Anew species of Crocodile Newt,genus Tylototriton(Amphibia,Caudata,Salamandridae)from the mountains of Kachin State,northern Myanmar
2019-06-28ThanZawPawLayParinyaPawangkhanantVladislavGorinNikolayPoyarkovJr
Than Zaw,Paw Lay,Parinya Pawangkhanant,Vladislav A.Gorin,Nikolay A.Poyarkov,Jr.,5,*
1Zoology Department,Mohnyin Degree College,Mohnyin,Kachin State,Myanmar
2Flora and Fauna International,Lon Ton Village,Indawgyi,Kachin State,Myanmar
3Bansomdejchaopraya Rajabhat University,Hiran Ruchi,Thon Buri,Bangkok 10600,Thailand
4Department of Vertebrate Zoology,Biological Faculty,Lomonosov Moscow State University,Moscow 119234,Russia
5Joint Russian-Vietnamese Tropical Research and Technological Center,Nghia Do,Cau Giay,Hanoi,Vietnam
ABSTRACT We describe a new species of the genus Tylototriton from Ingyin Taung Mt.,Mohnyin Township,Kachin State,Myanmar,based on morphological and molecular evidence.The new species is assigned to the subgenus Tylototriton s.str.and is clearly distinct from all known congeners by the following characters:medium body size;thin,long tail,lacking lateral grooves;rough skin;truncate snout;wide,protruding supratemporal bony ridges on head,beginning at anterior corner of orbit;weak,almost indistinct sagittal ridge;long,thin limbs,broadly overlapping when adpressed along body;distinct,wide,non-segmented vertebral ridge;13 or 14 rib nodules;brown to darkbrown background coloration with dull orange-brown to yellowish-brown markings on labial regions,parotoids,rib nodules,whole limbs,vent,and ventral tail ridge.We also briefly discuss biogeography and species diversity of the genus Tylototriton in Myanmar.
Keywords:Tylototriton kachinorum sp.nov.;mtDNA genealogy;ND2;16S rRNA;Shan;Biogeography;Endemism;Taxonomy
INTRODUCTION
The salamandrid genus Tylototriton Anderson,1871,or Crocodile Newts,currently includes 24 recognized species,inhabiting montane forest areas throughout the Asian monsoon climate zone from eastern Himalaya,southern and central China including Hainan Island,to northern Indochina including Vietnam,Laos,Thailand,and Myanmar(Hernandez,2016;Wang et al.,2018).Recent progress in phylogenetic studies of the genus Tylototriton has indicated that the genus is monophyletic(Nishikawa et al.,2013a,2013b;Phimmachak et al.,2015;Wang et al.,2018)and includes two major groups,corresponding to the subgenera Tylototriton s.str.and Yaotriton(Wang et al.,2018).Molecular taxonomy methods have proven to be useful for deciphering taxonomic diversity of the genus Tylototriton,with 13 species(over 50%)described in the past five years(Grismer et al.,2018a;Hou et al.,2012;Khatiwada et al.,2015;Nishikawa et al.,2013a,2013b,2014;Phimmachak et al.,2015;Shen et al.,2012;Yang et al.,2014;Zhao et al.,2012).However,recent molecular surveys indicate that our knowledge on taxonomic diversity of the genus Tylototriton is still far from complete,revealing several cryptic lineages likely corresponding to as yet undescribed species(Grismer et al.,2018a;Wang et al.,2018).
Myanmar,previously known as Burma,is the largest country of mainland Southeast Asia.Despite this,its herpetofauna remains one of the least explored in the region(Grismer et al.,2018a).Members of the genus Tylototriton have long been recorded from northern and eastern parts of Myanmar and have been traditionally classified as T.verrucosus Anderson,1871(Gyi,1969).Nishikawa et al.(2014),based on the examination of specimens assigned to T.verrucosus collected from the Shan Plateau in eastern Myanmar and pet-trade animals assumed to originate from Myanmar,recently described a new species,T.shanorum Nishikawa,Matsui&Rao,2014.Soon after,Phimmachak et al.(2015)published sequence data for specimens collected from the Sagaing Region and Kachin State in northern Myanmar,which were reported as T.verrucosus.More recently,Grismer et al.(2018a)demonstrated the presence of two morphologically and genetically distinct lineages of Tylototriton in the Shan Plateau and described a new species from its north-western edge,T.ngarsuensis Grismer,Wood,Quah,Thura,Espinoza,Grismer,Murdoch&Lin,2018.The same work of Grismer et al.(2018a)reanalyzed sequences of specimens from the Sagaing and Kachin regions of Myanmar and demonstrated that they belong to a distinct lineage-Tylototriton sp.1,distinct from T.verrucosus s.str.Recent work also indicated the presence of T.himalayanus Khatiwada,Wang,Ghimire,Vasudevan,Paudel&Jiang,2015(a species described from Nepalese Himalaya)in northern Myanmar,but without providing voucher specimen information or any other justification for this identification(Hernandez,2016;Hernandez et al.,2018).The recent monographic review of the genus Tylototriton by Hernandez(2016)also indicated the possibility of the occurrence of T.uyenoi Nishikawa,Khonsue,Pomchote&Matsui,2013 and T.shanjing Nussbaum,Brodie&Yang,1995 in parts of Myanmar adjacent to northern Thailand and the southwestern Yunnan Province of China;however,these records are not supported by voucher specimens.Thus,our knowledge on the taxonomic composition and diversity of the genus Tylototriton in Myanmar is still far from complete.In the present paper,we report on a new population of the genus Tylototriton from the Kachin Hills in the southern part of Kachin State,northern Myanmar.We applied morphological and molecular methods to evaluate its taxonomic status and describe it as a new species.We also discuss biogeography and taxonomy of the genus Tylototriton in Myanmar.
MATERIALS AND METHODS
Sample collection
Fieldwork was carried out in northern Myanmar,Kachin State,from 14 to 21 July 2018.Specimens of Tylototriton sp.were collected by hand in swamps in forest clearings surrounded by montane evergreen tropical forests of Ingyin Taung Mountain,Indawgyi Lake area,Kachin State(Figure 1;samples 22-23).Geographic coordinates and altitude were obtained using a Garmin GPSMAP 60CSx GPS receiver(Garmin Ltd.,USA)and recorded in datum WGS 84.Specimens were euthanized by 20%benzocaine and tissue samples for genetic analysis were taken and stored in 96%ethanol(femoral muscles)prior to preservation.Specimens were subsequently preserved in 70%ethanol and deposited in the herpetological collection of the Zoological Museum of Moscow State University(ZMMU)in Moscow,Russia;Zoological Institute of Russian Academy of Sciences(ZISP)in St.Petersburg,Russia;and Zoology Department of University of Mandalay(ZDUM),Mandalay,Myanmar.

Figure 1 Distribution of Tylototriton and sampling localities examined in this studyColors of dots correspond to clades 1-5 denoted in Figure 2.Sample numbers 1-115 refer to Table 1.Type locality of new species Tylototriton kachinorum sp.nov.is marked with a star.Photo by Nikolay A.Poyarkov.
Morphological description
Specimens of Tylototriton sp.were photographed in life and after preservation.The sex and maturity of the specimens and number of eggs were checked and counted by minor dissections.Measurements were taken using a digital caliper to the nearest 0.01 mm,subsequently rounded to 0.1 mm.We used a stereoscopic light binocular microscope when necessary.Statistical analyses were performed with Statistica 8.0(Version 8.0;StatSoft,Tulsa,OK,USA).
Adult morphology
Morphometrics followed Nishikawa et al.(2014),Khatiwada et al.(2015),and Okamiya et al.(2018)and included the following 24 measurements:(1)SVL(snout-vent length)from tip of snout to anterior tip of vent;(2)HL(head length);(3)HW(head width);(4)MXHW(maximum head width);(5)IND(internarial distance);(6)AGD(axilla-groin distance);(7)TRL(trunk length);(8)TAL(tail length)from anterior tip of vent to tail tip;(9)VL(vent length);(10)FLL(forelimb length);(11)HLL(hindlimb length);(12)VTW(vomerine tooth series width):greatest width of vomerine tooth series;(13)VTL(vomerine tooth series length):greatest length of vomerine tooth series;(14)LJL(lower jaw length from tip of lower jaw to articulation of upper and lower jaws);(15)SL(snout length from tip of snout to anterior tip of upper eyelid);(16)IOD(minimum interorbital distance);(17)UEW(maximum upper eyelid width);(18)UEL(upper eyelid length,distance between anterior and posterior angles);(19)OL(orbit length);(20)BTAW(basal tail width at level of anterior tip of cloaca);(21)MTAW(tail width at mid-level of tail);(22)MXTAH(maximum tail height);(23)MTAH(tail height at mid-level of tail);(24)ON(orbitonarial distance).For holotype description,we examined the following 12 morphometric and three meristic characters following Poyarkov etal.(2012)and Okamiya et al.(2018):additionalmorphometric characters:(25)ICD(intercanthal distance);(26)CW(chest width);(27)NSD(nostril-snout distance);(28)1FL(first finger length from base to tip);(29)2FL(second finger length from base to tip);(30)3FL(third finger length from base to tip);(31)4FL(fourth finger length from base to tip);(32)1TL(first toe length from base to tip);(33)2TL(second toe length from base to tip);(34)3TL(third toe length from base to tip);(35)4TL(fourth toe length from base to tip);(36)5TL(fifth toe length from base to tip);meristic characters:(37)UJTN(number of teeth onupperjaw);(38)LJTN(numberofteethonlowerjaw);(39)VTN,number of teeth on vomer.We also recorded the following characters as per Nishikawa et al.(2014)and Grismer et al.(2018a):shape of vomerine teeth and their positional relationship relative to choanae;skin texture;number and shape of rib nodules counted from posterior margin of vent to axilla;width and prominence of vertebral ridge and head ridges;and coloration of dorsum,venter,head,labial region,parotoid glands,rib nodules,limbs,soles,palms,tail surfaces,and vent region.
