High performance hybrid supercapacitor based on hierarchical MoS2/Ni3S2 metal chalcogenide
2019-06-20YingLiuDepengZhoHengqiLiuAhmdUmrXingWu
Ying Liu,Depeng Zho,Hengqi Liu,Ahmd Umr,Xing Wu,*
a School of Materials Science and Engineering, Shenyang University of Technology, Shenyang 110870, China
b Department of Chemistry, Faculty of Science and Arts and Promising Centre for Sensors and Electronic Devices (PCSED), Najran University, Najran 11001,Saudi Arabia
Keywords:
MoS2/Ni3S2
3D hierarchical structures
Hybrid supercapacitor
Energy storage device
Cycle stability
ABSTRACT
Recently,because of excellent electrical conductivities and many active sites,transition metal sulfides have been utilized as efficient electrodes for supercapacitors.Herein, we synthesize hierarchical MoS2/Ni3S2 structures grown on nickel foam by a facile one-pot hydrothermal process.The as-fabricated asymmetric hybrid capacitor based on hierarchical MoS2/Ni3S2 electrode exhibit a specific capacitance of ~1.033 C/cm2 at 1mA/cm2.Furthermore,the hybrid capacitor unveils an energy density of 35.93mWh/cm3 at a powerdensity of 1064.76mW/cm3.The observed results clearly revealed that the synthesized MoS2/Ni3S2 structure might be used as potential electrode material for future energy storage devices.
To solve the exhaustive fossil fuels delinquents, recently extensive research work is going on to develop renewable and sustainable energy storage systems [1-3].They have received a great attention owning to their importance in the clean renewable energy generation and hybrid automobile systems [2].However,the emerging energy storage devices require fast charge-discharge rate, high power density, low cost, long cycling life, and environment-benign behavior[1-7].Thus,to obtain such qualities in the energy storage devices, efficient and novel electrode materials are needed.Recently,transition metal oxides/hydroxides have been research focuses as efficient electrode materials due to the higher specific capacitance compared to carbon based materials and better electrochemical stabilities than conducting polymers [8-15].Interestingly, transition metal sulfides with rich redox reactions possess higher electronic conductivity and specific capacitance than corresponding metal oxides [16,17].
Among various metal sulfides, molybdenum sulfide (MoS2)possessing distinct place as it consists of covalently bonding S-Mo-S sheets attached by van der Waals force and weak interlayer coupling [18].Further, the large spacing of adjacent layers can provide a facile route for the intercalation and the ions transportation [19].However, due to low electrical conductivity during electrochemical processes, MoS2materials do not exhibit outstanding electrochemical properties and thus cannot be effectively utilized.Moreover, the specific capacitance of pure MoS2is very low for energy storage application[20,21].Therefore,it is an inevitable trend to synthesize metal disulfide composites to improve its conductivity.
To develop high performance metal disulfide based materials for electrochemical capacitors applications,nickel sulfide(Ni3S2)is a potential candidate own to its high theoretical capacitance and environmentally friendly characteristics[22].Previously,Yang and coworkers reported mushroom-like Ni3S2electrode which revealed the specific capacitance of 3.3 F/cm2at the current density of 4 A/g [23].Zhou et al.reported the sheet-like Ni3S2electrode on Ni foam with the capacitance of 1.342 F/cm2at current densities of 15 mA/cm2[24].Even though Ni3S2based electrodes are used for supercapacitance applications, but the total electrochemical performance was not satisfactory for the emerging energy storage devices.Therefore,it is necessary to design hybrid electrodes with unique spatial architecture because of their synergetic effect.In this direction, Zheng et al.prepared Ni3S2-CoS composite on nickel foam at 160°C with the capacitance of 722.12 F/g at 1 A/g [25].Zhang demonstrated Ni3S4@MoS2nanostructure for supercapacitor with a high capacitance of 1440.9 F/g at 2 A/g [26].Lei et al.reported MoS2/ Co3S4hybrid hollow structure with a specific capacitance of 1369 F/g at 1 A/g [27].
Herein, we synthesized hierarchical MoS2/ Ni3S2structures as electrode materials grown on nickel foam by a facile hydrothermal strategy.The hierarchical MoS2/ Ni3S2structures showed a capacitance of 1.033 C/cm2at a current density of 1 mA/cm2and specific capacitance of 490 C/g at 1 A/g.The fabricated hybrid device possesses an energy density of 35.93 mWh/cm3at 1064.76 mW/cm3.
