Surgical resection of early HCC is still the first choice for treatment, but the recurrence rate within five years is as high as 70%[2]. The multikinase inhibitor sorafenib is recognized as the most effective molecular targeted drug for the treatment of advanced HCC worldwide. Despite advancements in molecular therapy with the multikinase inhibitor sorafenib, the prognosis of advanced HCC cases remains poor,with five-year survival rates of 3%-11%[121]. The immune system plays a key role in controlling and eradicating cancer. Therefore, immunotherapy has received much attention in recent years. Additionally, although immunotherapy takes longer than conventional chemotherapy to produce a therapeutic effect, it persists for longer. Earlier immunotherapy mainly included cytokine-mediated immunotherapy, oncolytic virus therapy, TLR agonist therapy and DC vaccine. In recent years, emerging immunotherapies, such as immune checkpoint blockade and CAR T cell therapies, have shown better therapeutic effects on some tumors, thus giving us new hope for the treatment of HCC.
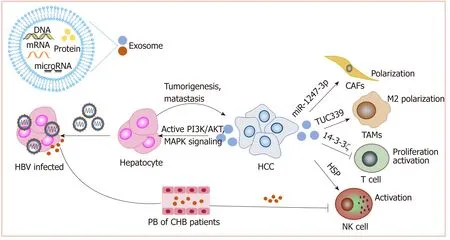
Figure 4 Exosomes play important roles in the development of hepatocellular carcinoma. Exosomes deliver a variety of biological molecules that have been proven to play important roles in hepatocellular carcinoma progression and immunosuppression. Lines ending with arrows or bars indicate activating or inhibitory effects, respectively. HCC: Hepatocellular carcinoma.
Immune checkpoint blockade
Checkpoint blockade, currently the top candidate in immunotherapy, has been shown to be effective for the treatment of many cancers, especially for chemotherapy resistant malignant tumors. Among the available immune checkpoint inhibitors, CTLA-4 and PD-1 display the most pronounced effects and have shown remarkable efficacy in the treatment of malignant melanoma[122,123]. Nivolumab, pembrolizumab (PD-1 inhibitor) and tremelimumab (CTLA-4 inhibitor) have been demonstrated to be safe and effective in clinical trials[124-126], and nivolumab has been approved by the U.S.Food and Drug Administration (commonly known as the FDA) as a second-line treatment for HCC[127].
Although good results have been achieved in the treatment of HCC with checkpoint blockade, the response rate to the treatment is relatively low due to the formation of an immunosuppressive microenvironment. The combination of checkpoint blockade inhibitors and other methods may enhance the efficacy[128,129].Recently, Zhou et al[130]found that the combined use of checkpoint inhibitors and myeloid-derived suppressor cell infiltration blockers can augment the therapeutic effect of anti-PD-L1 antibody in HCC. The use of tremelimumab combined with radio-frequency ablation to treat advanced HCC promoted the accumulation of CD8+T cells in tumor tissues, suggesting that this combined treatment might be a new therapeutic method[131]. The combined use of anti-CTLA-4 antibodies, anti-PD-L1 antibodies and the histone deacetylase inhibitor belinostat completely eliminated tumor load in HCC tumor-bearing mice[132]. An increasing number of researchers are investigating the therapeutic effect of combination therapy when immune checkpoint blockade alone is not effective. Thus, combined therapy may be a new strategy for the future application of checkpoint blockade in solid tumors, including liver cancer, but large amounts of clinical trial data are still needed to support the development of specific treatment strategies.
Adoptive transfer of genetically modified lymphocytes
Adoptive cell transfer is the most representative tumor immunotherapy at present,and it is mediated by cytolytic activity against tumor cells by the transfer of lymphocytes from the patient themselves or from donors. Before the emergence of CAR T cells, adoptive transfer therapy in HCC mainly focused on tumor-infiltrating lymphocytes and cytokine induced killer cells[133-135]. With the recent discovery of CAR T cells and their amazing therapeutic effect on hematological tumors[136], the effect of CAR T cell therapy in solid tumors such as HCC has attracted increasing attention.
CAR T cells:CARs provide T cells with the ability to directly recognize tumor antigens independent of the human leukocyte antigen. This allows CAR engineered T cells to recognize a wider range of targets than natural T cells. At present, the most widely used CAR structure consists of a single-chain antibody extracellular domain that recognizes and binds specific antigens, an extracellular hinge region, a transmembrane region, and an intracellular domain that provides proliferation and activation signals. Throughout the entirety of the immunotherapy process, the design and integration of CARs into T cells to generate CAR T cells are the most critical steps[137]. Multiple studies have demonstrated that glypican 3 (GPC3) is an attractive liver cancer-specific target, because its expression is high in HCC tissues but limited in normal tissues[138]. GPC3-specific CAR T cell therapy for HCC exhibits a strong killing effect on GPC3-positive HCC cells both in vivo and in vitro[139]. Furthermore, a relevant phase 1 clinical trial study (ClinicalTrials.gov identifier: NCT02395250) showed that autologous T cells bearing GPC3-specific CARs were safe and effective in patients with relapsed or refractory HCC. Meanwhile, another phase 1 clinical trial(ClinicalTrials.gov identifier: NCT02541370) involving CD133-directed CAR T cells for advanced HCC demonstrated feasibility, controllable toxicities and effectiveness[140].However, immunosuppressive microenvironments can hinder the infiltration of CAR T cells into tumor tissues, thereby reducing CAR T cell-mediated antitumor effects[141].In-terestingly, Guo et al[142]showed that further inhibition of PD-1 expression in GPC3-specific CAR T cells can enhance the killing effect of CAR T cells on HCC cells.