The characters of adult morphology chosen for comparison and data on other Tylototriton species were taken from the following sources:Anderson(1871);Böhme et al.(2005);Chen et al.(2010);Fang&Chang(1932);Fei et al.(1984);Grismer et al.(2018a);Hernandez(2016);Hou et al.(2012);Khatiwada et al.(2015);Le et al.(2015);Liu(1950);Nishikawa et al.(2013a;2013b;2014);Nussbaum et al.(1995);Phimmachak et al.(2015);Qian et al.(2017);Shen et al.(2012);Stuart et al.(2010);Unterstein(1930);Yang et al.(2014).
Larval morphology
Description of larval morphology followed Okamiya et al.(2018).For larval specimens,we recorded nine morphometric characters including(1)SVL,(2)HL,(3)HW,(4)OL,(5)AGD,(6)TAL,(7)FLL,(8)HLL,and(9)MXTAH(definition same as for adult morphology).Developmental stages were determined following Grosse(2013).
DNA isolation,PCR,and sequencing
Total genomic DNA was extracted from 95%ethanol-preserved muscle tissues using standard phenol-chloroform extraction protocols(Hillis et al.,1996).Total DNA concentration was estimated in 1μL using a NanoDrop 2000 spectrophotometer(Thermo Scientific,USA),and consequently adjusted to 100 ng DNA/μL.
We amplified two mtDNA fragments consisting of partial sequences of the ND2 and 16S rRNA mtDNA genes.These markers were chosen as they are usefulin studies of Tylototriton phylogeny and taxonomy(Nishikawa et al.,2013a,2013b,2014;Wang et al.,2018;Zhao et al.,2012 and references therein).We used the 16L-1(forward)(5'-CTGACCGTGCAAA GGTAGCGTAATCACT-3')and 16H-1(reverse)(5'-CTCCGG TCTGAACTCAGATCACGTAGG-3')primers to amplify the 16S rRNA fragments(Hedges,1994).For amplification and sequencing of the ND2 gene,we used the SL-1(forward)(5'-ATAGAGGTTCAAACCCTCTC-3')and SL-2(reverse)(5'-TTAAAGTGTCTGGGTTGCATTCAG-3')primers of Wang et al.(2018).Polymerase chain reaction(PCR)was performed in 20μL reactions using 50 ng genomic DNA,10 nmol of each primer,15 nmol of each d NTP,50 nmol additional MgCl2,Taq PCR buffer(10 mmol/L Tris-HCl,pH 8.3,50 mmol/L KCl,1.1 mmol/L MgCl2,and 0.01%gelatin),and 1 U of Taq DNA polymerase.PCR cycles included an initial denaturation step of 4 min at 94°C and 35 cycles of denaturation for 30 s at 94°C,primer annealing for 30 s at 48-58°C,and extension for 1 min 30 s at 72°C.PCR products were visualized by agarose gel electrophoresis in the presence of ethidium bromide and consequently purified using 2μL from a 1:4 dilution of ExoSap It(Amersham,UK)per 5 μL of PCR product prior to cycle sequencing.Sequencing was performed in both directions using the same primers as used in PCR on an ABI3730xl automated sequencer(Applied Biosystems,USA)at Evrogen Inc.,Moscow(Russia).
The newly obtained sequences were aligned and deposited in GenBank under the accession numbers MK095616-MK095619 and MK097271-MK097274(Table 1).Sequences of 25 other Tylototriton species used for comparisons were obtained from GenBank(Table 1).

Table 1 Sequences and voucher specimens of Tylototriton and outgroup taxa used in this study

Continued

Continued
Continued
Phylogenetic analyses
Sequences of partial fragments of ND2 and 16S rRNA mtDNA for119 Salamandridae specimens,including 115 representatives of Tylototriton(26 species)and four sequences of outgroup members of Salamandridae(Echinotriton and Pleurodeles)were included in the final alignment with a total length of up to 1 665 bp.Information on voucher specimens and GenBank accession Nos.used in phylogenetic analyses is summarized in Table 1.Nucleotide sequences were initially aligned in MAFFT v.6(Katoh et al.,2002)with default parameters,and then checked by eye in BioEdit 7.0.5.2(Hall,1999)and slightly adjusted.
The dataset was divided into four partitions:three codonpartitions for the ND2 gene and a single partition for 16S rRNA,with the optimal evolutionary models for each estimated using MODELTEST v.3.06(Posada& Crandall,1998).According to the Akaike information criterion(AIC),the TVM+G model was the best fit for the 16S rRNA partition;for the ND2 gene,however,the HKY+G model was considered the best fit for the first and second codon partitions,whereas the J2+G model was selected as the best fit for the third codon partition.Mean uncorrected genetic distances(P-distances)between sequences were determined with MEGA 7.0(Kumar et al.,2016).
The matrilineal genealogy was inferred using Bayesian inference(BI)and maximum likelihood(ML)algorithms.BI analyses were conducted in MrBayes v3.1.2(Huelsenbeck&Ronquist,2001;Ronquist&Huelsenbeck,2003).Metropoliscoupled Markov chain Monte Carlo(MCMCMC)analyses were run with one cold chain and three heated chains for twenty million generations and sampled every 2 000 generations. Five independent MCMCMC runs were performed and 1 000 trees were discarded as burn-in.We checked the convergence of the runs and that the effective sample sizes(ESS)were all above 200 by exploring the likelihood plots using TRACER v1.6(Rambaut et al.,2014).Confidence in tree topology was tested by posterior probability(PP)for the BItrees(Huelsenbeck&Ronquist,2001).Nodes with PP values over 0.95 were a-priori regarded as sufficiently resolved,those between 0.95 and 0.90 were regarded as tendencies,and values below 0.90 were considered to be not supported.
We conducted ML analyses using the RAxML web server(http://embnet.vital-it.ch/raxml-bb/;Stamatakis et al.,2008)and searched ML trees using the gamma model of rate heterogeneity option.Confidence in node topology was tested by non-parametric bootstrapping with 1 000 replicates(ML BS,see Felsenstein,1985).We a-priori regarded tree nodes with bootstrap(ML BS)values of 70%or greater and BI PP values over 0.95 as sufficiently resolved;ML BS values between 70%and 50%(BI PP between 0.95 and 0.90)were treated as tendencies,and nodes with ML BS values below 50% (BI PP below 0.90)were regarded as unresolved(Felsenstein,2004;Huelsenbeck&Hillis,1993).
RESULTS
Phylogenetic analyses
Sequences and statistics:The final alignment of the ND2
gene contained 1 157 aligned characters,including 712 conserved sites and 445 variable sites,of which 405 were parsimony-informative.The transition-transversion bias(R)was estimated to be 4.56(all data for ingroup only).Nucleotide frequencies were 37.5%(A),23.7%(T),28.3%(C),and 10.5% (G).The final alignment of the 16S rRNA gene contained 508 aligned characters,including 424 conserved sites and 82 variable sites,of which 69 were parsimonyinformative.The transition-transversion bias(R)was estimated to be 5.84(all data for ingroup only).Nucleotide frequencies were 36.8%(A),24.9%(T),20.3%(C),and 18.0%(G).
Position of Tylototriton sp.in matrilineal genealogy:BI and ML phylogenetic analyses resulted in essentially similar topologies(Figure 2).In general,the topology of the mtDNAbased matrilineal genealogy was consistent with the phylogeny of Tylototriton presented by Wang et al.(2018),suggesting that the genus is divided into five clades(1-5)grouped into two major reciprocally monophyletic groups(Figure 2):
(1)The first group joined two clades with sister relationships:clade 1(including T.verrucosus(type species of Tylototriton s.str.Anderson,1871),T.anguliceps,T.himalayanus,T.kweichowensis(type species of Qiantriton Fei,Ye&Jiang,2012),T.ngarsuensis,T.podichthys,T.pulcherrimus,T.shanjing,T.shanorum,T.uyenoi,T.yangi,and Tylototriton sp.1 from the western part of the Kachin and Sagaing States of Myanmar and the newly discovered population of Tylototriton sp.nov.from the Indawgyi Lake area in the southern part of Kachin State)and clade 2(including T.pseudoverrucosus and T.taliangensis,the latter being the type species of Liangshantriton Fei,Ye&Jiang,2012).