The typical synthesis process of MoS2/Ni3S2composite material was as follows: 0.2420 g of Na2MoO4·2H2O was dissolved into 50 mL DI water and stirred for 5 min.Then 0.3045 g thiourea was adding into the above solution.To get the homogeneous mixture of the resultant solution, it was further stirred for 30 min.Subsequently,the as-obtained solution and the pre-treated nickel foam were transferred into 80 mL autoclave and heated at 180°C for 12 h.After completion of reaction,the as-obtained product on taken out and rinsed with DI water and absolute ethanol, sequentially and dried at 60°C overnight.The average mass loading is 1.5 mg/cm2.
The prepared material was examined by various techniques.The crystal structure and phase purity of the as-prepared material were studied by power X-ray diffraction analyzer (XRD, 7000,Shimadzu).Scanning electron microscopy (SEM, Gemini 300-71-31) and transmission electron microscopy (TEM, JEM-2010 PLUS)were utilized to examine the morphology and structural properties of the material.Surface chemistry and element analysis of the synthesized material were investigated by X-ray photoelectron spectroscopy (XPS, ESCALAB 250 Xi, Thermo Scientific).
Electrochemical performance of the as-synthesized material was conducted out through a CHI660E electrochemical work station in a three-electrode system at room-temperature.Galvanostatic charge-discharge (GCD), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were conducted on a three-electrode system in 1 mol/L KOH aqueous electrolyte.The as-synthesized material was used as the working electrode, Pt foil as reference electrode and Hg/HgO as counter electrode.EIS measurements were used in a frequency ranges from 0.01 kHz to 100 kHz at a 10 mV open circuit potential.Areal and specific capacitance of the electrodes were calculated through GCD curves by following equations:Here, Ca, Cs, I, Δt, s and m are areal capacitance, specific capacitance, discharge current density (C/cm2), discharging time(s), active area and electrode mass, respectively.

The asymmetric capacitor was assembled using positive electrode, negative electrode and the electrolyte.The anode was prepared by mixing active carbon,carbon black and polyvinylidene fluoride binder in a weight ratio of 7:2:1 with a trace amount of Nmethyl-2-pyrrolidone as the solvent.The synthesized MoS2/Ni3S2composite material was applied as positive electrode.To prepare the electrolyte, typically 2 g of PVA (polyvinyl alcohol) was dissolved in 15 mL DI water and stirred at 80°C.Consequently,2 g KOH,dissolve in 5 mL DI water,was added in PVA solution and resultant mixture was vigorously stirred until it get clear and uniform.Before the assembly,the electrodes were immersed into PVA-KOH electrolyte for 10 min.After evaporating the excess water, an asymmetric capacitor was assembled by sandwiching KOH-PVA gel electrolyte between MoS2/Ni3S2and AC electrode.The loading mass of the negative electrode was studied by balancing the charges stored in each electrode (q+= q-), which based on the following equation [28]:

To obtain the charge balance, the mass of active carbon was calculated by the following equation:

Cm(C/g) is mass capacitance and ΔV is potential window for charge-discharge process.The mass ratio of negative electrode to positive electrode was about 5:1.
Fig.1a depicts XRD pattern of the as-synthesized material which revealed several well-defined diffraction peaks at 2θ=21.8°,31.1°, 37.8°, 55.3°, 69.3°and 73.0°which are well matched in accordance with the (101), (110), (003), (122), (131) and (214)crystal planes of Ni3S2(JCPDS No.44-1418).In addition, two diffraction peaks at 2θ=29.4°and 49.8°could be indexed to the(006) and (105) planes of cubic phase MoS2(PDF No.17-0744),respectively.The highest three diffraction peaks at 2θ=44.5°,51.8°and 78°are well in accordance with(011),(200)and(103)crystal planes of Ni(JCPDS No.45-1027).Among these,the diffraction peak belongs to crystal plane of (011) overlaps that of MoS2diffraction peaks.Elements of the as-prepared material is conducted by energy dispersive X-ray spectroscopy (EDS) and the observed result shows various well-defined peaks related with S,Ni and Mo(Fig.1b),confirming that the synthesized material is made of S,Ni and Mo.The uniformity of the as-synthesized MoS2/Ni3S2is proven by elemental mapping images in the inset of Fig.1b.