Treatment of HCC based on NK cells:Similar to T cells, NK cells can be modified with CARs that recognize antigens expressed by tumors and combine with signaling components that enhance NK cell activity. At present, clinical studies on CAR NK cells mostly focus on the treatment of lymphoma and hematological tumors, and only a few studies exist regarding the treatment of solid tumors such as HCC. Yu et al[143]found that GPC3-specific CAR NK cells constructed with NK-92 cells could effectively inhibit proliferation and promote apoptosis in HCC cells. Furthermore, CAR NK cells display lower toxicity than CAR T cells and do not need patient matching, which makes CAR NK cells more promising for cancer treatment[144]. Previously, we constructed gene-modified NK cells to augment NK cell activity and found that IL-15-or IFN-α-gene modification increased the production of TNF-α and IFN-γ by NK-92 or NKL cells, promoting apoptosis in HCC cells by upregulating the expression of NKG2D ligands and Fas on HCC cells. These NK cells also exerted enhanced antitumor effects in vivo[145-147].
TLR agonists
The role of TLR agonists as a vaccine adjuvant and tumor immunotherapeutic agent has been recently noted[148-150]. As a vaccine adjuvant, TLR agonists trigger antigen presentation by promoting the maturation of DCs. Multiple TLRs, such as TLR3 and TLR9, have been confirmed to be expressed on HCC cells[151-153], and the role of TLR agonists in tumor therapy has received much attention. The TLR2/4 agonist OM-174 has potential roles in the prevention of invasion and metastasis in HCC[154]. We also found that both TLR3 agonist poly (I:C) and TLR9 agonist ODN M362 can exert antitumor effects on HCC cells. Surprisingly, we found that simultaneous transfection of poly (I:C) and ODN M362 exhibits a lower proapoptotic effect on HCC than transfection of poly (I:C) alone. Further investigation demonstrated that ODN M362 blocks the entrance of poly (I:C) when simultaneously used to treat HCC cells and then decreases the activation of poly (I:C)-triggered cellular apoptosis; however, poly(I:C)-mediated proapoptotic effects could be enhanced by pretreating HCC cells with CpG ODN[155]. TLR agonists can also work as adjuvants to stimulate the immune system during tumor treatment, but their effects on tumor cells cannot be ignored. These therapeutic effects may thus be the overall outcome of various mechanisms.
Tumor vaccine
DCs are central regulators of the adaptive immune response and are thus necessary for T-cell-mediated antitumor immunity. DC vaccines have the characteristics of low complication rates and good tolerance, and DC-based tumor vaccines have been used for a variety of solid tumors[156]. Currently, there are many clinical studies on the use of DC vaccines for HCC treatment. The injection of DCs prestimulated by HCCspecific antigens can increase the number of CD8+T cells, promote antitumor immune responses and improve liver function[157-159]. The combined use of DC vaccines and other treatments, such as radiotherapy, can induce immunogenic death of tumor cells,which can prolong the overall survival of patients[160].
Exosomes display an array of HCC antigens. Rao et al[161]demonstrated that tumor cell-derived exosomes could trigger a stronger DC-mediated immune response than cell lysates and improve the HCC tumor microenvironment. Exosomes derived from α-fetoprotein (AFP)-expressing DCs (DEXAFP) elicited strong antigen-specific immune responses, resulting in significantly delayed tumor growth and prolonged survival rates in mice with HCC tumors[162]. Therefore, DEXAFPmight be a promising vaccine for HCC immunotherapy. AFP, a carcinoembryonic antigen, is highly expressed in HCC and serves as an important marker in the diagnosis of HCC, as well as a potential immunotherapy target for HCC. Zhang et al[163]prepared a mouse AFP recom-binant vaccine by genetic engineering and found that it could induce cellular and humoral immune responses in tumor-bearing mice and show obvious antitumor effects. Additionally, with the use of HCC cell lysates derived from STAT3-inhibited HCC cells to immunize healthy mice, we found that a variety of immune cells, such as T cells and NK cells, were significantly activated after challenge with murine HCC cells in these immunized mice, showing effective inhibition characteristics against the transplanted tumor and resulting in the formation of immune memory[164]. Therefore,as a target for HCC treatment and prevention, blocking STAT3 not only prevents tumor growth but also exerts an important effect on immune system activation.
Although immunotherapy for HCC has made significant progress, the clinical efficacy still needs to be further improved. Finding new targets for the treatment of HCC is still the direction of scientific researchers in the next few years. In recent years,studies about the roles of epigenetics and metabolomics on HCC progression have also become hot spots. Related drug development is also ongoing. A single treatment may not bring satisfactory therapeutic effect. Individual differences need to be more considered. Combined therapy and individualized therapy may be a promising option in HCC treatment.
CONCLUSION
The development of HCC results from the accumulation of many factors and the interaction among many mechanisms. Exploring the molecular mechanisms underlying the occurrence and development of HCC is important for us to obtain a more comprehensive understanding of the disease process and to identify more effective therapeutic targets and strategies. With continuous breakthroughs in research, in addition to traditional therapies, immunotherapies have shown good efficacy for HCC in both preclinical and clinical trials, offering hope for curing this disease. Thus, the combination of drugs acting on various pathways, targets and treatment methods might be effective strategies to achieve greater clinical benefits for the treatment of HCC.
杂志排行
World Journal of Gastroenterology的其它文章
- Advanced imaging in surveillance of Barrett’s esophagus: Is the juice worth the squeeze?
- Fate plasticity in the intestine: The devil is in the detail
- Revisiting the liver’s role in transplant alloimmunity
- Hepatocellular carcinoma: Therapeutic advances in signaling,epigenetic and immune targets
- Epidemiology of hepatitis E in South-East Europe in the "One Health" concept
- Infections with Helicobacter pylori and challenges encountered in Africa