(2)Thesecond group joined clade3(including T.broadoridgus,
T. dabienicus,T. liuyangensis,T.lizhenchangi,and T.wenxianensis), clade 4 (including T. panhai and T.vietnamensis),and clade 5(including T.asperrimus(type species of Yaotriton Dubois&Raffaëlli,2009),T.hainanensis,T.notialis,and T.ziegleri);the topological relationships between these three clades are essentially unresolved.
In accordance with the results of Wang et al.(2018)our analysis indicated deep phylogenetic structuring and paraphyly of T.asperrimus(consisting of two non-monophyletic lineages),T.wenxianensis(consisting of three lineages,not forming a monophyly),and T.dabienicus (consisting of two nonmonophyletic lineages),suggesting that taxonomy of this group is incomplete and further taxonomic and phylogenetic research is required.
The newly discovered population of Tylototriton sp.nov.from the Indawgyi Lake area belongs to clade 1(Figure 2),which occurs in western and northern Indochina,Himalaya,and Yunnan Province of China(Figure 1),and is grouped with T.himalayanus from Nepal,though not with significant node support(0.85/57,hereafter given for BI PP/ML BS,respectively).Tylototriton sp.nov.from the Indawgyi Lake area and T.himalayanus form a well-supported monophyletic group with Tylototriton species from the Shan Plateau in Myanmar(1.0/96),whereas populations of Tylototriton sp.1 from the Sagaing and Kachin states in northern Myanmar,reported by Grismer et al.(2018a),are clustered with other members of Tylototriton clade 1 from Yunnan and northern Indochina and belong to the T.verrucosus species complex(Figure 2).Our analyses suggest sister relationships of closely related T.verrucosus and T.shanjing but only with support from BI(1.0/-),suggesting that the taxonomic status of T.shanjing might need to be reconsidered.Finally,in our tree,the recently described Myanmar species T.ngarsuensis is nested within differentiation of T.shanorum,rendering the latter paraphyletic.
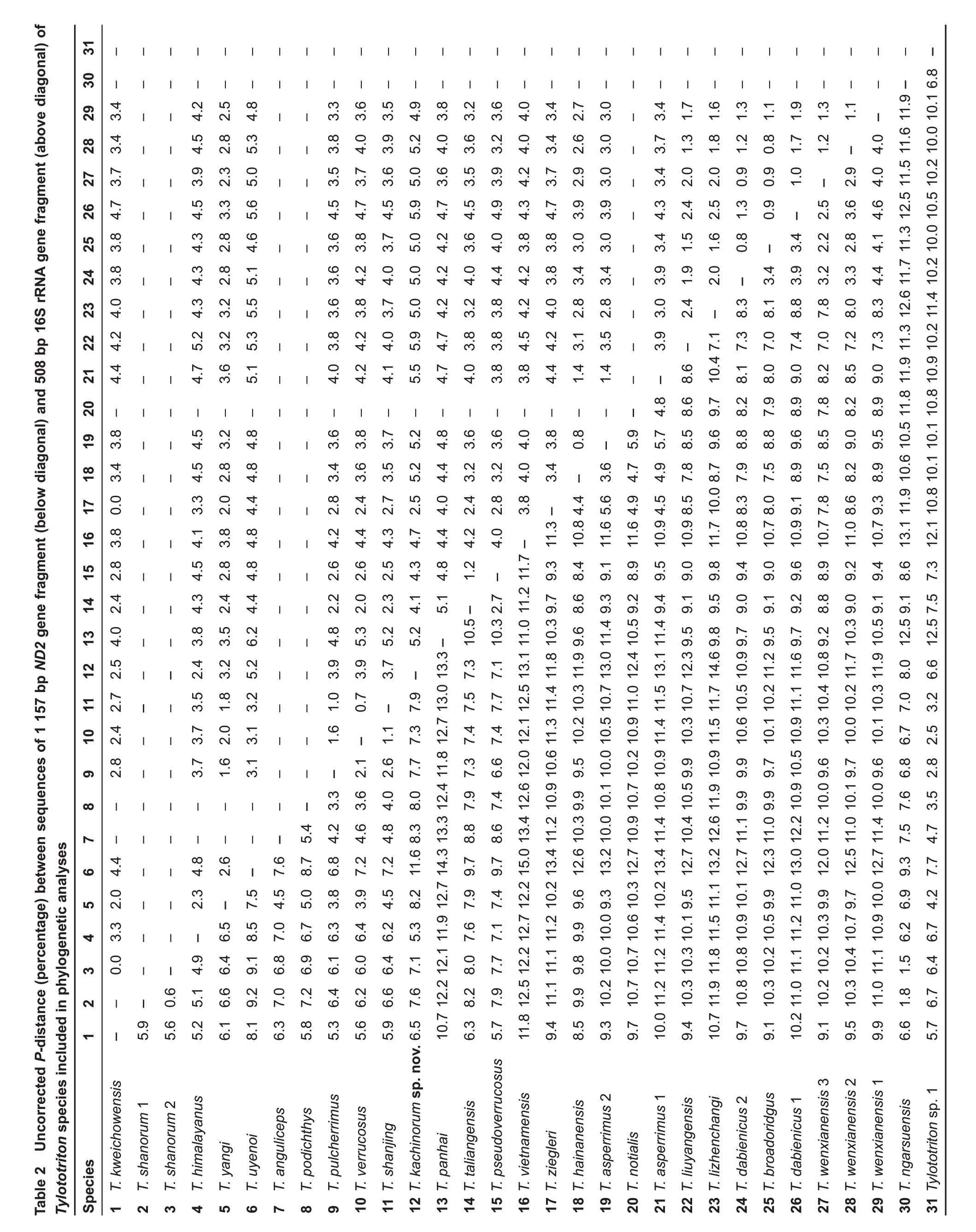
Sequence divergence:The uncorrected P-distances among and within the studied mtDNA fragments for the examined Tylototriton species are shown in Table 2(data for ingroup only).Theinterspecific uncorrected genetic P-distancesbetween the Tylototriton sp.from Kachin State of Myanmar and other congeners varied from 5.3%(between Tylototriton sp.and its sister species T.himalayanus)to 14.6%(between Tylototriton sp.and T.lizhenchangi)for the ND2 gene;and from 2.4%(between Tylototriton sp.and its sister species T.himalayanus)to 5.9%(between Tylototriton sp.and T.liuyangensis and T.dabienicus,lineage 1)for the 16S rRNA gene(Table 2).This degree of pairwise divergence is quite high,notably greater than the genetic divergence observed between many recognized species of Tylototriton(see Table 2 and Grismer et al.,2018a;Wang et al.,2018).
Taxonomy
Our mtDNA genealogy analyses based on the ND2 and 16S rRNA genes indicated that the newly discovered population of Tylototriton sp.nov.from the Indawgyi Lake area belongs to clade 1 of the subgenus Tylototriton s.str.and is clustered with two other species of the genus known from Myanmar,T.ngarsuensis and T.shanorum,and with T.himalayanus from Nepal(Figure 2).The lineage of Tylototriton sp.nov.from Indawgyi Lake is clearly distinct and notably divergent from all other congeners with the uncorrected genetic distance for interspecific comparisons exceeding P=5.3%in the ND2 gene and P=2.4%in the 16S rRNA gene.The observed differences in mtDNA sequences are congruent with evidence from diagnostic morphological characters(see“Comparisons”).These results support our hypothesis that the newly discovered population of Tylototriton sp.nov.from IndawgyiLake represents a previously unknown species,which we describe herein.Tylototriton kachinorum sp.nov.

Figure 2 Bayesian inference consensus tree of genus Tylototriton derived from analysis of 1 157 bp ND2 and 508 bp 16S rRNA gene fragmentsSpecimen numbers(1-115),corresponding voucher specimen information,and GenBank accession Nos.are given in Table 1.Color denotes five major clades revealed within genus Tylototriton(clades 1-5).Information on type species of subgeneric-level taxa Tylototriton s.str.,Qiantriton,Liangshantriton,and Yaotriton is provided.Number at tree nodes corresponds to BI PP/ML BS support values,respectively.Photo by Nikolay A.Poyarkov.