Fig.1.Structural characterization of the as-synthesized product:(a)XRD pattern;(b)EDX spectrum and the elemental mappings of Mo,Ni and S,respectively;(c)XPS survey spectrum; (d) XPS spectrum of Ni 2p; (e) XPS spectrum of Mo 3d; (f) XPS spectrum of S 2p.
XPS is used to further study surface chemical composition and chemical states of the synthesized MoS2/Ni3S2hierarchical structures.Fig.1c exhibits a survey scan spectrum,which consists of Ni 2p, Mo 3d, S 2p and O 1s.XPS spectra of Ni 2p (Fig.1d) are accordance with two spin-orbit doublets and two shake-up satellites (named “Sat.”).For Ni 2p spectrum, the peak at 854.4 eV is typically contributed to Ni3+, and the other peak at 872.2 eV is the characteristic peak of Ni2+ions.The satellite peaks at 861.2eV and 879.2 eV are two shake-up peaks of nickel element[29].As shown in Fig.1e, Mo 3d spectrum presents two peaks at 231.5 eV and 234.6 eV,which can be assigned to Mo 3d5/2and Mo 3d3/2, respectively [30].S 2p spectrum in Fig.1f shows two characteristic peaks at 162.59 eV and 163.2 eV, which could be ascribed to S 2p1/2and S 2p3/2, respectively [31].Based on aforementioned results, it can be identified that the synthesized material is MoS2/Ni3S2.
Morphology and microstructure of the synthesized material were tested through SEM and TEM.Fig.2a depicts typical SEM image of MoS2/Ni3S2material on surface of Ni foam.The products are composed of several thin sheets intermingled with each other and hence provide high surface to volume ratio.3D hierarchical structure with unique spatial architecture can provide large ion accessible surface for fast ion transport in energy storage device due to its specific surface area.The typical sizes of a single flowerlike structures are in the range of 2-5μm while the thickness of the nanosheets are ~15±3 nm(Fig.2b).The magnified SEM image indicates that the nanosheets possess rough surfaces throughout their dimensions (Fig.2b).TEM is used to further study the microstructure of the MoS2/Ni3S2hierarchical structure.Fig.2c exhibits the low magnification TEM image of the hierarchical structure which exhibited fill consistency with the observed SEM images in Figs.2a and b.As seen in TEM image, the hierarchical structures are composed of thin nanosheets which are connected and intermingled with each other and make flower-shaped morphologies.Interestingly, due to very low thickness, the nanosheets possess high transparency.Fig.2d depicts the typical high-resolution TEM (HRTEM) image which revealed two interplanar distances in the lattice fringes.The inter-planar spacings of 0.189 nm and 0.237 nm are consistent with the (107) and (003)planes of MoS2and Ni3S2, respectively (Fig.2d).

Fig.2.Morphology of the as-prepared hybrid structure:(a-b)SEM images;(c)TEM image; (d) HRTEM images.
To check the electrochemical performance, the synthesized MoS2/Ni3S2hierarchical structure are systematically studied by CV and GCD technique in a three-electrode configuration system as the electrolyte in a potential window from 0 to 0.58 V(vs.Hg/HgO).Fig.3a exhibits the typical CV studies of MoS2/Ni3S2hierarchical structure at various sweep rates which revealed the potential voltage range of the fabricated electrode with the voltage varying from 0 to 0.6 V.It can be found that a slight redox peak appears in the CV curve when the sweep speed gradually slows down(Fig.3a).It could be ascribed to the voltage change slowly with the sweep speed slowing,which is beneficial to the proximity and transfer of ions in the electrolyte to the electrode surface.Simultaneously,two obvious redox peaks are observed, which might be reduced by Faradic reaction.Fig.3c presents CV curves of Ni3S2, MoS2/Ni3S2and MoS2electrodes at a sweep rate of 40 mV/s,revealing that the influence of Ni3S2structure on capacitance of electrode material could be ignored.The integrated area of CV curve of hierarchical MoS2/Ni3S2structure is larger than that of MoS2,indicating that the hierarchical structure possesses high charge-storing ability.Fig.3b shows CP curves of hierarchical MoS2/Ni3S2structure at current densities from 1 mA/cm2to 8 mA/cm2.It is found that the curves are symmetric, revealing high reversibility of Faraday reaction.Also, it can be seen that MoS2/Ni3S2structures present discharge time of 1033s at the current density of 1 mA/cm2.The hybrid structure possesses capacitances of 1.033,1.018,0.993,0.973,0.926 and 0.894 C/cm2at current densities of 1,2,3,4,6 and 8 mA/cm2,respectively.The plateaus of the charge-discharge curves prove Faradaic redox conversion process related to Ni and Mo ions,which is consistent with CV curve.MoS2/Ni3S2hybrid structure shows longer discharge times than single structure, indicating its high specific capacitance (Fig.3d).A comparison of MoS2/Ni3S2electrode material with previous reports has been listed in Table 1.The results indicate that this work is better and competitive as compared to other electrode materials [32-34].