genefragment(abovediagonal)of 31-------------------------------30------------------------------6.8 29 3.4--4.2 2.5 4.8--3.3 3.6 3.5 4.9 3.8 3.2 3.6 4.0 3.4 2.7 3.0-3.4 1.7 1.6 1.3 1.1 1.9 1.3 1.1-11.9 28 3.4--4.5 2.8 5.3--3.8 4.0 3.9 5.2 4.0 3.6 3.2 4.0 3.4 2.6 3.0-3.7 1.3 1.8 1.2 0.8 1.7 1.2-4.0 11.6 10.1 27 3.7--3.9 2.3 5.0--3.5 3.7 3.6 5.0 3.6 3.5 3.9 4.2 3.7 2.9 3.0-3.4 2.0 2.0 0.9 0.9 1.0-2.9 4.0 11.5 10.0 26 4.7--4.5 3.3 5.6--4.5 4.7 4.5 5.9 4.7 4.5 4.9 4.3 4.7 3.9 3.9-4.3 2.4 2.5 1.3 0.9-2.5 3.6 4.6 12.5 10.2 25 3.8--4.3 2.8 4.6--3.6 3.8 3.7 5.0 4.2 3.6 4.0 3.8 3.8 3.0 3.0-3.4 1.5 1.6 0.8-3.4 2.2 2.8 4.1 11.3 10.5 10.0 rRNA 24 3.8--4.3 2.8 5.1--3.6 4.2 4.0 5.0 4.2 4.0 4.4 4.2 3.8 3.4 3.4-3.9 1.9 2.0-3.4 3.9 3.2 3.3 4.4 11.7 10.2 diagonal)and508bp16S 23 4.0--4.3 3.2 5.5--3.6 3.8 3.7 5.0 4.2 3.2 3.8 4.2 4.0 2.8 2.8-3.0 2.4-8.3 8.1 8.8 7.8 8.0 8.3 12.6 22 4.2--5.2 3.2 5.3--3.8 4.2 4.0 5.9 4.7 3.8 3.8 4.5 4.2 3.1 3.5-3.9-7.1 7.3 7.0 7.4 7.0 7.2 7.3 11.3 11.4 21 4.4--4.7 3.6 5.1--4.0 4.2 4.1 5.5 4.7 4.0 3.8 3.8 4.4 1.4 1.4--8.6 10.4 8.1 8.0 9.0 8.2 8.5 9.0 11.9 10.2 20--------------------4.8 8.6 9.7 8.2 7.9 8.9 7.8 8.2 8.9 11.8 10.9 19 3.8--4.5 3.2 4.8--3.6 3.8 3.7 5.2 4.8 3.6 3.6 4.0 3.8 0.8-5.9 5.7 8.5 9.6 8.8 8.8 9.6 8.5 9.0 9.5 10.5 10.8 18 3.4--4.5 2.8 4.8--3.4 3.6 3.5 5.2 4.4 3.2 3.2 4.0 3.4-3.6 4.7 4.9 7.8 8.7 7.9 7.5 8.9 7.5 8.2 8.9 10.6 10.1 10.1 Uncorrected P-distance(percentage)betweensequencesof1157bp ND2genefragment(below 17 0.0--3.3 2.0 4.4--2.8 2.4 2.7 2.5 4.0 2.4 2.8 3.8-4.4 5.6 4.9 4.5 8.5 10.0 16 3.8--4.1 3.8 4.8--4.2 4.4 4.3 4.7 4.4 4.2 4.0-11.3 8.3 8.0 9.1 7.8 8.6 9.3 11.9 10.8 15 2.8--4.5 2.8 4.8--2.6 2.6 2.5 4.3 4.8 1.2-11.7 10.8 11.6 11.6 10.9 10.9 11.7 10.8 10.7 10.9 10.7 11.0 10.7 13.1 12.1 14 2.4--4.3 2.4 4.4--2.2 2.0 2.3 4.1 5.1-2.7 11.2 9.3 8.4 9.1 8.9 9.5 9.0 9.8 9.4 9.0 9.6 8.9 9.2 9.4 8.6 7.3 13 4.0--3.8 3.5 6.2--4.8 5.3 5.2 5.2-10.5 9.7 8.6 9.3 9.2 9.4 9.1 9.5 9.0 9.1 9.2 8.8 9.0 9.1 9.1 7.5 12 2.5--2.4 3.2 5.2--3.9 3.9 3.7-13.3 10.3 11.0 10.3 9.6 11.4 13.0 7.3 7.1 13.1 11.8 11.4 10.5 9.5 9.8 9.7 9.5 9.7 9.2 10.3 10.5 12.5 12.5 11 2.7--3.5 1.8 3.2--1.0 0.7-7.9 13.0 12.4 7.5 7.7 12.5 13.1 11.9 12.3 11.4 10.3 10.7 11.0 11.5 10.7 14.6 10.9 11.2 11.6 10.8 11.7 11.9 8.0 6.6 10 2.4--3.7 2.0 3.1--1.6-1.1 7.3 12.7 11.7 10.5 10.2 11.1 10.4 10.2 10.3 7.0 3.2 9 2.8--3.7 1.6 3.1---2.1 2.6 7.7 11.8 7.4 7.4 12.1 11.3 10.2 10.5 10.9 11.4 10.3 11.5 10.6 10.1 10.9 10.3 10.0 10.1 6.7 2.5 8--------3.3 3.6 4.0 8.0 12.4 7.3 6.6 12.0 7.9 7.4 12.6 10.6 10.9 9.5 10.0 9.9 10.1 10.2 10.7 10.9 10.8 9.9 10.9 9.9 9.7 10.5 11.9 9.6 9.7 9.6 6.8 2.8 9.9 9.9 10.9 7-------5.4 4.2 4.6 4.8 8.3 13.3 10.0 11.2 10.1 10.3 10.0 10.0 7.6 3.5 10.9 6 4.4--4.8 2.6-7.6 8.7 6.8 7.2 7.2 11.6 11.4 10.5 14.3 Tylototriton speciesincludedinphylogeneticanalyses 8.8 8.6 13.4 10.4 12.6 11.1 11.0 12.2 11.2 11.0 11.4 7.5 4.7 5 2.0--2.3-7.5 4.5 5.0 3.8 3.9 4.5 8.2 12.7 9.7 9.7 15.0 13.4 12.6 13.2 12.7 13.4 12.7 13.2 12.7 12.3 13.0 12.0 12.5 12.7 9.3 7.7 4 3.3---6.5 8.5 7.0 6.7 6.3 6.4 6.2 5.3 11.9 7.9 7.4 12.2 10.2 9.6 9.3 10.3 10.2 9.5 11.1 10.1 9.9 11.0 9.9 9.7 10.0 6.9 4.2 3 0.0--4.9 6.4 9.1 6.8 6.9 6.1 6.0 6.4 7.1 12.1 7.6 7.1 12.7 11.2 9.9 10.0 10.6 11.4 10.1 11.5 10.9 10.5 11.2 10.3 10.7 10.9 6.2 6.7 2--0.6 5.1 6.6 9.2 7.0 7.2 6.4 6.2 6.6 7.6 12.2 8.0 7.7 12.2 11.1 9.8 10.0 10.7 11.2 10.3 11.8 10.8 10.2 11.1 10.2 10.4 11.1 1.5 6.4 1-5.9 5.6 5.2 6.1 8.1 6.3 5.8 5.3 5.6 5.9 6.5 10.7 8.2 7.9 12.5 6.3 5.7 11.8 11.1 9.9 10.2 10.7 11.2 9.4 8.5 9.3 9.7 10.0 10.3 11.9 9.4 10.7 10.8 10.3 11.0 9.7 9.1 10.2 10.2 10.3 11.0 1.8 6.7 9.1 9.5 9.9 6.6 5.7 T.shanorum 1 Species T.yangi 2 T.kweichowensis T.shanorum Table2 T.himalayanus T.uyenoi T.anguliceps T.podichthys T.pulcherrimus sp.nov.T.verrucosus T.shanjing T.kachinorum T.panhai T.taliangensis T.pseudoverrucosus T.vietnamensis T.ziegleri T.hainanensis T.asperrimus2 T.notialis T.asperrimus1 T.liuyangensis T.lizhenchangi T.dabienicus2 T.broadoridgus T.dabienicus1 T.wenxianensis3 T.wenxianensis2 T.wenxianensis1 T.ngarsuensis Tylototriton sp.1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31
Tables 3-4;Figures 3-6.
Holotype:ZMMU A5953(field number NAP-08318),adult male from a swamp in a forest clearing surrounded by montane evergreen tropical forest,Ingyin Taung Mountain,Indawgyi Lake area,Mohnyin Township,Kachin State,Myanmar(approximate coordinates N25.09°,E96.28°;elevation 1 000 m a.s.l.),collected on July 18,2018 at 2100 h by Than Zaw,Paw Lay,Parinya Pawangkhanant,Vladislav A.Gorin,and Nikolay A.Poyarkov.
Paratypes:ZMMU A5954(field number NAP-08320),ZISP 13721(field number NAP-08324),ZDUM-0101-0105(field numbers NAP-08325,NAP-08322,NAP-08319,NAP-08326 and NAP-08317,respectively),seven adult males from the same locality and with the same collection information as the holotype;and ZMMU A5955-A5956(field numbers NAP-08323 and NAP-08321,respectively),two adult females from the same locality and with the same collection information as the holotype.
Referred specimens:ZMMUA5957(field number NAP-08305),a larva(Grosse(2013)stage 40)from the same locality and with the same collection information as the holotype.
Diagnosis:The new species is assigned to the genus Tylototriton based on molecular data and by the following combination of morphological attributes:(1)presence of dorsal granules,(2)dorsolateral bony ridges on head,(3)presence of dorsolateral series of knob-like warts(rib nodules);and(4)absence of quadrate spine (Figure 2).Tylototriton kachinorum sp.nov.is distinguished from all other congeners by a combination of the following morphological attributes:(1)medium body size,adult SVL 62.3-74.1 mm in males,72.5-84.8 mm in females;(2)tail thin and long,longer than body in both sexes,lacking lateral grooves;(3)skin rough with fine granules;(4)snout truncate in dorsal view;(5)supratemporal bony ridges on head wide,protruding,beginning at anterior corner of orbit;(6)sagittal ridge on head very weak,almost indistinct;(7)limbs long and thin,tips of forelimb and hindlimb broadly overlapping when adpressed along body;(8)vertebral ridge distinct,wide,non-segmented;(9)rib nodules weakly distinct,13-14 along each side of body;(10)background coloration brown to dark-brown;(11)labial regions,parotoids,rib nodules,whole limbs,vent,ventral tail ridge with dull orange-brown to yellowish-brown markings.
The new species is also markedly distinct from all congeners for which comparable sequences are available of ND2(P≥5.3%)and 16S rRNA(P≥2.4%)mitochondrial DNAgenes.
Description of holotype:A medium-sized specimen in a good state of preservation(Figures 3-4).
Head:Head longer than wide(HW/HLratio 89.3%)(Figure 3C),head wider than body;angularly hexagonal in shape in dorsal view,slightly depressed,gently sloping in profile(Figure 3E);snout comparatively long,three times longer than eye(UEW/SL ratio 33.9%),sharply truncate in dorsal view(Figure 3C),slightly rounded in lateral view(Figure 3E),slightly projecting beyond lower jaw;nostrils on anterior margin of snout located notably closer to snout tip than to eye(NSD/ON ratio 58.8%),facing anterolaterally,not visible from dorsal view;labial folds absent;tongue oval,attached to anterior floor of mouth but free posteriorly and laterally;vomerine teeth arranged in inverted V-shaped almost straight series,converging and narrow anteriorly,gradually widening posteriorly,notably longer than wide(VTW/VTL ratio 77.7%),anteriorly reaching beyond level of choanae but not in contact with them,vomerine teeth 95(47/48 in right and left branches,respectively),upper jaw teeth 93,and lower jaw teeth 110;parotoids distinct,comparatively large,crescent-shaped,notably projecting posteriorly (Figure 3E);dorsolateral supratemporal bony ridges on head wide,notably protruding,from anterior corner of orbit to anterior end of parotoid,forming medially recurved projection on its posterior end(Figure 3C);sagittal bony ridge on head very weak,almost indistinct(Figure 3C);gular fold present(Figure 3D).
Body:Body habitus comparatively slender(Figure 3A);costal folds absent;vertebral middorsal ridge wide,non-segmented,running from occiput region to anterior one fifth of tail length,separated from sagittal head ridge on head with wide gap(Figure 3C);rib nodules weakly distinct,small,forming knoblike glandular warts,arranged in two longitudinal lines on dorsolateral surfaces of dorsum,14 on both sides of body from area posterior to axilla to level of posterior vent margin(base of tail)(Figure 3A);rib nodules almost of same size,rounded,those in posterior third of dorsum slightly ovalshaped,those on sacral area notably elongated,decreasing in size posteriorly on sacrum and tail basis.Limbs:Limbs comparatively long,slender(Figure 3A);forelimbs slightly shorter than hindlimbs;relative length of forelimb FLL/SVL ratio 26.2%,relative length of hindlimb ratio 28.0%;fore-and hindlimbs largely overlapping when adpressed towards each other along sides of body;fingers and toes well developed(Figure 3F-I),free of webbing;fingers four,comparative finger lengths:1FL<4FL<2FL<3FL;toes five,comparative toe lengths:1TL<5TL<2TL<3TL<4TL.Tail:Tail very long,notably exceeding body length(TAL/SVL ratio 114.9%);tail laterally compressed along entire length,tapering posteriorly,lateral grooves on tail absent;dorsal tail fin starting at anterior one fifth of tail length,more distinct posteriorly,with maximal tail height at posterior two thirds of tail length,dorsal tail fin slightly serrated;ventral tail fin smooth;tail tip pointed.Skin texture and skin glands:Skin rough,small granules present on dorsal surfaces of head and dorsum(Figure 3A,C),lateral sides of body and tail;on ventral surface granules become smaller,arranged in transverse striations(Figure 3B);small,sparse granules regularly arranged on throat(Figure 3D);head ridges with rough surface;skin on volar and plantar surfaces of hands and feet with tiny grooves forming reticulated pattern;metacarpal or metatarsal tubercles absent.Cloacal region notably swollen,vent as longitudinal slit(Figure 3J),vent edges with numerous transverse folds.
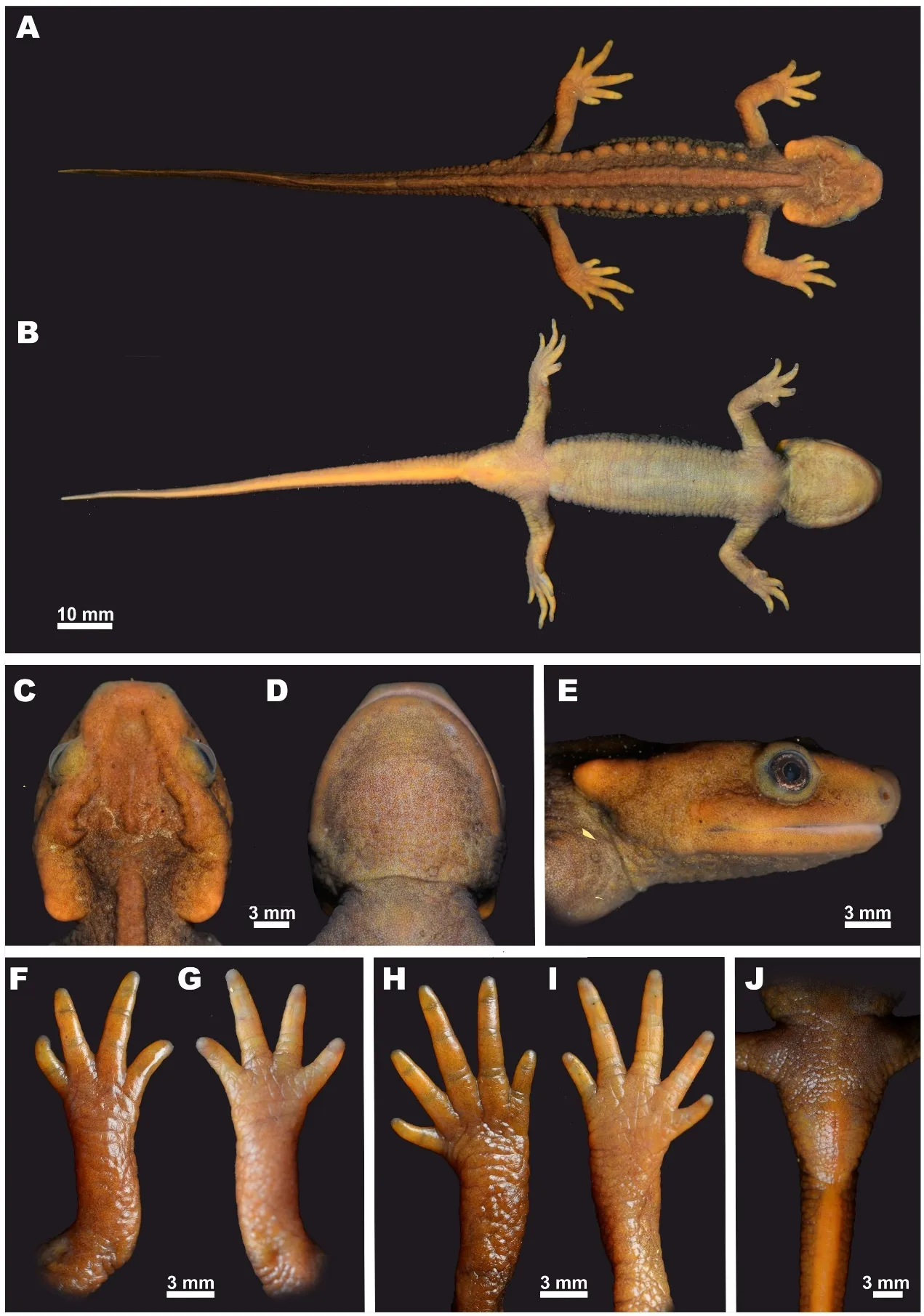
Figure 3 Holotype of Tylototriton kachinorum sp.nov.(ZMMU A5953,male)in lifeA:Dorsal view;B:Ventral view;C:Head,dorsal view;D:Head,ventral view;E:Head,lateral view;F:Opisthenar view of right hand;G:Volar view of right hand;H:Opisthenar view of right foot;I:Plantar view of right foot;J:Ventral view of cloacal area.Photos by Nikolay A.Poyarkov.
Color of holotype in life:Dorsalground color of dorsalsurfaces of head and trunk dark brown(Figure4);dorsal surfaces of limbs and lateral surfaces of tail light yellowish-brown(Figure 3A);iris brown with tiny bronze speckles along outer margins(Figure 3E);gular region,belly,and ventral surfaces of limbs light yellowish-gray(Figure 3B);anterior parts of head and parotoids light orange-brown;rib nodules and vertebral ridge yellowish-brown to orange-brown,barely discernable from dark brown trunk coloration;upper and lower lips,posteriormost corners of parotoids,palms,and soles light-orange to yellowish.