EIS is an important tool to study charge transfer behavior and capacitive characteristic between electrode and electrolyte interfaces within the applied frequency [35].EIS of MoS2, MoS2/Ni3S2and Ni3S2electrodes are measured in the range from 100 kHz to 0.01 Hz (Fig.3e).By equivalent circuit fitting, the Nyquist curve intercept on the real axis are 1.15 Ω for MoS2/Ni3S2structure,manifesting its low internal resistance.In low frequency zone,straight line shows diffusive resistance of ions and the slope shows an ideal capacitance behavior [36].The semi-circles in high frequency region indicate fast charge transfer process at the electrolyte-electrode interface [37].The intersection in real axis represents bulk resistance(Rs)and the diameters of the semicircles shows charge transfer resistance (Rct) in high frequency region[38].The image of equivalent circuit is shown in the inset of Fig.3e.The circuit consists of different parameters such as Rs, charge transfer resistance Rctand Warburg parameter(W).The Rs,Rctand W parameters of the electrode material is 0.003 Ω,1.15 Ω and 0.08 Ω,respectively.Rctvalues of MoS2, MoS2/Ni3S2and Ni3S2electrodes accord with the order of:MoS2/Ni3S2(1.15 Ω)<MoS2(1.85 Ω)<Ni3S2(2.15 Ω).

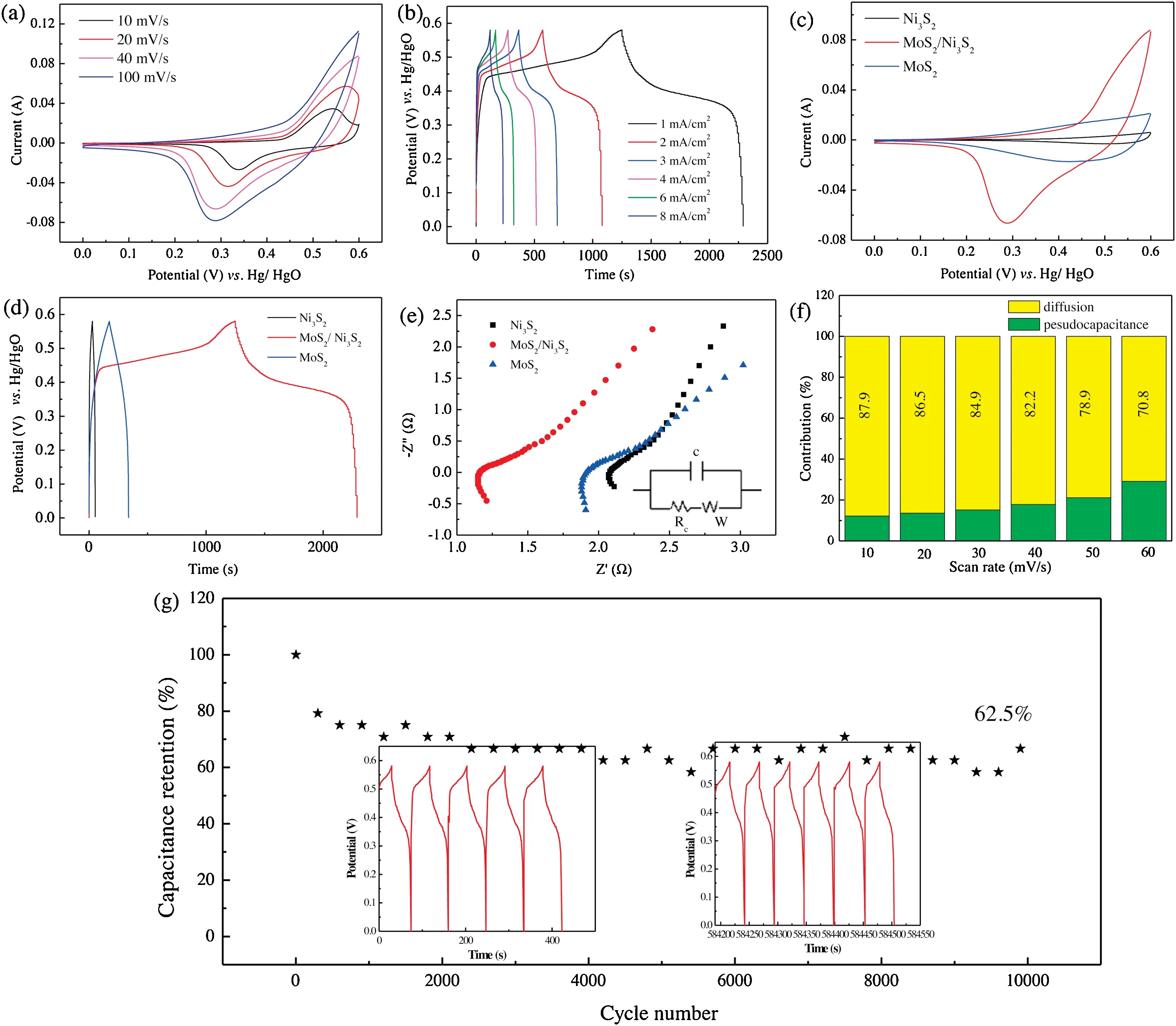