Figure 4 Holotype of Tylototriton kachinorum sp.nov.(ZMMU A5953,male)in situ(Photo by Nikolay A.Poyarkov and Than Zaw)
Color of holotype in preservative:After preservation in ethanol for six months,coloration pattern of holotype resembles that observed in life,however yellowish and orange tints faded to light brownish-gray.
Measurements and counts of holotype:Morphometric characters(all in mm):SVL 67.2;RHL 18.1;RHW 16.2;RMXHW 16.6;RIND 5.4;RAGD 34.1;RTRL 50.4;TAL 77.3;RVL 6.3;RFLL 26.2;RHLL 28.0;RVTW 6.0;RVTL 7.7;RLJL 13.5;RSL 6.8;RIOD 7.2;RUEW 2.3;RUEL 4.4;ROL 3.3;RBTAW 9.0;RMTAW 3.2;RMXTAH 10.8;RMTAH 7.0;RON 4.6;ICD 9.6;CW 11.5;NSD 2.7;1FL 2.9;2FL 4.8;3FL 5.5;4FL 3.0;1TL 2.5;2TL 5.4;3TL 7.0;4TL 7.7;5TL 3.6.Meristic characters:UJTN 93;LJTN 110;VTN 47/48(right/left).
Variation:Allindividuals in the type series were generally similar in morphology and agreed with the holotype description in body proportions and coloration;variation of morphometric characters within the type series is shown in Table 3.The variation of dorsal coloration in seven male and two female paratypes in life is presented in Figure 5.In general,males had more robust and slender bodies than females.The males were notably smaller in body size(SVL 62.3-74.1 mm,mean 68.6±2.9 mm)than the two females(SVL 72.5-84.8 mm)(Table 3).Body of the largest female(ZMMU A5956)was notably swollen and was wider than head width(Figure 5).
Male paratypes ZDUM-0105 and ZISP 13721 had notably shorter tails than other type specimens(Table 4)due to regeneration of tail tip after damage(Figure 5).Coloration within the type series slightly varied,from specimens lighter than the holotype,which appeared light-brown to yellowish-orange(ZDUM-0102),to darker specimens,which appeared dark-brown with duller orange-brown light markings(male ZISP 13721 and female ZMMUA5956)(Figure 5).
Eggs and clutch:The clutch size is unknown.The diameter of ripe eggs in the ovaries of a female paratype(ZMMUA5956)ranged from 1.6 to 1.7 mm(n=5,mean=1.7 mm).The animal pole was dark brown and the remaining area of ova was cream.
Larval morphology:Description of larval morphology is based on a single larval specimen(ZMMU A5957,Grosse(2013)stage 40)(see Referred specimens for details).Details of larval morphology are presented in Figure 6.
Larval measurements(all in mm):SVL 10.5;HL 3.8;HW 3.4;OL 0.8;AGD 5.9;TAL 9.7;FLL 3.6;HLL 2.8;MXTAH 2.6.

Figure 5 Variation of dorsal coloration in paratypes of Tylototriton kachinorum sp.nov.Scale bar:10 mm.Photos by Nikolay A.Poyarkov.
Larval external morphology:Body elongated,higher than wide.Head large,trapezoidal in shape,wide and slightly depressed with short,flattened snout,comprising 37%of snout-vent length(SVL),gently sloping in lateral view,two times wider than body in dorsal view.Snout truncate in dorsal view(Figure 6B),rounded in lateral view(Figure 6A).Tail subequal to body length comprising 93%of SVL;myotomes on body and tail not discernable in lateral view.Nostrils rounded,small,oriented anterolaterally,located much closer to snout tip than to eye.Eyes large,rounded,with lateral orientation but still visible in dorsal view(Figure 6B).Limbs thin,forelimbs longer than hindlimbs,HLL/FLL ratio 79.1%.Forelimbs with four well-developed elongated fingers;forelimb turned,palm facing ventrally;relative finger lengths:4FL<3FL<1FL<2FL.Hindlimbs with knee joint already formed and four well-developed toes,fifth toe as nub;relative toe lengths:5TL<4TL<3TL<1TL<2TL.Orbit diameter(OL)7.7%of SVL.Short longitudinal slit for vent.Height of tail musculature at highest portion comprises 50%-60%of tail height.Maximum height of dorsal tail fin 45%-55%of maximum tail height.Ventral tail fin two times lower than dorsal tail fin.Ventral tail fin roughly at level of vent,dorsal fin lower than head,at level of axilla and reaching maximum height mid tail.Tail tip sharply pointed(Figure 6A).Skin completely smooth;lateral line organs visible on ventral side of head;mouth open with well-developedteeth;no remains of yolk(Figure 6C);gills well-developed,much higher than body,with fimbriae clearly visible.

Table 3 Measurements of type series of Tylototriton kachinorum sp.nov.(all in mm)

Figure 6 Lateral(A),dorsal(B),and ventral(C)views of larval specimen(ZMMU A5957;Grosse(2013)stage 40)of Tylototriton kachinorum sp.nov.in lifeScale bar:3 mm.Photos by Nikolay A.Poyarkov.
Larval coloration in life:In life body background color ochre to golden(Figure 6A,B),ventral surfaces pinkish,translucent(Figure 6C).Body,tail,and head pigmented dorsally:tail almost uniform purple-gray with rare golden speckles,pigmentation forms tortoise-shell golden-dark-gray pattern on body and dorsal fin,head pigmentation less dense,reduced to grayish spots and dots.Few dark spots on ventral fin and limbs.Eyes,except for pupil,fully pigmented,iris golden(Figure 6A).
Position in mtDNA genealogy and sequence divergence:
According to our mtDNA data,Tylototriton kachinorum sp.nov.belongs to clade 1 of the subgenus Tylototriton s.str.(Figure 2)and is grouped with Tylototriton species from the Shan Plateau of Myanmar(T.shanorum,T.ngarsuensis)and Himalaya(T.himalayanus).Uncorrected genetic P-distances between Tylototriton kachinorum sp.nov.,16S rRNA sequences,and all homologous sequences of congeners available included in our analyses varied from 5.3%(with sister species T.himalayanus)to 14.6%(with T.lizhenchangi)(Table 2).
Distribution and biogeography:To date, Tylototriton kachinorum sp.nov.is known only from a single locality on the slopes of Ingyin Taung Mountain,Kachin State,Myanmar(Figure 1)at elevations from 900 to 1 050 m a.s.l.The Ingyin Taung Mountain belongs to the southernmost part of the Kachin Hills-a heavily forested group of highlands in the extreme northeastern area of Kachin State,consisting of a series of mountain ranges running mostly in a north-to-south direction.The actual distribution of Tylototriton kachinorum sp.nov.may be wider:it is anticipated that the new species occurs in the montane forests of adjacent mountains surrounding the largest inland lake of Kachin State,Indawgyi Lake,and possibly further northwards along the Kumon Bum subrange of the Kachin Hills.
Natural history notes:Our knowledge on the biology of Tylototriton kachinorum sp.nov.is scarce.Adult animals were encountered at night after 1900 h in flooded areas of shallow slow-moving streams and artificial ponds in forest clearings,which were used by local Kachin farmers as a watering place for cattle(Figure 7).Surrounding areas were covered by secondary bamboo forest and primary mixed evergreen tropical forest.Adult male newts were observed slowly moving along the clay bottom in clear water 20-40 cm deep;both females and the larval specimen were collected in deeper areas(60-100 cm deep)from dense water vegetation using a dip-net.Courtship behavior of male newts was observed in July.Local Kachin farmers reported that they often find adult newts walking in dense vegetation far from waterbodies,especially after rain;they are also often encountered in wells they construct.The new species is known to local Kachin people as“Lan Yan”literally meaning“water agama”in their native language.

Figure 7 Breeding habitats of Tylototriton kachinorum sp.nov.at type localityTemporary swamps in forest clearings within montane tropical forest on Ingyin Taung Mountain,Indawgyi Lake area,Kachin State,Myanmar,where larva and adult specimens were collected.Photos by Parinya Pawangkhanant.
Other species of amphibians recorded syntopically with the new species at the type locality include Microhyla heymonsi Vogt, Microhyla mukhlesuri Hasan, Islam, Kuramoto,Kurabayashi & Sumida, Microhyla butleri Boulenger,Limnonectes limborgi(Sclater),Limnonectes sp.,Fejervarya sp.,Kurixalus sp.,Feihyla vittata(Boulenger),Rhacophorus bipunctatus Ahl,Polypedates mutus(Smith),and Raorchestes parvulus(Boulenger).Comparisons:According to phylogenetic analyses,Tylototriton kachinorum sp.nov.falls into clade 1 of the subgenus Tylototriton s.str.and morphological comparisons with members of this group appear to be the most pertinent.The new species can be easily distinguished from members of the subgenus Yaotriton(clades 3-5 in Figure 2)by having light color markings on parotoids,lips,vertebral ridge,rib nodules,limbs,and ventral tail ridge(vs.dark body coloration except for palms and soles,vent region,and ventral ridge of tail in most members of the subgenus Yaotriton,with the exception of T.panhai).The new species can be further distinguished from T.panhai by having light color markings on entire limbs(vs.distinct light markings only on palms,soles,and fingers).
Morphometric comparisons and morphological differences inseveraldiagnostic charactersbetween Tylototritonkachinorum sp.nov.and the closely related species of the subgenus Tylototriton are summarized in Table 4.In particular,the new species can be distinguished from T.taliangensis(in clade 2 of the subgenus Tylototriton,sometimes regarded as a separate subgenus or full genus Liangshantriton;see Gong et al.,2018)by having distinct rib nodules,light markings on rib nodules,lips,and parotids(vs.lack of distinct rib nodules,mostly dark charcoal-black body coloration with light orange to red markings only on posterior part of parotoids,digits,palms,soles,vent,and ventral tail ridge).From T.pseudoverrucosus(clade 2)and T.kweichowensis(clade 1 of the subgenus Tylototriton,sometimes regarded as a separate subgenus Qiantriton), Tylototriton kachinorum sp. nov. can be distinguished by having isolated light markings on rib nodules(vs.connected markings forming light dorsolateral lines).

Table 4 Morphological comparison between Tylototriton species found in Myanmar and adjacent territories

Continued

Continued
Tylototriton kachinorum sp.nov.can be distinguished from T.uyenoi,T.pulcherrimus,T.shanjing,and T.yangi by having dull orange-brown to yellowish-brown light markings(vs.much brighter orange to bright-yellow light markings).In particular,T.pulcherrimus has a series of bright-orange glandular spots located ventrolaterally and on flanks(vs.dark-brown coloration of flanks lacking light spots in new species);T.yangi has contrasting charcoal-black coloration of head and lips with only posteriormost part of parotoid colored bright orange,and no light ventral markings on body and tail(vs.all head dull orange-brown with slightly lighter lips and parotoids,light markings present on ventral tail ridge and vent in new species).Tylototriton kachinorum sp.nov.can be distinguished from T.uyenoi by relatively longer head in males(RHL 27.6 vs.24.7),greater internarial distance(RIND 7.7-8.0 vs.7.0-7.1 for both sexes),and longer tail in both sexes(RTAL 120.5 vs.115.0 for males;100.5-114.0 vs.88.0-97.0 for females)(Table 4).Males of the new species can be further diagnosed from males of T.shanjing by having comparatively wider head(RHW 25.1 vs.22.2),longer tail(RTAL 120.5 vs.104.4),greater internarial distance(RIND 7.7 vs.7.1),comparatively wider(RVTW 9.0 vs.6.7)and longer vomerine tooth series(RVTL 12.8 vs.8.8)(Table 4).The new species can be further differentiated from T.shanjing by non-segmented vertebral ridge(vs.well-segmented)and brown to dark-brown background coloration of body(vs.blackish background coloration).
Tylototriton kachinorum sp.nov.can be distinguished from T.verrucosus by having light ventral markings on body and tail(vs.no ventral markings on body and tail),comparatively longer head(RHL 27.6 vs.24.3 in males,RHL 23.9-24.9 vs 21.7 in females),comparatively wider head in both sexes(RHW 25.1-25.4 vs.20.5-23.7),greater internarial distance in both sexes(RIND 7.7-8.0 vs.6.2-7.0),longer tail in both sexes(RTAL 120.5 vs.104.9 for males;100.5-114.0 vs.102.5 for females)(Table 4),non-segmented vertebral ridge(vs.wellsegmented),and brown to dark-brown background coloration of body(vs.blackish background coloration).
The new species can be distinguished from T.podichthys by having comparatively shorter head in both sexes(RHL 23.9-27.6 vs 28.1-34.3),much longer tail in both sexes(RTAL 100.5-120.5 vs.79.2-104.8),truncate snout(vs.rounded),comparatively smoother skin on parotoids and dorsal surface of head,comparatively longer limbs(limbs widely overlap when adpressed to body in the new species vs.digit tips touch when limbs are adpressed to body in T.podichthys),13-14 rib nodules(vs.15-16 rib nodules),and duller coloration with orange-brown light markings and brown to dark-brown background (vs.orange to dark-red light markings and blackish background)(Table 4).
Tylototriton kachinorum sp.nov.can be distinguished from T.anguliceps by having larger body size in both sexes(SVL 68.6±2.9 mm in males and 72.5-84.8 mm in females of new species vs.61.1-62.5 mm and 70.6±3.4 mm in T.anguliceps),comparatively wider head in both sexes(RHW 25.1-25.4 vs.22.7-23.4),greater internarial distance in both sexes(RIND 7.7-8.0 vs.6.5-7.4),longer tail in both sexes(RTAL 100.5-120.5 vs.91.2-102.3),and comparatively wider(RVTW 8.8-9.0 vs.6.4-7.8)and longer vomerine tooth series(RVTL 11.4-12.8 vs.9.2-10.8)(Table 4).The new species can be further diagnosed from T.anguliceps by having wide protruding supratemporal ridges(vs.narrow and steep supratemporal ridges),very small and almost indiscernible sagittal ridge(vs.long and notably protruding sagittal ridge),wide and nonsegmented vertebral ridge(vs.narrow and weakly segmented vertebral ridge),less distinct 13-14 rib nodules(vs.rib nodules more distinct and protruding,not less than 15),and duller coloration with orange-brown light markings and brown to dark-brown background(vs.bright-orange light markings and blackish background)(Table 4).
Phylogenetically and morphologically,Tylototriton kachinorum sp.nov.is most closely related to other species of Tylototriton inhabiting Myanmar(e.g.,T.shanorum and T.ngarsuensis)from the Shan Plateau and T.himalayanus from Nepalese Himalaya(Table 4).The new species can be readily distinguished from T.shanorum by having longer head in males(RHL 27.6 vs.22.4),greater internarial distance in both sexes(RIND 7.7-8.0 vs.6.0-6.7),notably longer tail in both sexes(RTAL 120.5 vs.111.2 in males,100.5-114.0 vs.97.0-97.8 in females),wider vomerine tooth series in both sexes(RVTW 8.7-9.0 vs.6.1),longer vomerine tooth series in males(RVTL 12.8 vs.10.7)(Table 4),supratemporal bony ridges beginning at anterior corner of orbit(vs.supratemporalbony ridges beginning at loreal region),and wide,non-segmented vertebral ridge(vs.narrow,weakly segmented vertebral ridge).Tylototriton kachinorum sp.nov.has generally lighter coloration than T.shanorum:brownish ground color with orange-brown light markings(vs.more contrasting dark brown to black background color with yellow to reddish-brown light markings)and light markings present only on ventral tail ridge(vs.lateral sides of tail with light markings)(Table 4).
Tylototriton kachinorum sp.nov.can be easily distinguished from T.ngarsuensis by having generally smaller body size in males(SVL 68.6±2.9 mm vs.74.9-76.4 mm)and females(SVL 72.5-84.8 mm vs.102.3 mm),comparatively longer head in males(RHL 27.6 vs.24.0-26.0)and females(RHL 23.9-24.9 vs.21.5),longer tail in males(RTAL 120.5 vs.98.0-103.5),generally longer tail in females(RTAL 100.5-114.0 vs.104.6),and comparatively shorter vent length(in males RVL 10.2 vs.10.7-12.3,in females 5.6-6.8 vs.8.0)(Table 4).The new species can be further distinguished from T.ngarsuensis by having truncate snout(vs.rounded snout),supratemporal ridges starting at anterior corner of orbit(vs.supratemporal bony ridges beginning posterior to orbit),non-segmented vertebral ridge(vs.weakly segmented),and 13-14 weakly distinct rib nodules(vs.15 well-distinct rib nodules)(Table 4).
Tylototriton kachinorum sp.nov.also has much lighter and duller colorationthan T.ngarsuensis:background color brown to light brown (vs.nearly black)with light orange-brown markings on rib nodules,parotids,and whole limbs(vs.no light markings on rib nodules or parotids,dark-yellow coloration present only on palms and soles)(Table 4).
Morphologically,Tylototriton kachinorum sp.nov.most resembles its sister species,T.himalayanus from Nepal;however,it can be readily distinguished by the following morphological attributes:comparatively longer head in males(RHL 27.6 vs.24.5),notably wider head in both sexes(RHW 25.1-25.4 vs.23.0-23.6),shorter internarial distance in both sexes(RIND 7.7-8.0 vs.8.2-8.4),and longer tail in both sexes(RTAL 100.5-120.5 vs.98.0-100.6)(Table 4).The new species has generally smoother skin than T.himalayanus and can be distinguished from the latter species by having weakly distinct 13-14 rib nodules(vs.large and prominent 16 rib nodules)and absence of lateral grooves on tail(vs.very distinct).Coloration of the new species is similar to T.himalayanus,but light markings are also present on labial regions,parotoids,rib nodules,and whole limbs(vs.no light markings on head and rib nodules,on limbs only on ventral surfaces)(Table 4).
Etymology:The specific name“kachinorum”is a Latin adjective in the genitive plural(masculine gender),derived from the name of the Kachin people who inhabit the montane areas of northern Myanmar and adjacent territories(Kachin Hills),including the type locality of the new species.