Fig.3.Electrochemical performance:(a)CV curves at different scan rates;(b)GCD curves at different current density;(c)CV curves of MoS2,Ni3S2 and MoS2/Ni3S2 at a scan of 40 mV/s;(d)GCD curves of MoS2,Ni3S2 and MoS2/Ni3S2 at 1 mA/cm2;(e)Nyquist plots of MoS2,Ni3S2 and MoS2/Ni3S2;(f)Contribution ratio between capacitive capacities and diffusion-limited capacities; (g) Cycling performance at the current density of 20 mA/cm2.
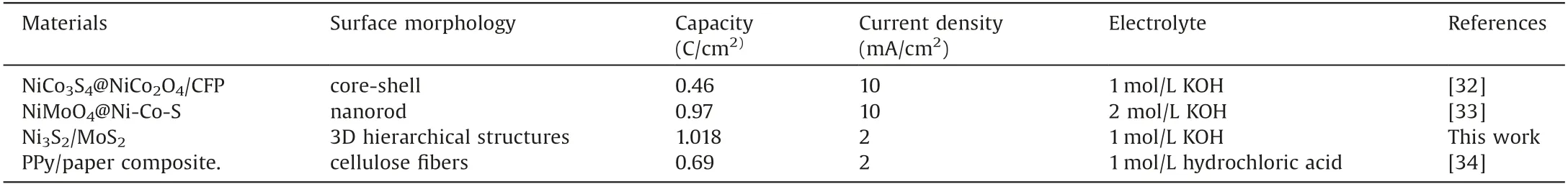
Table 1 A comparison of MoS2/Ni3S2 with previous reported electrode materials.
Capacitive contribution can be studied by the following formula[39]:where i,v,k1and k2denotes for the measured current,sweep rate and constant, respectively.The calculated capacitance and diffusion-controlled redox values are presented in Fig.3f.
Pseudo-capacitance controlled capacitance proportions of MoS2/Ni3S2are 12.1%,13.5%,15.1%,17.8%,21.1%and 29.2%at sweep rates of 1, 2, 3, 4, 6 and 10 mV/s, respectively.It shows that diffusion-controlled reaction is predominant in the total capacitance for hybrid electrode,indicating that hybrid structure benefit the permeation of OH-.To further study the stability of fabricated electrode based on hierarchical MoS2/Ni3S2structure, the cycling stability test is conducted at a current density of 20 mA/cm2.After 10,000 cycles,the capacitance of hybrid electrode maintains 62.5%of it first capacitance which revealed high stability of the fabricated electrode (Fig.3g).
To investigate the practical application,an asymmetric capacitor is assembled by utilizing hierarchical MoS2/Ni3S2structure as a cathode and AC as an anode one.To obtain optimal property of the fabricated device, the mass loadings of the electrodes should match well based on charge balance.Mass ratio of the electrodes is calculated to be 5:1 according to Eq.(4).CV curves of MoS2/Ni3S2and active carbon electrodes are compared,as shown in Fig.4a.It was found that two electrodes possess a potential window from 0 V to 0.6 V and -1 V to 0 V, respectively.Fig.4b shows CV curves of MoS2/Ni3S2electrode at scan rates from 10 mV/s to 100 mV/s.With the sweep rate increasing,the shape of the curve does not change,revealing that the fabricated device behaves excellent capacitive characteristic.The performance of the device is then evaluated by GCD curves at different current densities (Fig.4c).The device possesses a voltage window from 0 to 1.58 V.Energy and power density of the fabricated device were studied by the equations[40]:
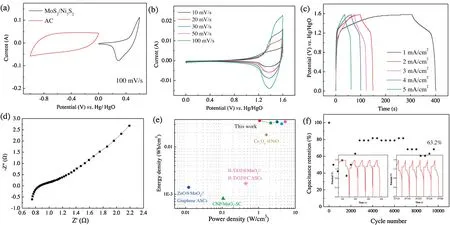
Fig.4.Electrochemical performance in two-electrode system:(a)CV curves of MoS2/Ni3S2 and AC electrodes at 100 mV/s;(b)CV curves at different scan rates;(c)GCD curves at different current densities; (d) Nyquist plots; (e) Ragone plot; (f) cycling performance at a current density of 8 mA/cm2.

where,E(mWh/cm3)is energy density,P(W/cm3)denotes power density, C is specific capacitance, V is the voltage and Δt refers to discharge time.Energy and power densities of asymmetric supercapacitor devices could be compared with previously reports(Fig.4d).According to the above mentioned equations, the calculated energy density of the fabricated device was 35.93 mWh/cm-3at 1064.76 mW/cm3,which is superior to the literature[41-44].Firstly,Liu et al.constructed high-capacitance conductive yarns with hierarchical structures composed of deposited rGO,MnO2, and PPy.The device delivers an energy densities of 0.0092 mWh/cm2and 1.1 mWh/cm3[45].In addition, Xing et al.synthesized Co3O4nanowires@NiO nanosheet arrays,the energy density of the device was 0.152 mWh/cm3[42].At the same time,cycling stability of the device also is tested at the current density of 8 mA/cm2, revealing that the fabricated asymmetric supercapacitor device possesses 62.5% retention even after 10,000 cycles, as shown in Fig.4f.
In summary,hierarchical MoS2/Ni3S2structures are successfully synthesized by a facile solution route and used to fabricate asymmetric supercapacitor electrode material.Interestingly, the fabricated electrode delivers a capacitance of 1.033 C/cm2at current density of 1 mA/cm2.Due to synergistic effect of hybrid structure, the as-fabricated device exhibits high energy density and long-term cycle stability.Clearly, the observed results demonstrate that hierarchical MoS2/Ni3S2structures based hybrid electrodes are promising and efficient electrode material for highperformance energy storage devices.
Acknowledgment
This project is supported by State Key Laboratory of New Ceramic and Fine Processing Tsinghua University (No.KF201807).
杂志排行
Chinese Chemical Letters的其它文章
- Synthesis of aza-BODIPY dyes bearing the naphthyl groups at 1,7-positions and application for singlet oxygen generation
- Flower-like Cu5Sn2S7/ZnS nanocomposite for high performance supercapacitor
- Degradation of p-nitrophenol(PNP)in aqueous solution by mFe/Cu-air-PS system
- The “Fingerprint” of a freshwater microalga Scenedesmus sp.LX1:Visualizing the composition of its soluble algal products
- Surface modulated hierarchical graphene film via sulfur and phosphorus dual-doping for high performance flexible supercapacitors
- Effects on the thermal expansion coefficient and dielectric properties of CLST/PTFE filled with modified glass fiber as microwave material