Recommended vernacular name:We recommend the following name in English: Kachin Crocodile Newt.Recommended vernacular name in Burmese(Myanmar)language:Kachin Yae Poke Thin.
Conservation status:Tylototriton kachinorum sp.nov.is,to date,known from a single locality in the southern part of the Kachin Hills of northern Myanmar;the actual range of the new species is unknown.The new species is anticipated to inhabit elevations above 900 m a.s.l.on mountains surrounding the Indawgyi Lake valley and may be found in other parts of the Kachin Hills.Further research is required to estimate the actual distribution,population trends,and possible threats to the new species.Tylototriton kachinorum sp.nov.appears to be associated with montane forests and may be affected by growing anthropogenic pressure and forest destruction observed in different areas of Kachin State in Myanmar.Given the available information,we suggest Tylototriton kachinorum sp.nov.to be tentatively considered as a Vulnerable(VU)species following IUCN’s Red List categories(IUCN,2001).
DISCUSSION
Our phylogenetic data largely confirmed the phylogeny of Tylototriton as presented in the earlier studies of Phimmachak et al.(2015),Khatiwada et al.(2015),Wang et al.(2018),and Grismer et al.(2018a).In particular,our data support the subdivision of Tylototriton into two major groups,traditionally regarded as subgenera:Tylototriton s.str.(clades 1 and 2)and Yaotriton(clades 3-5).Two other subgenera recently proposed by Fei et al.(2012),namely Qiantriton and Liangshantriton are nested within the radiation of Tylototriton s.str.(Figure 2)and possibly should not be regarded as independent subgenera or even genera(see Gong et al.,2018;Hernandez,2016).Our mtDNA-based genealogy is insufficiently resolved in a number of deeper tree nodes;however,it unambiguously suggests that clade 1 of Tylototriton s.str.is subdivided into three main subclades with prominent geographic structuring.The northernmost member of this group-T.kweichowensis,occurring in the Guizhou Plateau of China(Figure 1,samples 7-10),is distant from other members of clade 1 and its phylogenetic position is unresolved.Most other species of clade 1,which occur in northern Indochina and Yunnan Province of China,form a monophyly(including T.verrucosus,T.shanjing,T.uyenoi,T.anguliceps,T.podichthys,T.pulcherrimus,T.yangi,and Tylototriton sp.1)(Figure 1,samples 17-18 and 24-48).Finally,four species from the northern part of Myanmar and Himalaya form a distinct monophyletic group(including T.shanorum,T.ngarsuensis,T.himalayanus,and Tylototriton kachinorum sp.nov.).
Studies on the taxonomic status of Tylototriton species in Myanmar have been delayed,even though the genus has been reported from the country for long time(see Gyi,1969).Nishikawa et al.(2014)assigned Tylototriton from the Shan Plateau of eastern Myanmar to a new species,T.shanorum,whereas Grismer et al.(2018a)recently demonstrated the presence of divergent lineages of Tylototriton within different parts of the Shan Plateau and described a second species for the country-T.ngarsuensis.Contrary to the tree of Grismer et al.(2018a),our analysis suggests that T.shanorum is paraphyletic with respect to T.ngarsuensis and is subdivided into two lineages(Figure 2).However,it is worth noting that the second lineage of T.shanorum is based on data from a single specimen,reported by Nishikawa et al.(2014)(KUHE42348),which was obtained through the pet trade and presumed to come from Myanmar.Its assignment to T.shanorum is thus tentative and this specimen possibly represents a new lineage of Tylototriton from the Shan Plateau,yet undiscovered in the wild.Hence,considering the profound morphological differences between T.ngarsuensis and T.shanorum reported by Grismer et al.(2018a),though genetic divergence between these species was low(P=1.8%for ND2 gene),we consider that T.ngarsuensis indeed represents a distinct species of Tylototriton.
Our description of Tylototriton kachinorum sp.nov.thus represents the third species of Tylototriton endemic to Myanmar.Tylototriton kachinorum sp.nov.is more closely related to T.himalayanus than to T.shanorum and T.ngarsuensis,occurring on the Shan Plateau in the eastern part of the country.This fact can be explained from a biogeographic viewpoint:the Kachin Hills represent the southernmost outcrop of the Great Himalaya ridge(Hadden,2008)and are isolated from the Shan Plateau by the Irrawaddy(Ayeyarwaddy)River valley,which may serve as an important biogeographic border for forest-dwelling taxa.Tylototritonhimalayanus,which can be found in Nepal and possibly along the Great Himalaya Ridge,was previously reported for northern Myanmar(Hernandez,2016,2018),but withoutanyinformationonvoucherspecimensorreasonsforsuch identification.There is a possibility that these records are based on the misidentification of Tylototriton kachinorum sp.nov.
The present work indicates that Tylototriton diversity in Myanmar is still underestimated.Phimmachak et al.(2015)and Grismer et al.(2018a)reported sequences of Tylototriton cf.verrucosus from two regions of northern Myanmar:Sagaing Region(sample 18,see Figure 1)and the eastern part of Kachin State(sample 17,see Figure 1).Surprisingly,these populations were found to be distantly related to Tylototriton kachinorum sp.nov.,despite the geographical proximity to the southern part of Kachin Hills where the new species occurs.Sagaing and eastern Kachin Tylototriton populations(indicated herein as Tylototriton sp.1)were reconstructed as members of the T.verrucosus species complex;however,they formed an mtDNA lineage clearly distinct from all currently recognized species.These data suggest that Tylototriton cf.verrucosus from northern Myanmar might represent a currently undescribed species and further morphological and phylogenetic studies are required to evaluate its taxonomic status.In agreement with the results of Wang et al.(2018),our analysis also shows deep subdivision and the presence of several lineages of possibly full-species status within T.asperrimus,T.dabienicus,and T.wenxianensis of the subgenus Yaotriton.
Our study indicates that knowledge on amphibian diversity in montane regions of northern Myanmar is still far from complete and further diversity is likely to be revealed with additional survey efforts.A number of recent studies have shown that species diversity of amphibians and reptiles is widely underestimated across Myanmar(Grismer et al.,2017a,2017b,2018a,2018b,2018c;Mulcahy et al.,2018),and it is likely that further surveys on this geographically complex and insufficiently studied region will lead to more discoveries.Recent economic development of Myanmar has led to increasing habitat loss and modification across the country(Li&Quan,2017).Further intensified survey efforts and biodiversity assessments are urgently required for effective conservation management of yet unrealized herpetofaunal diversity in Myanmar.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’CONTRIBUTIONS
T.Z.and N.A.P.designed the study;T.Z.,P.L.,P.P.,V.A.G.,and N.A.P.collected study materials;T.Z.,P.L.,P.P.,V.A.G.,and N.A.P.discussed the results,T.Z.,V.A.G.,and N.A.P.prepared the manuscript;P.P.and N.A.P.provided study photographs;V.A.G.and N.A.P.performed molecular and phylogenetic analyses;N.A.P.provided funding for the study and revised the manuscript.All authors read and approved the final version of the manuscript.
NOMENCLATURAL ACTS REGISTRATION
The electronic version of this article in portable document format will represent a published work according to the International Commission on Zoological Nomenclature(ICZN),and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone(see Articles 8.5-8.6 of the Code).This published work and the nomenclatural acts it contains have been registered in ZooBank,the online registration system for the ICZN.The ZooBank LSIDs(Life Science Identifiers)can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefixhttp://zoobank.org/.Publication LSID:
urn:lsid:zoobank.org:pub:FC2F997C-2E7B-4B22-9376-E335E7A84AB1.
Tylototriton kachinorum LSID:
urn:lsid:zoobank.org:act:FF992F77-436E-4164-A1C2-3EB8D220648F.
ACKNOWLEDGEMENTS
We thank the Ministry of Natural Resources and Environmental Conservation Forest Department for the collection and export permits and the staff of the Indawgyi National Park for help with organization of fieldwork.We thank the staff of the guesthouse in Lon Ton Village for their hospitality.N.A.P.thanks Duong Van Tang and Anna S.Dubrovskaya for help during lab work and to Evgeniy Popov for help with map design.For permission to study specimens under their care and permanent support,we thank Valentina F.Orlova(ZMMU),Roman A.Nazarov(ZMMU),and Konstantin D.Milto(ZISP).We thank Natalia Ershova for proofreading.We are sincerely grateful to L.Lee Grismer and Bryan Stuart for their kind help and useful comments,which helped us to improve the previous version of this manuscript.
杂志排行
Zoological Research的其它文章
- Specific function and modulation of teleost monocytes/macrophages:polarization and phagocytosis
- A new species of the endemic Himalayan genus Liurana(Anura,Ceratobatrachidae)from southeastern Tibet,China,with comments on the distribution,reproductive biology,and conservation of the genus
- Duplication and diversif ication of insulin genes in ray-f inned f ish
- Effects of C-terminal amidation and heptapeptide ring on the biological activities and advanced structure of amurin-9KY,a novel antimicrobial peptide identif ied from the brown frog,Rana kunyuensis
- Purification and characterization of a novel anti-coagulant from the leech Hirudinaria manillensis
- Passive eye movements induced by electromagnetic force(EMF)in rats
